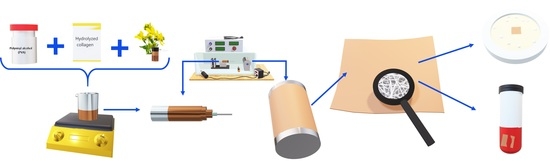
PVA-Based Electrospun Biomembranes with Hydrolyzed Collagen and Ethanolic Extract of Hypericum perforatum for Potential Use as Wound Dressing: Fabrication and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ethanolic Extract of Hypericum perforatum L. (EEHP)
2.3. Solutions Preparation
2.4. Fabrication of Electrospun Membranes
2.5. Physicochemical Characterization of the Polymeric Solutions
2.6. Morphological and Physical Characterization
2.6.1. Morphological Characterization
2.6.2. Porosity Evaluation
2.6.3. Thickness
2.6.4. Barrier Properties
2.7. Chemical, Thermal, and Structural Characterization
2.7.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.7.2. X-ray Diffraction
2.7.3. Differential Scanning Calorimetry (DSC)
2.8. Biological Characterization
2.8.1. Anti-Inflammatory Activity In Vitro Assessment
2.8.2. Antimicrobial Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Solutions Characterization
3.2. Fabrication and Microstructural Characterization of the Electrospun Membranes
3.3. Barrier Properties: Water Vapor Permeability (WVP) and Water Vapor Transmission Rate (WVTR)
3.4. Chemical, Structural, and Thermal Characterization
3.5. X-ray Diffraction (XRD)
3.6. Differential Scanning Calorimetry
3.7. Biological Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Lin, T.; Fang, J.; Yao, G.; Zhao, H.; Dodson, M.; Wang, X. In vivo wound healing and antibacterial performances of electrospun nanofibre membranes. J. Biomed. Mater. Res. A 2010, 94, 499–508. [Google Scholar] [PubMed]
- Liakos, I.; Rizzello, L.; Hajiali, H.; Brunetti, V.; Carzino, R.; Pompa, P.P.; Athanassiou, A.; Mele, E. Fibrous wound dressings encapsulating essential oils as natural antimicrobial agents. J. Mater. Chem. B 2015, 3, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
- Pourhojat, F.; Sohrabi, M.; Shariati, S.; Mahdavi, H.; Asadpour, L. Evaluation of poly e-caprolactone electrospun nanofibers loaded with Hypericum perforatum extract as a wound dressing. Res. Chem. Intermed. 2017, 43, 297–320. [Google Scholar] [CrossRef]
- Eğri, Ö.; Erdemir, N.; Gevrek, F. Effect of H. perforatum oil containing membranes on the second degree burn wound in rats. Mater. Today Commun. 2020, 24, 10095424. [Google Scholar] [CrossRef]
- Thakur, R.A.; Florek, C.A.; Kohn, J.; Michniak, B.B. Electrospun nanofibrous polymeric scaffold with targeted drug release profiles for potential application as wound dressing. Int. J. Pharm. 2008, 364, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Kim, K.; Fang, D.; Ran, S.; Hsiao, B.S.; Chu, B. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer 2002, 43, 4403–4412. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Ouyang, J.; Zhang, L.; Xue, J.; Zhang, H.; Tao, W. Electroactive electrospun nanofibers for tissue engineering. Nano Today 2021, 39, 101196. [Google Scholar] [CrossRef]
- Ziai, Y.; Petronella, F.; Rinoldi, C.; Nakielski, P.; Zakrzewska, A.; Kowalewski, T.A.; Augustyniak, W.; Li, X.; Calogero, A.; Sabała, I.; et al. Chameleon-inspired multifunctional plasmonic nanoplatforms for biosensing applications. NPG Asia Mater. 2022, 14, 1–17. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospun formulation of acyclovir/cyclodextrin nanofibers for fast-dissolving antiviral drug delivery. Mater. Sci. Eng. C 2020, 118, 111514. [Google Scholar] [CrossRef]
