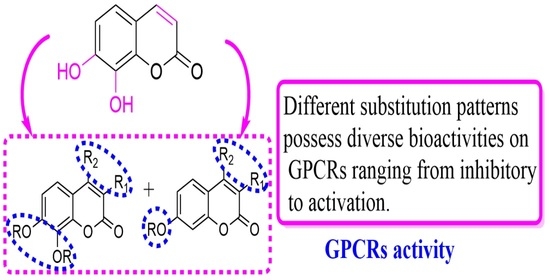
Synthesis of Coumarin Derivatives: A New Class of Coumarin-Based G Protein-Coupled Receptor Activators and Inhibitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. General Procedure for Preparation the Derivatives 1–25
2.2.1. General Procedure for Preparation of Derivatives 1–6
2.2.2. General Procedure for Preparation of Derivatives 7 and 8
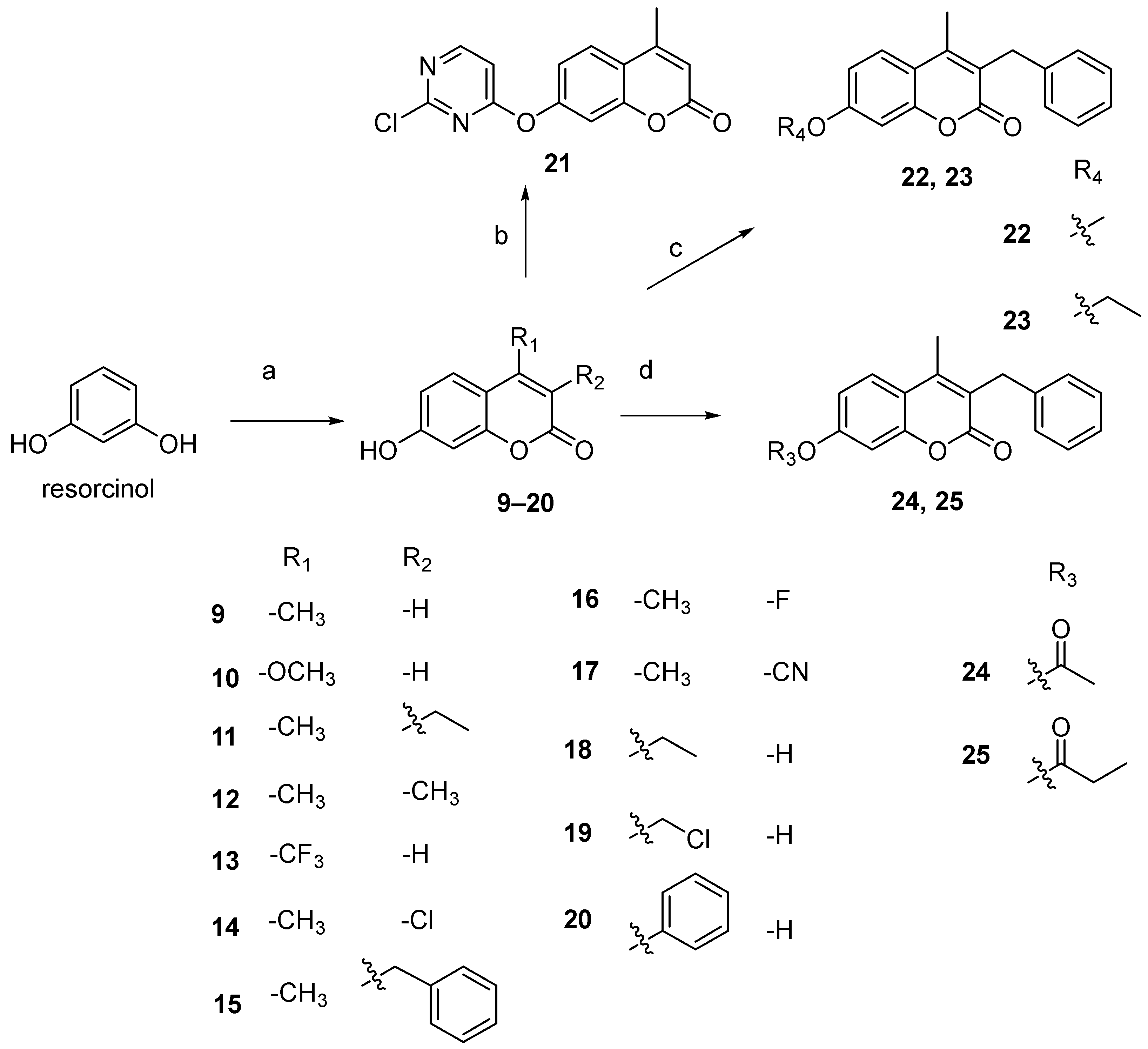
2.2.3. General Procedure for Preparation of Derivatives 9–20
2.2.4. General Procedure for Preparation of Derivatives 21
2.2.5. General Procedure for Preparation of Derivatives 22 and 23
2.2.6. General Procedure for Preparation of Derivatives 24 and 25
2.3. Sample Pretreatment
2.4. DAS-ELISA Evaluation of Coumarin Derivatives on GPCRs
3. Results and Discussion
3.1. Results
Chemistry
3.2. Discussion
Biological Evaluation of The Synthesized Coumarin Derivatives at GPCRs in Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katritch, V.; Cherezov, V.; Stevens, R.C. Structure-function of the G protein-coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 531–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wettschureck, N.; Offermanns, S. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 2005, 85, 1159–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Qiao, A.; Yang, D.; Yang, L.; Dai, A.; de Graaf, C.; Reedtz-Runge, S.; Dharmarajan, V.; Zhang, H.; Han, G.W.; et al. Structure of the full-length glucagon class B G-protein-coupled receptor. Nature 2017, 546, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schioth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug. Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef]
- Sriram, K.; Insel, P.A. G Protein-Coupled receptors as targets for approved drugs: How many targets and how many drugs? Mol. Pharmacol. 2018, 93, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.C.; Bruner, H.C. Naloxegol in opioid-induced constipation: A new paradigm in the treatment of a common problem, Patient. Prefer. Adherence 2017, 11, 1265–1271. [Google Scholar] [CrossRef] [Green Version]
- Hauser, R.A.; Hewitt, L.A.; Isaacson, S. Droxidopa in patients with neurogenic orthostatic hypotension associated with Parkinson’s disease (NOH306A). J. Parkinsons. Dis. 2014, 4, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Turncliff, R.; Hard, M.; Du, Y.; Risinger, R.; Ehrich, E.W. Relative bioavailability and safety of aripiprazole lauroxil, a novel once-monthly, long-acting injectable atypical antipsychotic, following deltoid and gluteal administration in adult subjects with schizophrenia. Schizophr. Res. 2014, 159, 404–410. [Google Scholar] [CrossRef]
