Repeatable Self-Healing of a Protective Coating Based on Vegetable-Oil-Loaded Microcapsules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Reaction Behavior Study of Soybean and Olive Oil Mixtures
2.4. Intrinsic Self-Healing Behavior Study of Coated Oil Mixtures
2.5. Microencapsulation
2.6. Preparation of Self-Healing and Control Coatings
2.7. Anticorrosion Test
2.8. Electrochemical Test
3. Results and Discussion
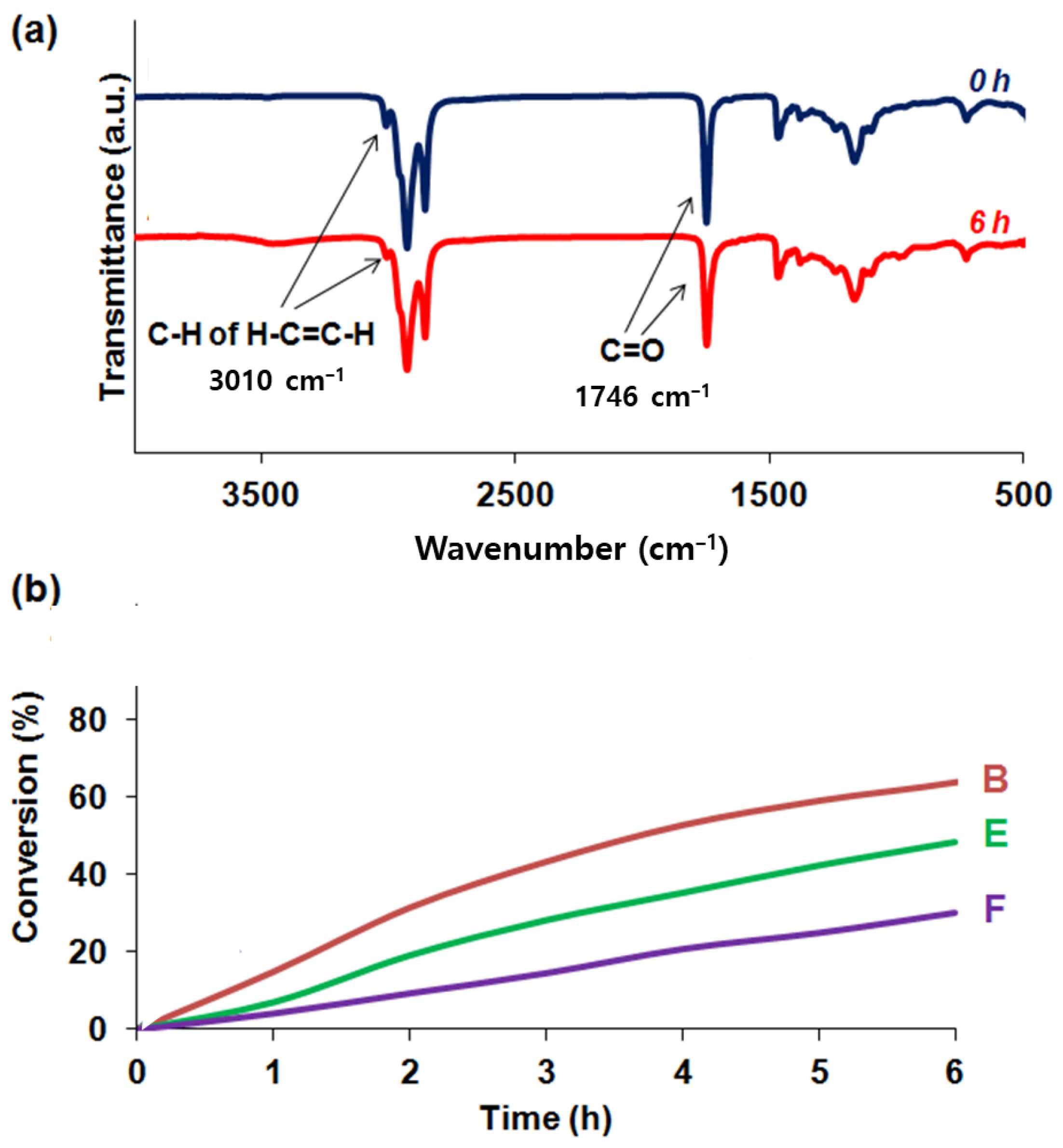
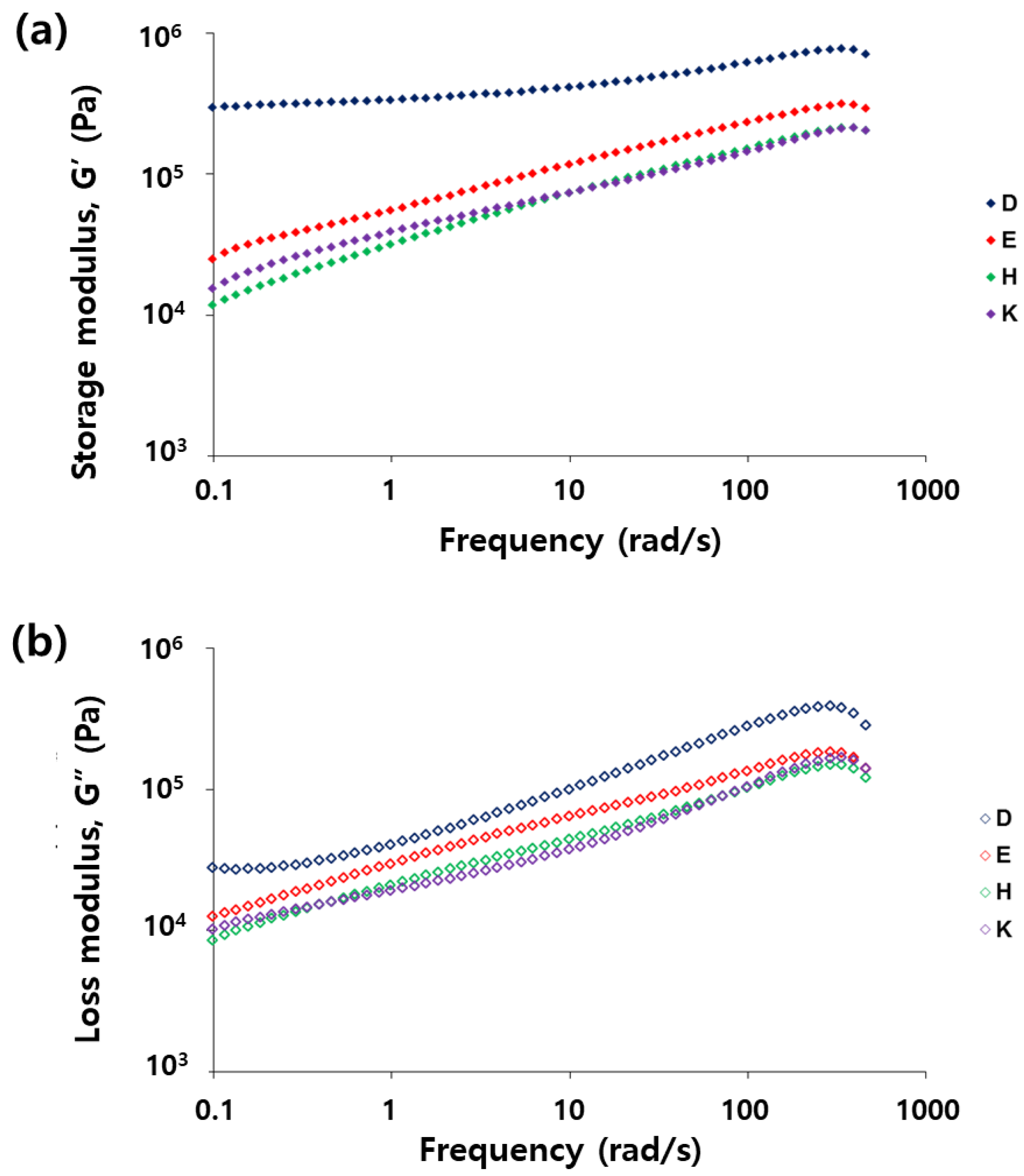
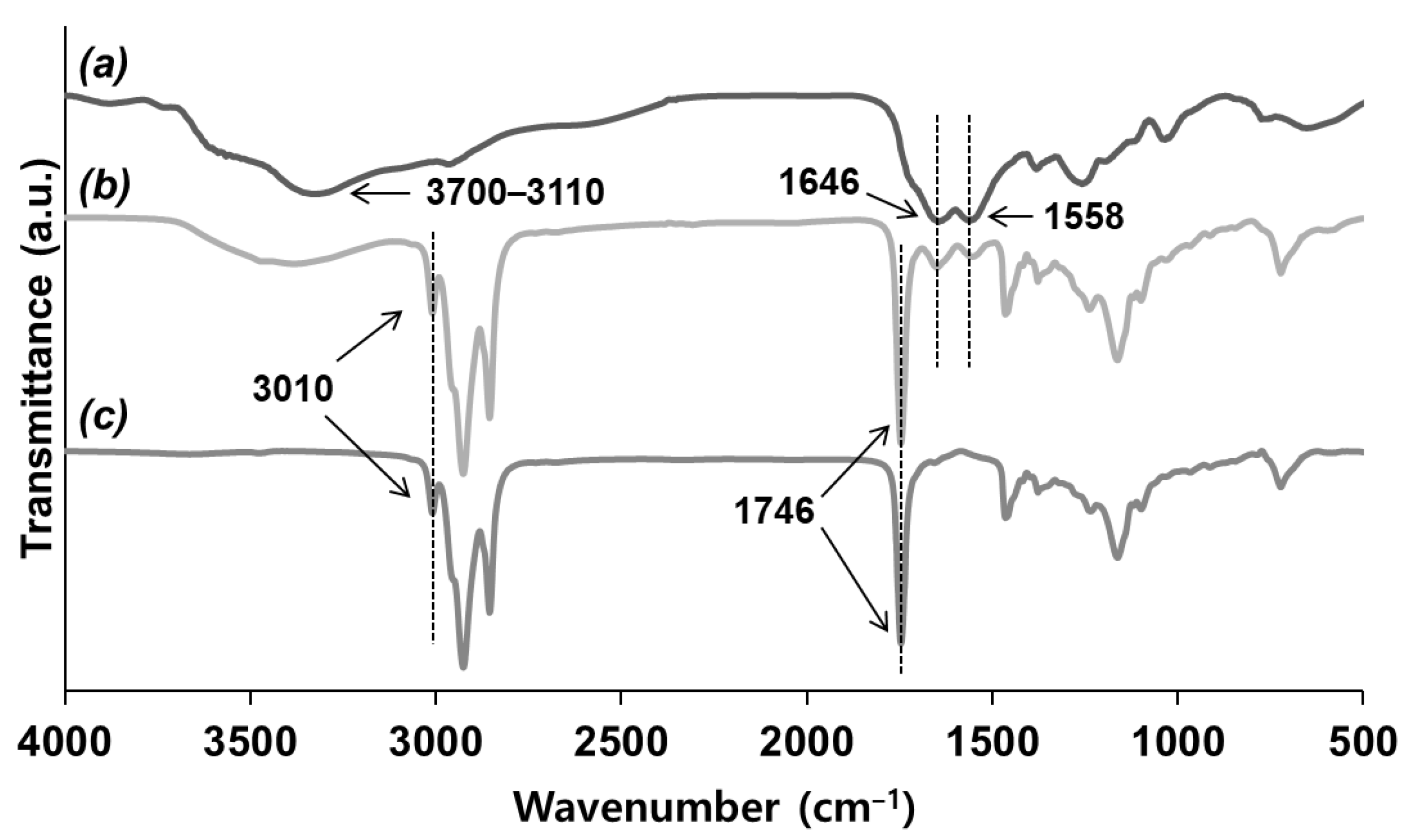
3.1. Reaction of Vegetable Oil Mixtures and Properties of Their Reaction Products
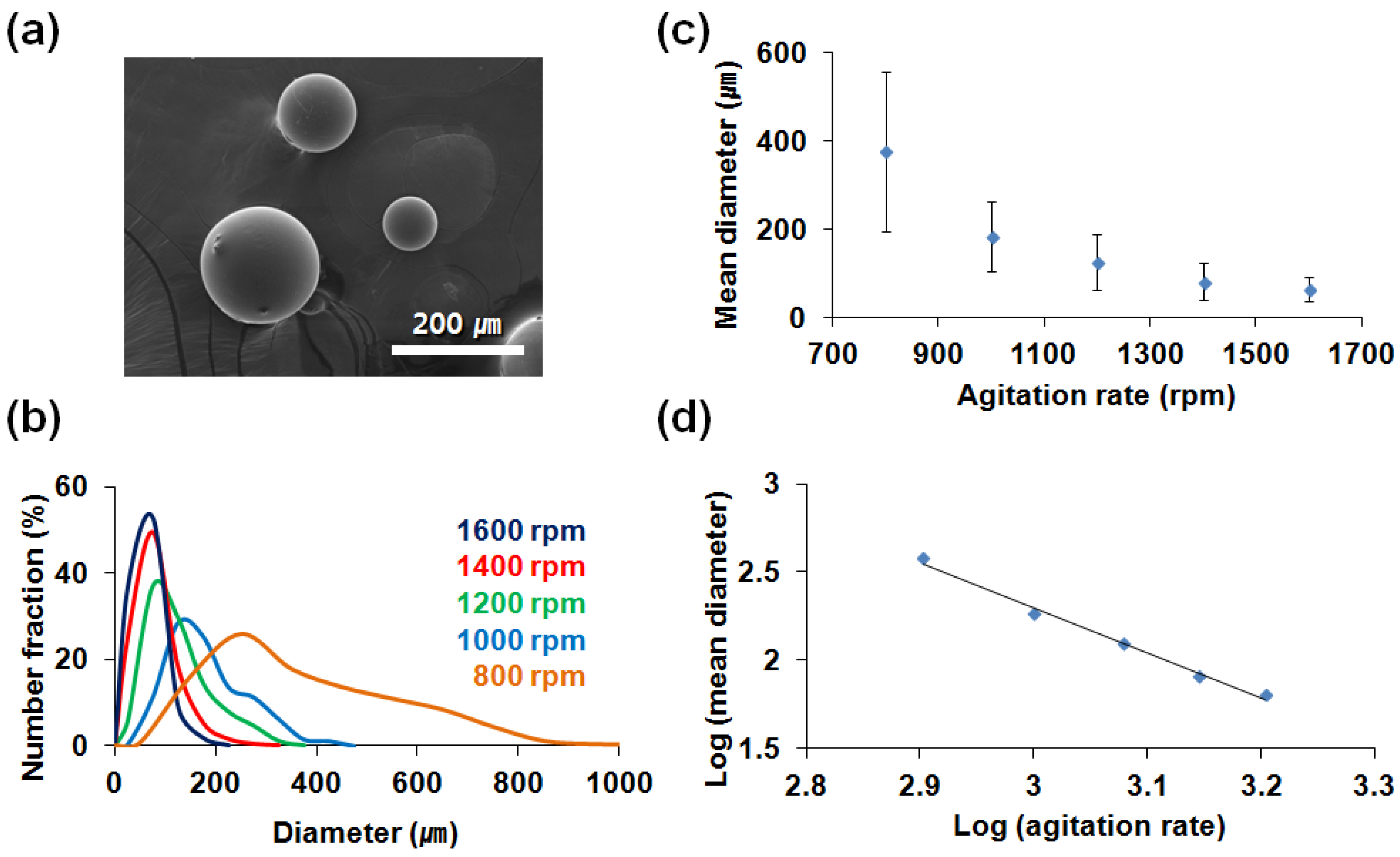
3.2. Microencapsulation
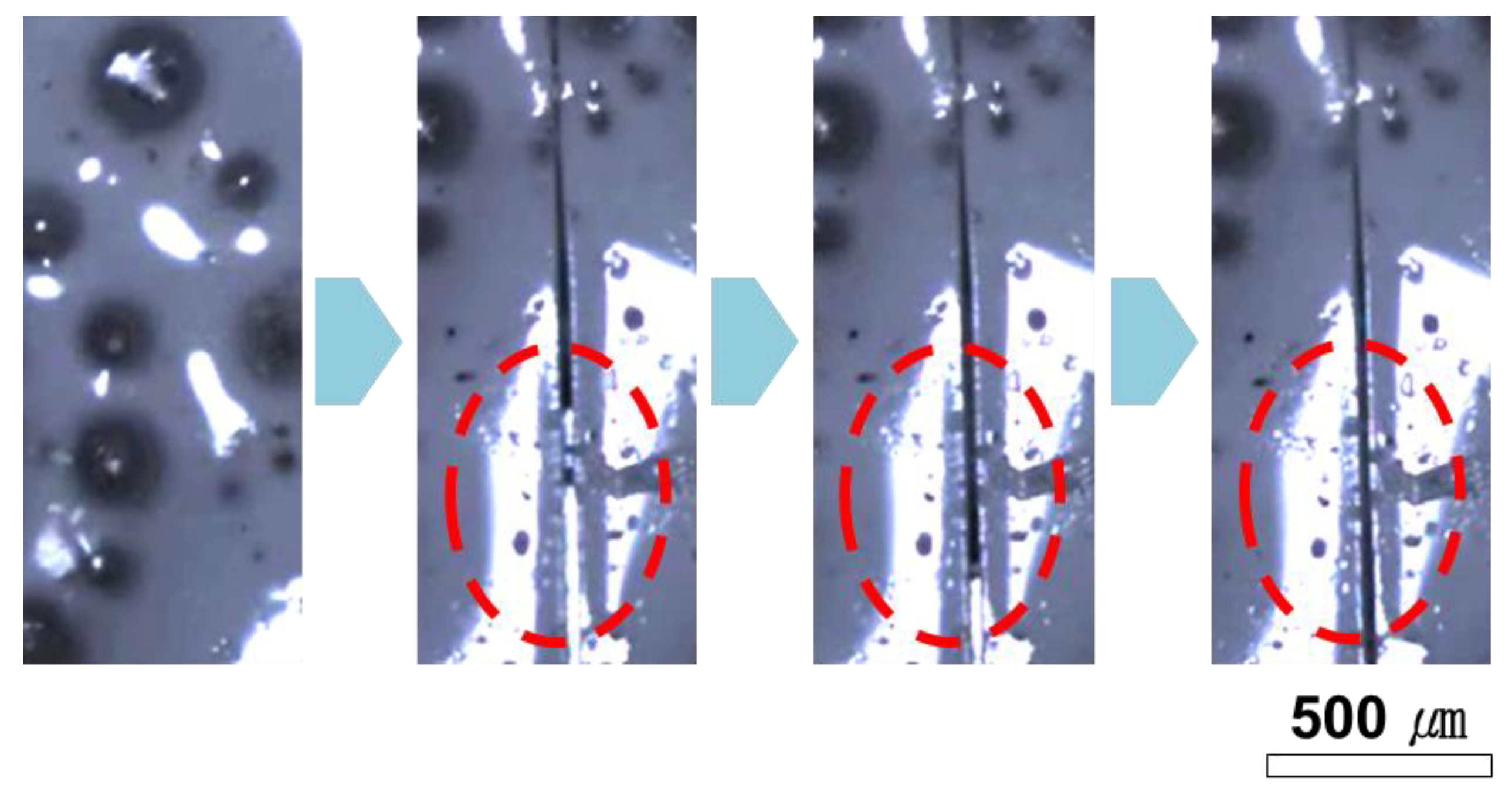
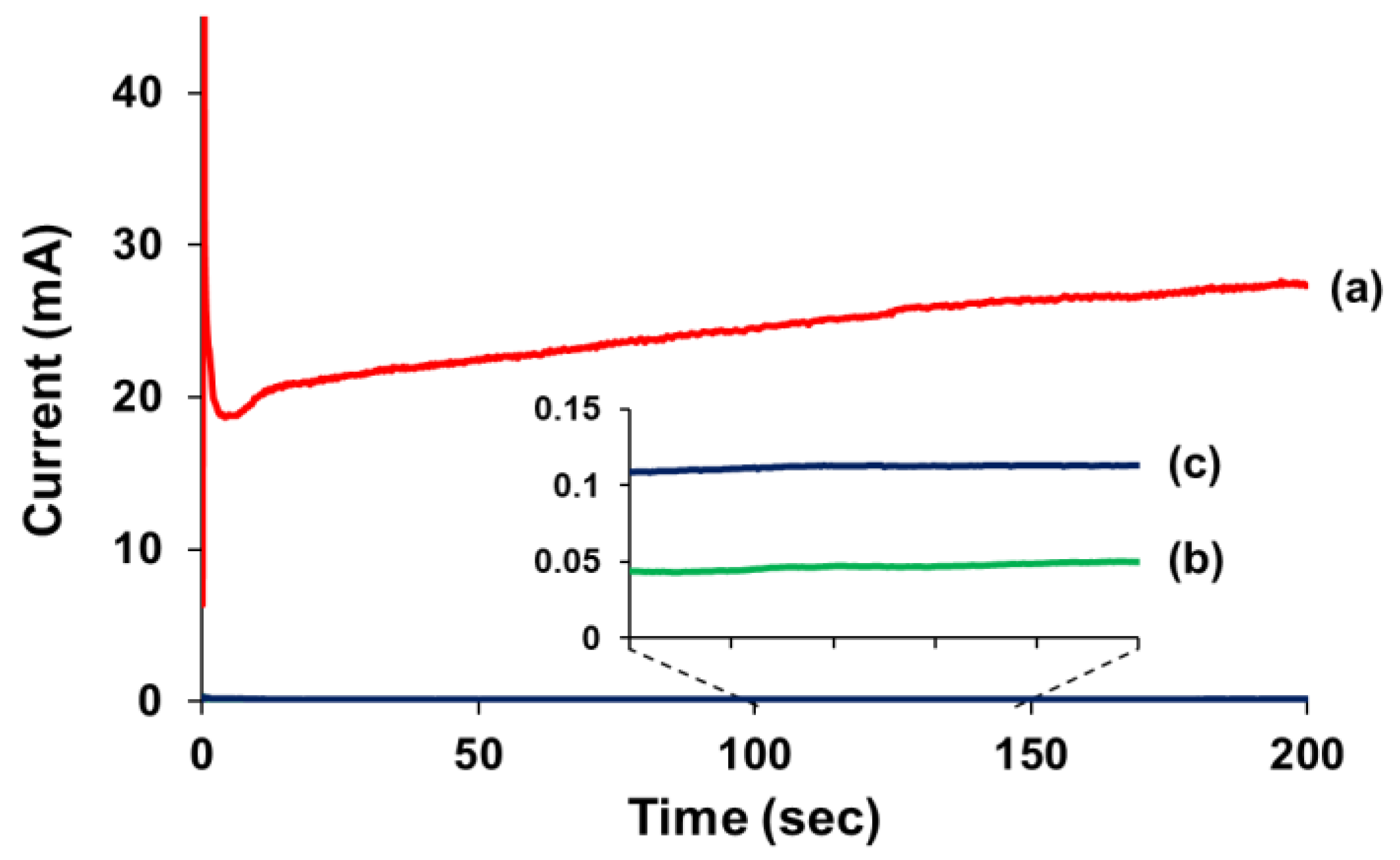