- Azimi, B.; Bafqi, M.S.S.; Fusco, A.; Ricci, C.; Gallone, G.; Bagherzadeh, R.; Donnarumma, G.; Uddin, M.J.; Latifi, M.; Lazzeri, A.; et al. Electrospun ZnO/Poly(Vinylidene Fluoride-Trifluoroethylene) Scaffolds for Lung Tissue Engineering. Tissue Eng. Part A 2020, 26, 1312–1331. [Google Scholar] [CrossRef]
- Sill, T.J.; Von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Unnithan, A.F.; Joshi, M.K.; Tiwari, A.P.; Hong, S.T.; Park, C.H.; Kim, C.N. Electrospun bioactive poly (e-caprolactone)—cellulose acetate—dextran antibacterial composite mats for wound dressing applications. Colloid Surf. A Physicochem. Eng. Asp. 2015, 469, 194–201. [Google Scholar] [CrossRef]
- Aguirre-Chagala, Y.E.; Altuzar, V.; León-Sarabia, E.; Tinoco-Magaña, J.C.; Yañez-Limón, J.M.; Mendoza-Barrera, C. Physicochemical properties of polycaprolactone/collagen/elastin nanofibers fabricated by electrospinning. Mater. Sci. Eng. C 2017, 76, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.A.; Schiffman, J.D. Electrospinning an essential oil: Cinnamaldehyde enhances theantimicrobial efficacy of chitosan/poly(ethylene oxide) nanofibers. Carbohydr. Polym. 2014, 113, 561–568. [Google Scholar] [CrossRef]
- Azimi, B.; Maleki, H.; Gigante, V.; Bagherzadeh, R.; Mezzetta, A.; Milazzo, M.; Guazzelli, L.; Cinelli, P.; Lazzeri, A.; Danti, S. Cellulose-based fiber spinning processes using ionic liquids. Cellulose 2022, 29, 3079–3129. [Google Scholar] [CrossRef]
- Danti, S.; Anand, S.; Azimi, B.; Milazzo, M.; Fusco, A.; Ricci, C.; Zavagna, L.; Linari, S.; Donnarumma, G.; Lazzeri, A.; et al. Chitin Nanofibril Application in Tympanic Membrane Scaffolds to Modulate Inflammatory and Immune Response. Pharmaceutics 2021, 13, 1440. [Google Scholar] [CrossRef]
- Saxena, S. Polyvynyl alcohol (PVA). In Chemical and Technical Assessment; FAO: Rome, Italy, 2004. [Google Scholar]
- Gule, N.P.; de Kwaadsteniet, M.; Cloete, T.E.; Klumperman, B. Electrospun Poly(vinyl alcohol) Nanofibres with Biocidal Additives for Application in Filter Media, 1-Properties Affecting Fibre Morphology and Characterisation. Macromol. Mater. Eng. 2012, 297, 609–617. [Google Scholar] [CrossRef]
- Naseri, N.; Algana, C.; Jacobs, V.; John, M.; Oksmana, K.; Mathew, A.P. Electrospun chitosan-based nanocomposite mats reinforced withchitin nanocrystals for wound dressing. Carbohydr. Polym. 2014, 109, 7–15. [Google Scholar] [CrossRef]
- Li, T.T.; Yan, M.; Zhonga, Y.; Ren, H.T.; Lou, C.W.; Huang, S.Y.; Lin, J.H. Processing and characterizations of rotary linear needleless electrospun polyvinyl alcohol (PVA)/ chitosan (CS)/ graphene (Gr) nanofibrous membranes. J. Mater. Res. Technol. 2019, 8, 5124–5132. [Google Scholar] [CrossRef]
- Kang, Y.O.; Yoon, I.-S.; Lee, S.Y.; Kim, D.-D.; Lee, S.J.; Park, W.H.; Hudson, S.M. Chitosan-coated poly(vinyl alcohol) nanofibers for wound dressings. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 9999B, 568–576. [Google Scholar] [CrossRef]