- Hope, J.; Castle, D.; Keks, N.A. Brexpiprazole: A new leaf on the partial dopamine agonist branch. Australas Psychiatry 2018, 26, 92–94. [Google Scholar] [CrossRef] [Green Version]
- Pola, S.; Shah, S.R.; Pingali, H.; Zaware, P.; Thube, B.; Makadia, P.; Patel, H.; Bandyopadhyay, D.; Rath, A.; Giri, S.; et al. Discovery of a potent G-protein-coupled receptor 119 agonist for the treatment of type 2 diabetes. Bioorg. Med. Chem. 2021, 35, 116071. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Sheng, R.; Fu, Z.; Fan, J.; Wu, W.H.; Tu, Q.; Guo, R. Synthesis and bioactivities of marine pyran-isoindolone derivatives as potential antithrombotic agents. Mar. Drugs. 2021, 19, 218. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, S.; Sheng, R.; Fan, J.; Wu, W.; Guo, R. Structure-activity relationships of natural and synthetic indole-derived scaffolds as alpha-glucosidase inhibitors: A mini-review, Mini. Rev. Med. Chem. 2020, 20, 1791–1818. [Google Scholar] [CrossRef] [PubMed]
- Bian, C.; Wang, J.; Zhou, X.; Wu, W.; Guo, R. Recent advances on marine alkaloids from sponges. Chem. Biodivers. 2020, 17, e2000186. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Sheng, R.; Fang, Y.; Guo, R. Novel bioactive polyketides isolated from marine actinomycetes: An update review from 2013 to 2019. Chem. Biodivers. 2020, 17, e2000562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, K.; Xu, B.; Shi, J.; Qiang, J.; Shi, S.; Yi, Y.; Li, H.; Jin, T.; Guo, R.; et al. Epigenetic input dictates the threshold of targeting of the integrin-dependent pathway in non-small cell lung cancer. Front. Cell. Dev. Biol. 2020, 8, 652. [Google Scholar] [CrossRef]
- Wang, J.; Su, S.; Zhang, S.; Zhai, S.; Sheng, R.; Wu, W.; Guo, R. Structure-activity relationship and synthetic methodologies of alpha-santonin derivatives with diverse bioactivities: A mini-review. Eur. J. Med. Chem. 2019, 175, 215–233. [Google Scholar] [CrossRef]
- Guo, R.; Duan, D.; Hong, S.; Zhou, Y.; Wang, F.; Wang, S.; Wu, W.; Bao, B. A marine fibrinolytic compound FGFC1 stimulating enzymatic kinetic parameters of a reciprocal activation system based on a single chain urokinase-type plasminogen activator and plasminogen. Process. Biochem. 2018, 68, 190–196. [Google Scholar] [CrossRef]
- Guo, R.; Guo, C.; He, D.; Zhao, D.; Shen, Y. Two new C19-diterpenoid alkaloids with anti-inflammatory activity from Aconitum iochanicum. Chin. J. Chem. 2017, 35, 1644–1647. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, Y.; Duan, D.; Fu, Q.; Zhang, X.; Yu, X.; Wang, S.; Bao, B.; Wu, W. Fibrinolytic evaluation of compounds isolated from a marine fungus stachybotrys longispora FG216. Chin. J. Chem. 2016, 34, 1194–1198. [Google Scholar] [CrossRef]