3.3. Evaluation of Repeatable Self-Healing Capability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blaiszik, B.J.; Kramer, S.L.B.; Olugebefola, S.C.; Moore, J.S.; Sottos, N.R.; White, S.R. Self-Healing Polymers and Composites. Annu. Rev. Mater. Res. 2010, 40, 179–211. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, X.; Urban, M.W. Chemical and physical aspects of self-healing materials. Prog. Polym. Sci. 2015, 49–50, 34–59. [Google Scholar] [CrossRef] [Green Version]
- Wen, N.; Song, T.; Ji, Z.; Jiang, D.; Wu, Z.; Wang, Y.; Guo, Z. Recent advancements in self-healing materials: Mechanicals, performances and features. React. Funct. Polym. 2021, 168, 105041. [Google Scholar] [CrossRef]
- Song, M.-M.; Wang, Y.-M.; Liang, X.-Y.; Zhang, X.-Q.; Zhang, S.; Li, B.-J. Functional materials with self-healing properties: A review. Soft Mater. 2019, 15, 6615–6625. [Google Scholar] [CrossRef]
- Utrera-Barrios, S.; Verdejo, R.; López-Manchado, M.A.; Hernández Santana, M. Evolution of self-healing elastomers, from extrinsic to combined intrinsic mechanisms: A review. Mater. Horiz. 2020, 7, 2882–2902. [Google Scholar] [CrossRef]
- Song, Y.-K.; Chung, C.-M. Repeatable self-healing of a microcapsule-type protective coating. Polym. Chem. 2013, 4, 4940–4947. [Google Scholar] [CrossRef]
- Gao, L.; He, J.; Hu, J.; Wang, C. Photoresponsive Self-Healing Polymer Composite with Photoabsorbing Hybrid Microcapsules. ACS Appl. Mater. Interfaces. 2015, 7, 25546–25552. [Google Scholar] [CrossRef]
- Toohey, K.S.; Sottos, N.R.; Lewis, J.A.; Moore, J.S.; White, S.R. Self-healing materials with microvascular networks. Nat. Mater. 2007, 6, 581–585. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, H.-W.; Lee, J.-S.; An, H.-S.; Chung, C.-M. Microcapsule-Type Self-Healing Protective Coating that Can Maintain Its Healed State upon Crack Expansion. Materials 2021, 14, 6198. [Google Scholar] [CrossRef]