- Zine, R.; Sinha, M. Nanofibrous poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/collagen/graphene oxide scaffolds for wound coverage. Mater. Sci. Eng. C 2017, 80, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, C.; Zhao, Y.; Hu, J.; Zhang, L.-M. Co-electrospun Nanofibrous Membranes of Collagen and Zein for Wound Healing. ACS Appl. Mater. Interfaces 2012, 4, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.J.; Phan, T.T.; Lim, I.J.; Zhang, Y.; Bay, B.H.; Ramakrishna, S.; Lim, C.T. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, P.; Nikhil, K.; Roy, P.; Pruthi, P.A.; Singh, R.P.; Pruthi, V. A novel gellan–PVA nanofibrous scaffold for skin tissue regeneration: Fabrication and characterization. Carbohydr. Polym. 2016, 136, 851–859. [Google Scholar] [CrossRef]
- Sibilla, S.; Godfrey, M.; Brewer, S.; Budh-Raja, A.; Genovese, L. An Overview of the Beneficial Effects of Hydrolysed Collagen as a Nutraceutical on Skin Properties: Scientific Background and Clinical Studies. Open Nutraceuticals J. 2015, 8, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Guillerminet, F.; Fabien-Soulé, V.; Even, P.; Tomé, D.; Benhamou, C.-L.; Roux, C.; Blais, A. Hydrolyzed collagen improves bone status and prevents bone loss in ovariectomized C3H/HeN mice. Osteoporos. Int. 2011, 23, 1909–1919. [Google Scholar] [CrossRef]
- De la Ossa, J.G.; Felice, F.; Azimi, B.; Salsano, J.E.; Digiacomo, M.; Macchia, M.; Danti, S.; Di Stefano, R. Waste Autochthonous Tuscan Olive Leaves (Olea europaea var. Olivastra seggianese) as Antioxidant Source for Biomedicine. Int. J. Mol. Sci. 2019, 20, 5918. [Google Scholar] [CrossRef] [Green Version]
- De la Ossa, J.G.; Fusco, A.; Azimi, B.; Esposito Salsano, J.E.; Digiacomo, M.; Coltelli, M.-B.; De Clerck, K.; Roy, I.; Macchia, M.; Lazzeri, A.; et al. Immunomodulatory Activity of Electrospun Polyhydroxyalkanoate Fiber Scaffolds Incorporating Olive Leaf Extract. Appl. Sci. 2021, 11, 4006. [Google Scholar] [CrossRef]
- Nazlı, O.; Baygar, T.; Dönmez, Ç.E.D.; Dere, Ö.; Uysal, A.İ.; Aksözek, A.; Işık, C.; Aktürk, S. Antimicrobial and antibiofilm activity of polyurethane/Hypericum perforatum extract (PHPE) composite. Bioorg. Chem. 2019, 82, 224–228. [Google Scholar] [CrossRef]
- Güneş, S.; Tıhmınlıoğlu, F. Hypericum perforatum incorporated chitosan films as potential bioactive wound dressing material. Int. J. Biol. Macromol. 2017, 102, 933–943. [Google Scholar] [CrossRef]
- Bölgen, N.; Demir, D.; Yalçın, M.S.; Özdemir, S. Development of Hypericum perforatum oil incorporated antimicrobial and antioxidant chitosan cryogel as a wound dressing material. Int. J. Biol. Macromol. 2020, 161, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Correia, I.J. Electrospun Polycaprolactone/Aloe Vera_Chitosan Nanofibrous Asymmetric Membranes Aimed for Wound Healing Applications. Polymers 2017, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Mouro, C.; Dunne, C.P.; Gouveia, I.C. Designing New Antibacterial Wound Dressings: Development of a Dual Layer Cotton Material Coated with Poly(Vinyl Alcohol)_Chitosan Nanofibers Incorporating Agrimonia eupatoria L. Extract. Molecules 2020, 26, 83. [Google Scholar] [CrossRef] [PubMed]
- Rad, Z.P.; Mokhtari, J.; Abbasi, M. Calendula officinalis extract/PCL/Zein/Gum arabic nanofibrous bio-composite scaffolds via suspension, two-nozzle and multilayer electrospinning for skin tissue engineering. Int. J. Biol. Macromol. 2019, 135, 530–543. [Google Scholar]
- Lin, L.; Dai, Y.; Cui, H. Antibacterial poly(ethylene oxide) electrospun nanofibers containing cinnamon essential oil/beta-cyclodextrin proteoliposomes. Carbohydr. Polym. 2017, 178, 131–140. [Google Scholar] [CrossRef]