- Wang, G.; Wu, W.; Zhu, Q.; Fu, S.; Wang, X.; Hong, S.; Guo, R.; Bao, B. Identification and fibrinolytic evaluation of an isoindolone derivative isolated from a rare marine fungus Stachybotrys longispora FG216. Chin. J. Chem. 2015, 33, 1089–1095. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.M.; Jiang, W.; Hu, Q.Q.; Wang, X.J.; Xu, H.H. Recent Advances on Anticancer Effects and Molecular Mechanisms of Maslinic Acid. Med. Res. 2022, 6, 220002. [Google Scholar]
- Wang, X.W.; Yang, Y.Q.; Li, Y.; Ba, C.F. Acteoside Attenuates Retinal Ganglion Cell Damage in Acute High Intraocular Pressure-Induced Rats via the Bax/Bcl-xL Axis. Med. Res. 2022, 6, 220001. [Google Scholar]
- Wang, H.; Dong, H.Y.; Liu, Y.F.; Ma, Y.X.; Wang, W.; Guo, Z.M.; Wang, C.R. Chemical Research on Limonoids Isolated from the Fruits of Melia toosendan. Med. Res. 2022, 6, 210014. [Google Scholar]
- Zheng, S.D.; Fang, H.; Liu, J.F. Study on Chemical Constituents and Bioactivities from Eucalyptus globulus. Med. Res. 2021, 5, 210007. [Google Scholar]
- Fang, H.; Zheng, S.D.; Liu, J.F. Progress in Chemical Constituents and Bioactivities of Perilla frutescens (L.). Britt. Med. Res. 2021, 5, 210008. [Google Scholar]
- Molnar, M.; Lončarić, M.; Kovač, M. Green chemistry approaches to the synthesis of coumarin derivatives. Curr. Org. Chem. 2020, 24, 4–43. [Google Scholar] [CrossRef]
- Bourgaud, F.; Poutaraud, A.; Guckert, A. Extraction of coumarins from plant material (Leguminosae). Phytochem. Anal. 1994, 5, 127–132. [Google Scholar] [CrossRef]
- Qu, D.; Hou, Z.; Li, J.; Luo, L.; Su, S.; Ye, Z.; Bai, Y.; Zhang, X.; Chen, G.; Li, Z.; et al. A new coumarin compound DCH combats methicillin-resistant Staphylococcus aureus biofilm by targeting arginine repressor. Sci. Adv. 2020, 6, 9597–9611. [Google Scholar] [CrossRef]
- Mangasuli, S.N.; Hosamani, K.M.; Devarajegowda, H.C.; Kurjogi, M.M.; Joshi, S.D. Synthesis of coumarin-theophylline hybrids as a new class of anti-tubercular and anti-microbial agents. Eur. J. Med. Chem. 2018, 146, 747–756. [Google Scholar] [CrossRef]
- Salvador, J.P.; Tassies, D.; Reverter, J.C.; Marco, M.P. Enzyme-linked immunosorbent assays for therapeutic drug monitoring coumarin oral anticoagulants in plasma. Anal. Chim. Acta. 2018, 1028, 59–65. [Google Scholar] [CrossRef]