- Shields, Y.; De Belie, N.; Jefferson, A.; Van Tittelboom, K. A review of vascular networks for self-healing applications. Smart Mater. Struct. 2021, 30, 063001. [Google Scholar] [CrossRef]
- Kim, S.; Kim, B.-H.; Oh, M.; Park, D.H.; Lee, S. Repeatable Crack Self-Healing by Photochemical [2 + 2] Cycloaddition of TCE-co-DCE Monomers Enclosed in Homopolymer Microcapsules. Polymers 2019, 11, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee Hia, I.; Chan, E.-S.; Chai, S.-P.; Pasbakhsh, P. A novel repeated self-healing epoxy composite with alginate multicore microcapsules. J. Mater. Chem. A 2018, 6, 8470–8478. [Google Scholar] [CrossRef]
- Xue, Y.; Li, C.; Liu, J.; Tan, J.; Su, Z.; Yang, Y.; Zhang, G.; Zhang, Q. Fabrication and characterization of hierarchical microcapsules with multi-storage cells for repeatable self-healing. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125201. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Rao, K.C.; Kumar, D. Preparation and characterization of microcapsules containing linseed oil and its use in self-healing coatings. Prof. Org. Coat. 2008, 63, 72–78. [Google Scholar] [CrossRef]
- Szabó, T.; Telegdi, J.; Nyikos, L. Linseed oil-filled microcapsules containing drier and corrosion inhibitor—Their effects on self-healing capability of paints. Prog. Org. Coat. 2015, 84, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Lang, S.; Zhou, Q. Synthesis and characterization of poly(urea-formaldehyde) microcapsules containing linseed oil for self-healing coating development. Prog. Org. Coat. 2017, 105, 99–110. [Google Scholar] [CrossRef]