- Swarup RJong-Whan, R. Preparation of bioactive functional poly(lactic acid)/curcumin composite film for food packaging application. Int. J. Biol. Macromol. 2020, 162, 1780–1789. [Google Scholar]
- Azimi, B.; Thomas, L.; Fusco, A.; Kalaoglu-Altan, O.I.; Basnett, P.; Cinelli, P.; De Clerck, K.; Roy, I.; Donnarumma, G.; Coltelli, M.-B.; et al. Electrosprayed Chitin Nanofibril/Electrospun Polyhydroxyalkanoate Fiber Mesh as Functional Nonwoven for Skin Application. J. Funct. Biomater. 2020, 11, 62. [Google Scholar] [CrossRef]
- Hashmi, M.; Ullah, S.; Kim, I.S. Electrospun Momordica charantia incorporated polyvinyl alcohol (PVA) nanofibers for antibacterial applications. Mater. Today Commun. 2020, 24, 101161. [Google Scholar] [CrossRef]
- Naeimi, A.; Payandeh, M.; Ghara, A.R.; Ghadi, F.E. In vivo evaluation of the wound healing properties of bio-nanofiber chitosan/ polyvinyl alcohol incorporating honey and Nepeta dschuparensis. Carbohydr. Polym. 2020, 240, 116315. [Google Scholar] [CrossRef]
- Güzel, A.; Akyüz, M.; Sanda, M.A. Determination of Antioxidant activity of Hypericum perforatum. J. Integr. Med. 2019, 1, 9–18. [Google Scholar]
- Nikolic, G.S.; Zlatkovic, S.Z. Assaying the variation in secondary metabolites of St.John’s wort for its better use as an antibiotic. J. Med. Plant Res. 2010, 4, 211–224. [Google Scholar]
- Galeotti, N. Hypericum perforatum (St. John’s wort) beyond depression: A therapeutic. J. Ethnopharmacol. 2017, 200, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Pakolpakçıl, A.; Draczyński, Z.; Szulc, J.; Stawski, D.; Tarzyńska, N.; Bednarowicz, A.; Sikorski, D.; Hernandez, C.; Sztajnowski, S.; Krucińska, I.; et al. An In Vitro Study of Antibacterial Properties of Electrospun Hypericum perforatum Oil-Loaded Poly(lactic Acid) Nonwovens for Potential Biomedical Applications. Appl. Sci. 2021, 11, 8219. [Google Scholar] [CrossRef]
- Guleken, Z.; Depciuch, J.; Ege, H.; Ilbay, G.; Kalkandelen, C.; Ozbeyli, D.; Bulut, H.; Sener, G.; Tarhan, N.; Kuruca, S.E. Spectrochemical and biochemical assay comparison study of the healing effect of the Aloe vera and Hypericum perforatum loaded nanofiber dressings on diabetic wound. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 254, 119639. [Google Scholar] [CrossRef]
- García-Hernández, A.B.; Calderón-Domínguez GMorales-Sánchez, E.; Salgado-Cruz, M.P.; Farrera-Rebollo, R.R.; García-Bórquez, A.; Vega-Cuellar, M.A. Hydrolyzed collagen on PVA-based electrospun membranes: Synthesis and characterization. J. Appl. Polym. Sci. 2021, 138, e551197. [Google Scholar] [CrossRef]
- National Institute of Health. ImageJ/FIJI Software; National Institute of Health: New York, NY, USA, 2018. [Google Scholar]
- Hotaling, N.A.; Bharti, K.; Kriel, H.; Simon, C.G. DiameterJ: A validated open source nanofiber diameter measurement tool. Biomaterials 2015, 61, 327–338. [Google Scholar] [CrossRef] [Green Version]
- Cazón, P.; Vázquez, M.; Velazquez, G. Novel composite films based on cellulose reinforced with chitosan and polyvinyl alcohol: Effect on mechanical properties and water vapour permeability. Polym. Test. 2018, 69, 536–544. [Google Scholar] [CrossRef]