- Emam, S.H.; Sonousi, A.; Osman, E.O.; Hwang, D.; Kim, G.D.; Hassan, R.A. Design and synthesis of methoxyphenyl- and coumarin-based chalcone derivatives as anti-inflammatory agents by inhibition of NO production and down-regulation of NF-kappaB in LPS-induced RAW264.7 macrophage cells. Bioorg. Chem. 2021, 107, 104630. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Yang, X.W.; Wu, B.F.; Shang, J.H.; Liu, Y.P.; Zhi, D.; Luo, X.D. Anti-inflammatory effect of Pomelo Peel and its bioactive coumarins. J. Agric. Food. Chem. 2019, 67, 8810–8818. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Su, Z.; Yang, J.; Liu, H.; Tang, M.; Hu, Q.; Bai, P.; Wen, J.; He, J.; Niu, T.; et al. Anti-tumor study of H6, a 4-substituted coumarins derivative, loaded biodegradable self-assembly nano-micelles in vitro and in vivo. J. Biomed. Nanotechnol. 2019, 15, 1515–1531. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, J.; Liu, Y.; Zeng, Y.; Wu, G. A review on anti-tumor mechanisms of coumarins. Front. Oncol. 2020, 10, 592853. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, I.; Ali, Z.; Baek, S.H.; Mirza, B.; Ahn, K.S. Assessment of the antitumor potential of umbelliprenin, a naturally occurring sesquiterpene coumarin. Biomedicines 2020, 8, 126. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Y.; Zhang, L.; An, L.; Chen, R.; Liu, Y.; Luo, Q.; Li, Y.; Wang, H.; Xue, Y. Computational study on the antioxidant property of coumarin-fused coumarins. Food. Chem. 2020, 304, 125446. [Google Scholar] [CrossRef]
- Safakish, M.; Hajimahdi, Z.; Aghasadeghi, M.R.; Vahabpour, R.; Zarghi, A. Design, synthesis, molecular modeling and anti-HIV assay of novel quinazolinone Incorporated coumarin derivatives. Curr. HIV Res. 2020, 18, 41–51. [Google Scholar] [CrossRef]
- Gao, Y.; Cheng, H.; Khan, S.; Xiao, G.; Rong, L.; Bai, C. Development of coumarine derivatives as potent anti-filovirus entry inhibitors targeting viral glycoprotein. Eur. J. Med. Chem. 2020, 204, 112595. [Google Scholar] [CrossRef]
- Hu, Y.H.; Yang, J.; Zhang, Y.; Liu, K.C.; Liu, T.; Sun, J.; Wang, X.J. Synthesis and biological evaluation of 3-(4-aminophenyl)-coumarin derivatives as potential anti-alzheimer’s disease agents, J. Enzyme. Inhib. Med. Chem. 2019, 34, 1083–1092. [Google Scholar] [CrossRef]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef]
- Loncaric, M.; Strelec, I.; Pavic, V.; Subaric, D.; Rastija, V.; Molnar, M. Lipoxygenase inhibition activity of coumarin derivatives-QSAR and molecular docking study. Pharmaceuticals 2020, 13, 154. [Google Scholar] [CrossRef]
- Tian, D.; Wang, F.; Duan, M.; Cao, L.; Zhang, Y.; Yao, X.; Tang, J. Coumarin analogues from the citrus grandis (L.) osbeck and their hepatoprotective activity. J. Agric. Food. Chem. 2019, 67, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Potdar, M.K.; Mohile, S.S.; Salunkhe, M.M. Coumarin syntheses via pechmann condensation in lewis acidic chloroaluminate ionic liquid. Cheminform 2002, 42, 9285–9287. [Google Scholar] [CrossRef]