- Kim, D.-M.; Song, I.-H.; Choi, J.-Y.; Jin, S.-W.; Nam, K.-N.; Chung, C.-M. Self-Healing Coatings Based on Linseed-Oil-Loaded Microcapsules for Protection of Cementitious Materials. Coatings 2018, 8, 404. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, M.S.; Gite, V.V. Self-healing polyurethane coatings of eugenol-based polyol incorporated with linseed oil encapsulated cardanol-formaldehyde microcapsules: A sustainable approach. Prog. Org. Coat. 2022, 162, 106534. [Google Scholar] [CrossRef]
- Yang, H.; Mo, Q.; Li, W.; Gu, F. Preparation and Properties of Self-Healing and Self-Lubricating Epoxy Coatings with Polyurethane Microcapsules Containing Bifunctional Linseed Oil. Polymers 2019, 11, 1578. [Google Scholar] [CrossRef] [Green Version]
- Shisode, P.S.; Patil, C.B.; Mahulikar, P.P. Preparation and Characterization of Microcapsules Containing Soybean Oil and Their Application in Self-Healing Anticorrosive Coatings. Polym.-Plast. Technol. Eng. 2018, 57, 1334–1343. [Google Scholar] [CrossRef]
- Ataei, S.; Hassan, A.; Azari, P.; Pingguan-Murphy, B.; Yahya, R.; Basirun, W.J.; Shahabudin, N. Electrosprayed PMMA microcapsules containing green soybean oil-based acrylated epoxy and a thiol: A novel resin for smart self-healing coatings. Smart Mater. Struct. 2020, 29, 085037. [Google Scholar] [CrossRef]
- Li, H.; Cui, Y.; Wang, H.; Zhu, Y.; Wang, B. Preparation and application of polysulfone microcapsules containing tung oil in self-healing and self-lubricating epoxy coating. Colloids Surf. A Physicochem. Eng. Asp. 2017, 518, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.-I.; Kim, D.-M.; Yu, H.-C.; Chung, C.-M. Microcapsule-Type Organogel-Based Self-Healing System Having Secondary Damage Preventing Capability. ACS Appl. Mater. Interfaces 2016, 8, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Mallégol, J.; Lemaire, J.; Gardette, J.-L. Drier influence on the curing of linseed oil. Prog. Org. Coat. 2000, 39, 107–113. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.-K.; Kim, H.-W.; Chung, C.-M. Repeatable Self-Healing of a Protective Coating Based on Vegetable-Oil-Loaded Microcapsules. Polymers 2022, 14, 2013. https://doi.org/10.3390/polym14102013
Song Y-K, Kim H-W, Chung C-M. Repeatable Self-Healing of a Protective Coating Based on Vegetable-Oil-Loaded Microcapsules. Polymers. 2022; 14(10):2013. https://doi.org/10.3390/polym14102013
Chicago/Turabian StyleSong, Young-Kyu, Hyun-Woo Kim, and Chan-Moon Chung. 2022. "Repeatable Self-Healing of a Protective Coating Based on Vegetable-Oil-Loaded Microcapsules" Polymers 14, no. 10: 2013. https://doi.org/10.3390/polym14102013