- Valdespino León, M.; Calderón-Domínguez, G.; Salgado Cruz, M.D.L.P.; Rentería Ortega, M.; Farrera Rebollo, R.R.; Morales Sánchez, E.; Gaona Sánchez, V.A.; Terrazas Valencia, F. Biodegradable electrosprayed pectin films: An alternative to valorize cofee mucilage. Waste Biomass Valorization 2020, 12, 2477–2494. [Google Scholar] [CrossRef]
- Tretinnikov, O.Z.; Zagorskaya, S.A. Determination of the degree of crystallinity of poly(vinyl alcohol) by FTIR spectroscopy. J. Appl. Spectrosc. 2012, 79, 521–526. [Google Scholar] [CrossRef]
- Islam, M.; Karim, M. Fabrication and characterization of poly(vinyl alcohol)/alginate blend nanofibers by electrospinning method. Colloids Surf. A Physicochem. Eng. Asp. 2010, 366, 135–140. [Google Scholar] [CrossRef]
- Kumar, S.; Bajwa, B.; Kuldeep, S.; Kalia, A. Anti-Inflammatory Activity of Herbal Plants: A review. Int. J. Pharm. Pharm. Sci. Biol. Chem. 2013, 2, 272–281. [Google Scholar]
- Kardile, M.V.; Mahajan, U.B.; Shaikh, H.M.; Goyal, S.N.; Patil, C.R. Membrane stabilization assay for anti-inflammatory activity yields false positive results for samples containing traces of ethanol and methanol. World J. Pharm. Pharm. Sci. 2016, 5, 493–497. [Google Scholar]
- Chaitanya Chippada, S.; Suresh Volluri, S.; Bammidi, R.; Sand Vangalapati, M. In vitro anti-inflammatory activity of methanolic extract of Centella asiatica by HBRC membrane stabilization. J. Chem. 2011, 4, 457–460. [Google Scholar]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2015, 34, 422–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çakmak, H.; Özselek, Y.; Turan, O.Y. Fıratlıgil, E.; Karbancioğlu-Güler, F. Whey protein isolate edible films incorporated with essential oils: Antimicrobial activity and barrier properties. Polym. Degrad. Stab. 2020, 179, 109285. [Google Scholar] [CrossRef]
- Pujol, M.; Limón, E. Epidemiología general de las infecciones nosocomiales. Sist. Y Programas Vigilancia 2013, 31, 108–113. [Google Scholar] [CrossRef]
- Alonso-Ibarra, M.R.; Silva-Lucero, M.C.; Zacapala-Gómez, A.E.; Barrios-Casarrubias, A.; Muñoz-Castillo, M.S. Frecuencia de infecciones bacterianas de heridas quirúrgicas en dos hospitales de Chilpancingo, Guerrero. Mediagraphic 2009, 34, 99. [Google Scholar]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun nanofibers: New generation materials for advanced applications. Mater. Sci. Eng. B 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Dong, H.; Wang, N.; Wang, L.; Bai, H.; Wu, J.; Zheng, Y.; Jiang, L.; Zhao, Y. Bioinspired Electrospun Knotted Microfibers for Fog Harvesting. Chem. Phys. Chem. 2012, 13, 1153–1156. [Google Scholar] [CrossRef]
- Amariei, N.; Manea, L.R.; Bertea, A.P.; Bertea, A.; Popa, A. The Influence of Polymer Solution on the Properties of Electrospun 3D Nanostructures. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, 012092. [Google Scholar] [CrossRef] [Green Version]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Tan, N.C.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Zhao, H.; Chi, H. Electrospun Bead-on-String Fibers: Useless or Something of Value. In Novel Aspects of Nanofibers; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Li, T.; Ding, X.; Tian, L.; Hu, J.; Yang, X.; Ramakrishna, S. The control of beads diameter of bead-on-string electrospun nanofibers and the corresponding release behaviors of embedded drugs. Mater. Sci. Eng. C 2017, 74, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Structure and properties of nanohydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.; Yannas, I.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. 2004, 71, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, A.; Matic, A.; Jacob, P.; Borjesson, L.; Navarra, M.; Fernicola, A.; Panero, S.; Scrosati, B. Structural analysis of PVA-based proton conducting membranes. Solid State Ion. 2006, 177, 2431–2435. [Google Scholar] [CrossRef]