- Rosen, T. The perkin reaction. In Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Sciencedirect: Amsterdam, The Netherlands, 1991; Volume 2, pp. 395–408. [Google Scholar]
- Watson, B.T.; Christiansen, G.E. Solid phase synthesis of substituted coumarin-3-carboxylic acids via the Knoevenagel condensation. Tetrahedron. Lett. 1998, 39, 6087–6090. [Google Scholar] [CrossRef]
- Jadhav, N.H.; Sakate, S.S.; Rasal, N.K.; Shinde, D.R.; Pawar, R.A. Heterogeneously catalyzed pechmann condensation employing the tailored Zn0.925Ti0.075O NPs: Synthesis of coumarin. ACS. Omega 2019, 4, 8522–8527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouellette, R.J.; Rawn, J.D. Aldehydes and Ketones: Nucleophilic Addition Reactions; Sciencedirect: Amsterdam, The Netherlands, 2018; pp. 595–623. [Google Scholar]
- Laufer, M. Synthesis of 7-hydroxycoumarins by Pechmann reaction using Nafion resin/silica nanocomposites as catalysts. J. Catal. 2003, 218, 315–320. [Google Scholar] [CrossRef]
- Ryabukhin, D.S.; Vasilyev, A.V. Synthesis of (iso)quinoline, (iso)coumarin and (iso)chromene derivatives from acetylene compounds. Russ. Chem. Rev. 2016, 85, 637–665. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Fu, Z.; Sheng, R.; Wu, W.; Fan, J.; Guo, R. Syntheses and evaluation of daphnetin derivatives as novel G protein-coupled receptor inhibitors and activators. Bioorg. Chem. 2020, 104, 104342. [Google Scholar] [CrossRef]
- Graaf, C.D.; Song, G.; Cao, C.; Zhao, Q.; Wang, M.W.; Wu, B.; Stevens, R.C. Extending the Structural View of Class B GPCRs. Trends Biochem. Sci. 2017, 42, 946–960. [Google Scholar] [CrossRef]
- Katkevi, S.M.; Kontijevskis, A.; Mutule, I. Microwave-Promoted Automated Synthesis of a Coumarin Library. Chem. Heterocycl. Compd. 2007, 43, 151–159. [Google Scholar] [CrossRef]
- Tiftikçi, E.; Erk, Ç. The synthesis of novel crown ethers, Part X—4-propyl- and 3-ethyl-4-methylchromenone-crown ethers. J. Heterocyclic. Chem. 2010, 41, 867–871. [Google Scholar] [CrossRef]
- Madhav, J.V.; Kuarm, B.S.; Someshwar, P. ChemInform Abstract: Dipyridine Cobalt Chloride: A Novel Catalyst for the Synthesis of Coumarins via Pechmann Condensation. J. Chem. Res. 2010, 2008, 867–871. [Google Scholar] [CrossRef]
- Lu, Z.L.; Neverov, A.A.; Brown, R.S. An ortho-palladated dimethylbenzylamine complex as a highly efficient turnover catalyst for the decomposition of P=S insecticides. Mechanistic studies of the methanolysis of some P=S-containing phosphorothioate triesters. Org. Biomol. Chem. 2005, 3, 3379–3387. [Google Scholar] [CrossRef]