- Matsuura, H.N.; Fett-Neto, A.G. Plant Alkaloids: Main Features, Toxicity, and Mechanisms of Action. Plant Toxins 2015, 2, 1–15. [Google Scholar]
- Bonilla, J.; Fortunati, E.L.E.N.A.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, structural and antimicrobial properties of poly vinyl alcohol–chitosan biodegradable films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Morgado, P.I.; Lisboa, P.F.; Ribeiro, M.P.; Miguel, S.P.; Simões, P.C.; Correia, I.J.; Aguiar-Ricardo, A. Poly(vinyl alcohol)/chitosan asymmetrical membranes: Highly controlled morphology toward the ideal wound dressing. J. Membr. Sci. Res. 2014, 469, 262–271. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, L.; Yuan, T.; Zhang, Q.; Fan, H. Asymmetric polyurethane membrane with in situ-generated nano-TiO2 as wound dressing. J. Appl. Polym. Sci. 2011, 19, 1532–1541. [Google Scholar] [CrossRef]
- Meral, G.; Karabay, N.Ü. In vitro antibactrial activities of three Hypericum species from West Anatolia . Turk. Electron. J. Biotechnol. 2002, 19, 6–10. Available online: https://www.maltawildplants.com/CLUS/Docs/HYPTQ/Antimicrobialproperties.pdf (accessed on 27 February 2022).
- Calderón-Arenas, J.M.; Martínez-Rincón, H.A. Obtención de Fibras Poliméricas a Partir de la Técnica de Electrospinning Para Aplicaciones Biomédicas; Universidad Autónoma de Occidente: Santiago de Cali, Colombia, 2012. [Google Scholar]
- Miller, E.J.; Rhodes, R.K. Preparation and Characterization of the Different Types of Collagen. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1982; Volume 82, pp. 33–64. [Google Scholar]
- Asran, A.S.; Henning, S.; Michler, G.H. Polyvinyl alcohol–collagen–hydroxyapatite biocomposite nanofibrous scaffold: Mimicking the key features of natural bone at the nanoscale level. Polymer 2010, 51, 868–876. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, V. Design of psyllium–PVA–acrylic acid based novel hydrogels for use in antibiotic drug delivery. Int. J. Pharm. 2010, 389, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Abureesh, M.A.; Oladipo, A.A.; Gazi, M. Facile synthesis of glucose-sensitive chitosan–poly(vinyl alcohol) hydrogel: Drug release optimization and swelling properties. Int. J. Biol. Macromol. 2016, 90, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Sizeland, K.H.; Hofman, K.A.; Hallett, I.C.; Martin, D.E.; Potgieter, J.; Kirby, N.M.; Hawle, A.; Mudie, S.T.; Ryan, T.M.; Haverkamp, R.G.; et al. Nanostructure of electrospun collagen: Do electrospun collagen fibers form native structures. Materaialia 2018, 3, 90–96. [Google Scholar] [CrossRef]
- Schmidt, M.; Fontoura, A.; Vidal, A.; Dornelles, R.; Kubota, E.; Mello, R.; Cansian, R.D.I.O.C. Characterization of hydrolysates of collagen from mechanically separated chicken meat residue. J. Food Sci. Technol. 2019, 40, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Dhayabaran, V.; Margre, A. Nanoparticulated formulations of St. John’s wort (Hypericum perforatum L.) as smart drug delivery system combating depression incited in mice models. Pharmacogn. J. 2017, 5, 187–199. [Google Scholar]
- Taraj, K.; Ciko, L.; Malollari, I.; Andoni, A.; Ylli, F.; Ylli, A.; Plaku, E.; Llupa, J.; Borshi, X. Eco-extraction of essential oil from albanian Hypericum perforatum L. and characterisation by spectroscopy techniques. J. Environ. Prot. Ecol. 2019, 20, 188–195. [Google Scholar]
- Gitea, D.; Terodorescu, A.; Pantis, C.; Tit, D.; Bungau, A.; Bogdan, M.; Fodor, I.; Bustea, C. Green Synthesis of Silver Nanoparticles Using Hypericum perforatum L. Extract and Evaluation of Their Antibacterial Activity. Rev. Chim. 2020, 71, 273–279. [Google Scholar] [CrossRef]
- Cheng, W.; Fan, F.; Zhang, Y.; Pei, Z.; Wang, W.; Pei, Y. A Facile Approach for Fabrication of Core-Shell Magnetic Molecularly Imprinted Nanospheres towards Hypericin. Polymers 2017, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Rudko, G.Y.; Kovalchuk, A.O.; Fediv, V.I.; Chen, W.M.; Buyanova, I.A. Interfacial bonding in a CdS/PVA nanocomposite: A Raman scattering study. J. Colloid Interface Sci. 2015, 452, 33–37. [Google Scholar] [CrossRef]
- Goroškaitė, S. The Formation and Analysis of Electrospun Materials from Nano-Microfibers with Hemp Extract. Master’s Thesis, Kaunas University of Technology, Kaunas, Lithuania, 2020. [Google Scholar]
- Colvin, B.G. Crystal structure of polyvinyl alcohol. Nature 1974, 5451, 248. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Yao, H.; Wang, J.; Wang, D.; Liu, Q. Greener synthesis of electrospun collagen/hydroxyapatite composite fibers with an excellent microstructure for bone tissue engineering. Int. J. Nanomed. 2015, 10, 3203–3215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koosha, M.; Mirzadeh, H. Electrospinning, mechanical properties, and cell behavior study of chitosan/PVA nanofibers. J. Biomed. Mater. Res. Part A 2015, 103, 3081–3093. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Fujii, A.; Saito, A.; Saomoto, H.; Kamada, K. Effect of Crystallinity in Stretched PVA Films on Triplet–Triplet Annihilation Photon Upconversion. ACS Appl. Polym. Mater. 2020, 2, 1422–1428. [Google Scholar] [CrossRef]
- Foltran, I.; Foresti, E.; Parma, B.; Sabatino, P.; Roveri, N. Novel Biologically Inspired Collagen Nanofibers Reconstituted by Electrospinning Method. Macromol. Symp. 2008, 269, 111–118. [Google Scholar] [CrossRef]
- Gauza-Włodarczyk, M.; Kubisz, L.; Mielcarek, S.; Włodarczyk, D. Comparison of thermal properties of fish collagen and bovine collagen in the temperature range 298–670 K. Sci. Eng. C 2017, 80, 468–471. [Google Scholar] [CrossRef]
- Basu, P. Gasification Theory. In Biomass Gasification, Pyrolysis and Torrefaction; Academic Press: Cambridge, MA, USA, 2018; pp. 211–262. [Google Scholar]
- Ding, W.; Wei, S.; Zhu, J.; Chen, X.; Rutman, D. Manipulated Electrospun PVA Nanofibers with Inexpensive Salts. Macromol. Mater. Eng. 2010, 295, 958–965. [Google Scholar] [CrossRef]
- Alakanandana, K.; Subrahmanyam, A.R.; Sayanna, R.; Kumar, J.S. Characterization of C2H2O4 doped PVA solid polymer electrolyte. Polym. Eng. Sci. 2014, 34, 859–866. [Google Scholar] [CrossRef]