- Qing, S.; Quan, P.; Jialiang, S.; Xuefeng, L. Synthesis and biological evaluation of functionalized coumarins as acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2005, 40, 1307–1315. [Google Scholar]
- Fall, Y.; Santana, L.; Uriarte, E. Synthesis and characterization of some coumarins with two hydroxy or methoxy substituents. J. Heterocyclic. Chem. 2001, 38, 1231–1232. [Google Scholar] [CrossRef]
- Timonen, J.M.; Nieminen, R.M.; Sareila, O. Synthesis and anti-inflammatory effects of a series of novel 7-hydroxycoumarin derivatives. Eur. J. Med. Chem. 2011, 46, 3845–3850. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Muncipinto, G.; Miscioscia, T.F.; Nicolloti, O.; Leonetti, F.; Catto, M.; Caccia, C.; Salvati, P.; Soto-Otero, R.; Mendez-Alvarez, E.; et al. Discovery of a Novel Class of Potent Coumarin Monoamine Oxidase B Inhibitors: Development and Biopharmacological Profiling of 7-[(3-Chlorobenzyl)oxy]-4-[(methylamino)methyl]-2H-chromen-2-one Methanesulfonate (NW-1772) as a Highly Potent, Selective, Reversible, and Orally Active Monoamine Oxidase B Inhibitor. J. Med. Chem. 2009, 52, 6685–6706. [Google Scholar]
- Sun, W.; Hu, S.; Fang, S.; Yan, H. Design, synthesis and biological evaluation of pyrimidine-based derivatives as VEGFR-2 tyrosine kinase inhibitors. Bioorg. Chem. 2018, 78, 393–405. [Google Scholar] [CrossRef]
- Kim, S.; Kang, D.; Lee, C.H. ChemInform Abstract: Synthesis of Substituted Coumarins via Broensted Acid Mediated Condensation of Allenes with Substituted Phenols or Anisoles. J. Org. Chem. 2012, 77, 6530–6537. [Google Scholar] [CrossRef]
- Wang, J.; Bian, C.; Wang, Y.; Shen, Q.; Bao, B.; Fan, J.; Zuo, A.; Wu, W.; Guo, R. Syntheses and bioactivities of songorine derivatives as novel G protein-coupled receptor antagonists. Bioorg. Med. Chem. 2019, 27, 1903–1910. [Google Scholar] [CrossRef]
- Zhuang, Y.; Xu, G.; Zhang, L.; Zha, J.K.; Ji, P.P.; Li, Y.N.; Liu, B.Y.; Zhang, J.F.; Zhao, Q.; Sun, Y.N.; et al. Development of a double monoclonal antibody–based sandwich enzyme-linked immunosorbent assay for detecting canine distemper virus. Appl. Microbiol. Biot. 2020, 104, 10725–10735. [Google Scholar] [CrossRef] [PubMed]
- Kimman, T.G.; Leeuw, D.O.; Kochan, G.; Szewczyk, B.; Rooij, V.E.; Jacobs, L.; Kramps, J.; Peeters, V. An indirect double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) using baculovirus-expressed antigen for the detection of antibodies to glycoprotein E of pseudorabies virus and comparison of the method with blocking ELISAs. Clin. Diagn. Lab. Immun. 1996, 3, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.; Park, Y.J.; Ryu, J.S. Scandium(III) triflate–catalyzed coumarin synthesis. Synth. Commun. 2008, 38, 4395–4406. [Google Scholar] [CrossRef]