- Hammer, K.D.P.; Birt, D.F. Evidence for contributions of interactions of constituents to the anti-inflammatory activity of Hypericum perforatum. Crit. Rev. Food Sci. Nutr. 2013, 54, 781–789. [Google Scholar] [CrossRef]
- Wölfle, U.; Seelinger, G.; Schempp, C.M. Topical Application of St. John’s Wort (Hypericum perforatum). Planta Med. 2014, 80, 109–120. [Google Scholar]
- Cole, G.B.; Bateman, T.J.; Moraes, T.F. The surface lipoproteins of gram-negative bacteria: Protectors and foragers in harsh environments. J. Biol. Chem. 2021, 296, 100147. [Google Scholar] [CrossRef] [PubMed]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2009, 1794, 808–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Concentration [%] * | PVA:PepColl:EEHP Ratio (v:v:v) | Nomenclature | ||
|---|---|---|---|---|
| PVA | HC | EEHP | ||
| 6 | 0 | 0 | 10:0:1 ** | 6-0-0 |
| 6 | 5 | 0 | 10:1:0 | 6-5-0 |
| 6 | 0 | 8 | 10:0:1 | 6-0-8 |
| 6 | 5 | 8 | 10:0.5:0.5 | 6-5-8 |
| 6 | 0 | 16 | 10:0:1 | 6-0-16 |
| 6 | 5 | 16 | 10:0.5:0.5 | 6-5-16 |
| 6 | 0 | 32 | 10:0:1 | 6-0-32 |
| 6 | 5 | 32 | 10:0.5:0.5 | 6-5-32 |
| Tube | Solutions |
|---|---|
| Blank | 0.5 mL of NaCl + 0.25 mL of PBS + 0.375 of DW |
| Blood control (BC) | 0.5 mL of NaCl + 0.25 mL of PBS + 0.125 mL of HRBC + 0.25 mL of isosaline |
| Positive control | 0.5 mL of NaCl + 0.25 mL of PBS + 0.125 mL of HRBC + 0.25 mL of diclofenac |
| Sample | 0.5 mL of NaCl + 0.25 mL of PBS + 0.125 mL of HRBC + 0.25 mL of isosaline solution + membrane |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Hernández, A.B.; Morales-Sánchez, E.; Berdeja-Martínez, B.M.; Escamilla-García, M.; Salgado-Cruz, M.P.; Rentería-Ortega, M.; Farrera-Rebollo, R.R.; Vega-Cuellar, M.A.; Calderón-Domínguez, G. PVA-Based Electrospun Biomembranes with Hydrolyzed Collagen and Ethanolic Extract of Hypericum perforatum for Potential Use as Wound Dressing: Fabrication and Characterization. Polymers 2022, 14, 1981. https://doi.org/10.3390/polym14101981
García-Hernández AB, Morales-Sánchez E, Berdeja-Martínez BM, Escamilla-García M, Salgado-Cruz MP, Rentería-Ortega M, Farrera-Rebollo RR, Vega-Cuellar MA, Calderón-Domínguez G. PVA-Based Electrospun Biomembranes with Hydrolyzed Collagen and Ethanolic Extract of Hypericum perforatum for Potential Use as Wound Dressing: Fabrication and Characterization. Polymers. 2022; 14(10):1981. https://doi.org/10.3390/polym14101981
Chicago/Turabian StyleGarcía-Hernández, Alitzel Belem, Eduardo Morales-Sánchez, Blanca M. Berdeja-Martínez, Monserrat Escamilla-García, Ma. Paz Salgado-Cruz, Minerva Rentería-Ortega, Reynold R. Farrera-Rebollo, Miguel A. Vega-Cuellar, and Georgina Calderón-Domínguez. 2022. "PVA-Based Electrospun Biomembranes with Hydrolyzed Collagen and Ethanolic Extract of Hypericum perforatum for Potential Use as Wound Dressing: Fabrication and Characterization" Polymers 14, no. 10: 1981. https://doi.org/10.3390/polym14101981
APA StyleGarcía-Hernández, A. B., Morales-Sánchez, E., Berdeja-Martínez, B. M., Escamilla-García, M., Salgado-Cruz, M. P., Rentería-Ortega, M., Farrera-Rebollo, R. R., Vega-Cuellar, M. A., & Calderón-Domínguez, G. (2022). PVA-Based Electrospun Biomembranes with Hydrolyzed Collagen and Ethanolic Extract of Hypericum perforatum for Potential Use as Wound Dressing: Fabrication and Characterization. Polymers, 14(10), 1981. https://doi.org/10.3390/polym14101981