- Xiao, F.L. The Study of General Toxicology for a New Anticoccidial Triazine Grug Ethanamizuril; Chinese Academy of Agricultural Sciences: Beijing, China, 2014. [Google Scholar]




 | ||||||
| No. | R1 | R2 | R3 | R4 | EC50(nM) (% Activation) | IC50(nM) (% INHIBITION) |
| Daphnetin | –OH | –OH | –H | –H | >10 (49.43%) | |
| 1 | –OH | –OH | –H |  | 0.56 ± 0.02 | – |
| 2 | –OH | –OH | –H |  | >43.83 (39.82%) | – |
| 3 | –OH | –OH | –H |  | >39.37 (35.75%) | – |
| 4a | –OH | –OH | –H | –CH3 | 2.65 ± 0.25 | – |
| 4 | –OH | –OH | –H |  | – | 0.15 ± 0.01 |
| 5 | –OH | –OH | –H |  | – | 3.77 ± 0.58 |
| 6 | –OH | –OH | –H |  | 0.49 ± 0.32 | – |
| 7 |  |  | –H |  | – | 0.02 ± 0.01 |
| 8 |  |  | –H |  | – | 6.86 ± 0.04 |
| 9 | –OH | –H | –H | –CH3 | 1.25 ± 0.89 | – |
| 10 | –OH | –H | –H | –OCH3 | – | >48.54 (33.58%) |
| 11 | –OH | –H |  | –CH3 | 1.28 ± 1.07 | – |
| 12 | –OH | –H | –CH3 | –CH3 | 1.07 ± 0.33 | – |
| 13 | –OH | –H | –H | –CF3 | – | 7.75 ± 7.23 |
| 14 | –OH | –H | –Cl | –CH3 | 0.03 ± 0.02 | – |
| 15 | –OH | –H |  | –CH3 | 3.60 ± 0.11 | – |
| 16 | –OH | –H | –F | –CH3 | 0.24 ± 0.21 | – |
| 17 | –OH | –H | –CN | –CH3 | 0.03 ± 0.02 | – |
| 18 | –OH | –H | –H |  | 0.03 ± 0.01 | – |
| 19 | –OH | –H | –H |  | 4.22 ± 3.49 | – |
| 20 | –OH | –H | –H |  | >41.97 (34.41%) | – |
| 21 |  | H | H | –CH3 | 0.02 ± 0.01 | – |
| 22 |  | –H |  | –CH3 | – | 9.67 ± 2.59 |
| 23 |  | –H |  | –CH3 | – | 0.76 ± 0.70 |
| 24 |  | –H |  | –CH3 | >35.67 (32.82%) | – |
| 25 |  | –H |  | –CH3 | 11.98 ± 9.47 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Z.; Zhang, L.; Hang, S.; Wang, S.; Li, N.; Sun, X.; Wang, Z.; Sheng, R.; Wang, F.; Wu, W.; et al. Synthesis of Coumarin Derivatives: A New Class of Coumarin-Based G Protein-Coupled Receptor Activators and Inhibitors. Polymers 2022, 14, 2021. https://doi.org/10.3390/polym14102021
Fu Z, Zhang L, Hang S, Wang S, Li N, Sun X, Wang Z, Sheng R, Wang F, Wu W, et al. Synthesis of Coumarin Derivatives: A New Class of Coumarin-Based G Protein-Coupled Receptor Activators and Inhibitors. Polymers. 2022; 14(10):2021. https://doi.org/10.3390/polym14102021
Chicago/Turabian StyleFu, Zhe, Linjie Zhang, Sijin Hang, Shiyi Wang, Na Li, Xiaojing Sun, Zian Wang, Ruilong Sheng, Fang Wang, Wenhui Wu, and et al. 2022. "Synthesis of Coumarin Derivatives: A New Class of Coumarin-Based G Protein-Coupled Receptor Activators and Inhibitors" Polymers 14, no. 10: 2021. https://doi.org/10.3390/polym14102021