Dithiocarbamates as Effective Reversible Addition–Fragmentation Chain Transfer Agents for Controlled Radical Polymerization of 1-Vinyl-1,2,4-triazole
Abstract
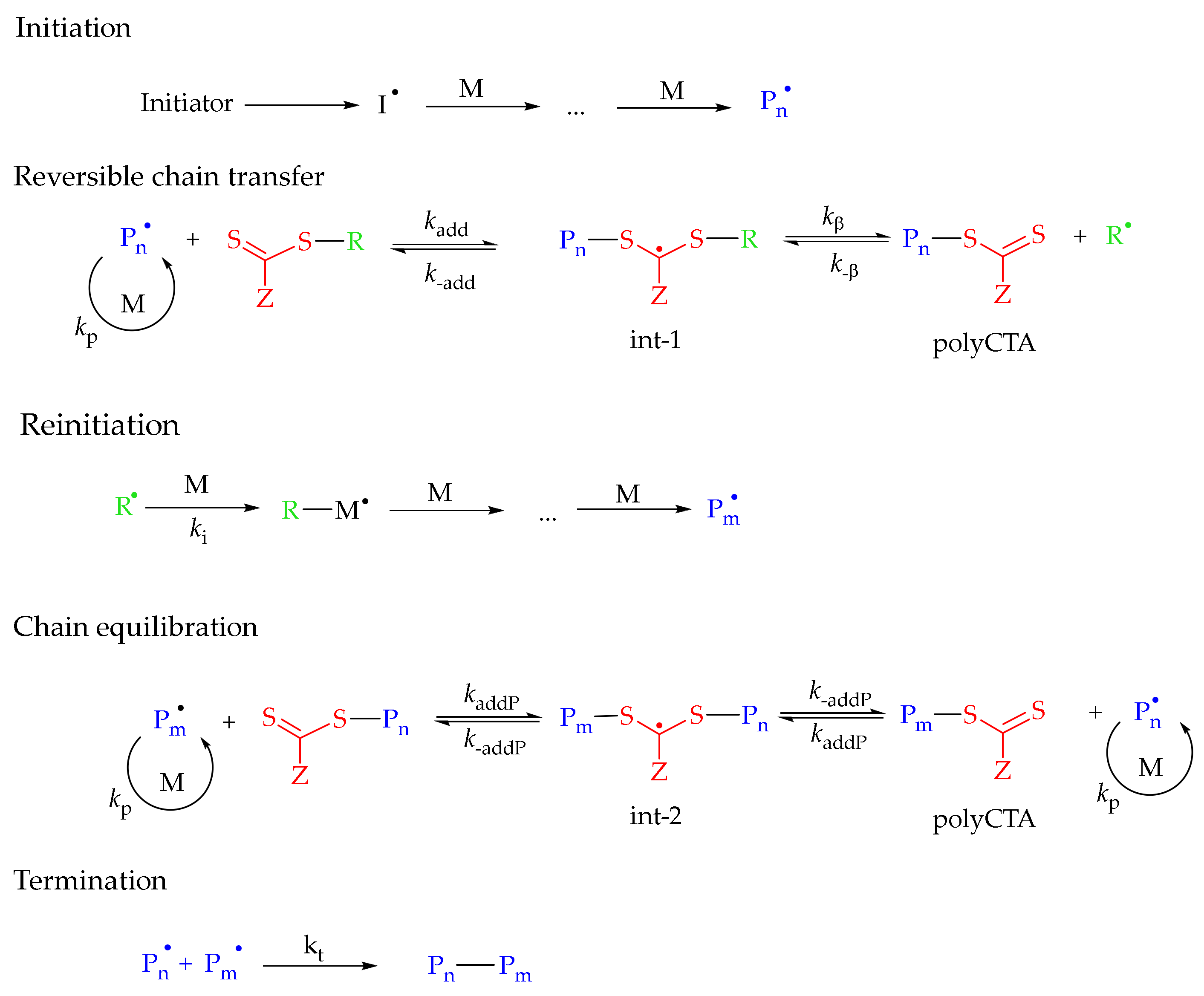
:1. Introduction
2. Materials and Methods
2.1. Materials
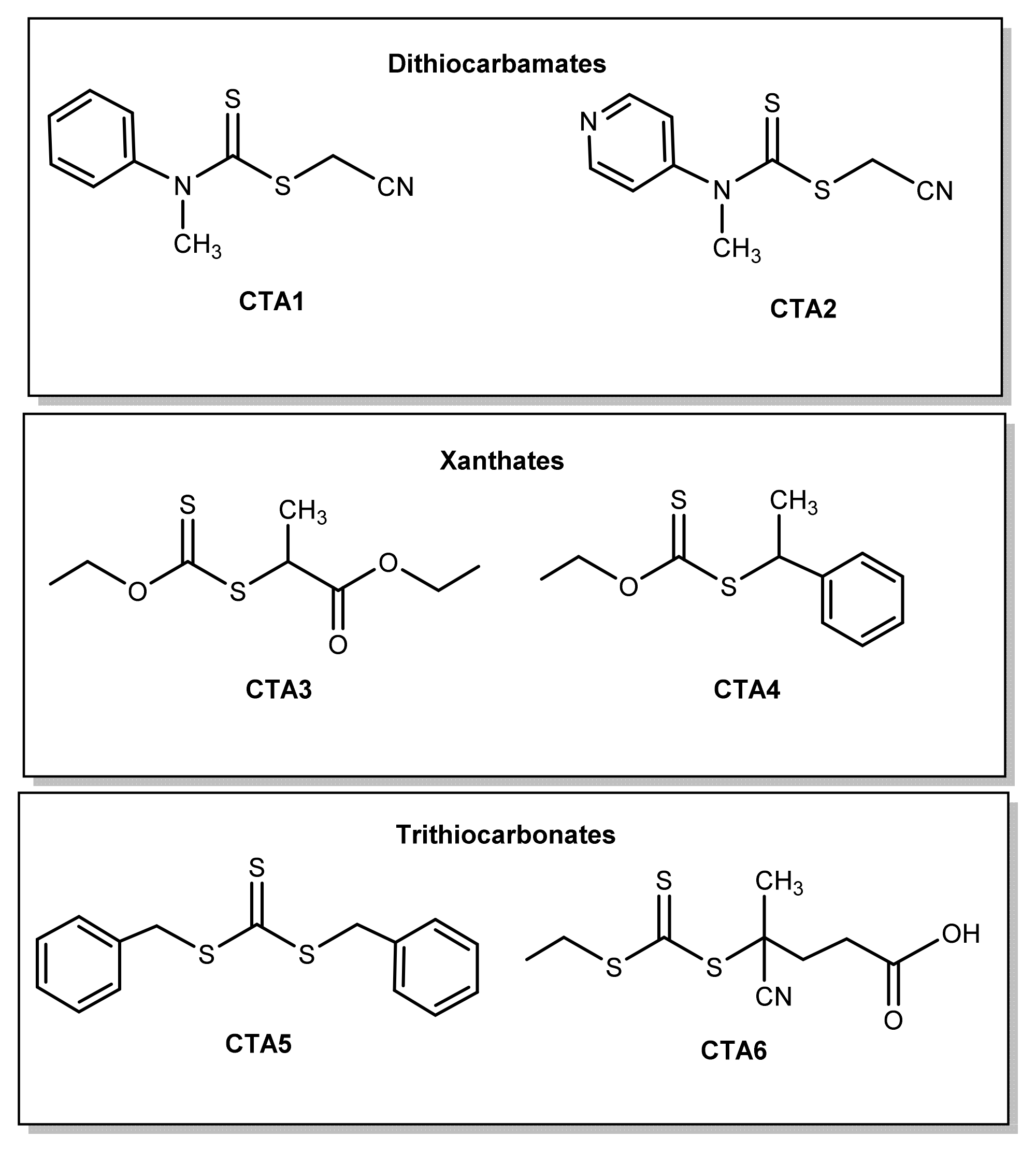
2.2. Procedure for the Synthesis of Chain Transfer Agent (CTA)
2.2.1. Synthesis of Cyanomethyl Methyl(Pyridine-4-yl)Dithiocarbamate (CTA2)
2.2.2. Synthesis of Ethyl 2-[(Ethoxycarbonothiolyl)thio]propanoate (CTA3)
2.2.3. Synthesis of O-Ethyl-S-(1-phenylethyl)dithiocarbonate (CTA4)
2.2.4. Synthesis of Dibenzyl Trithiocarbonate (CTA5)
2.2.5. Synthesis of 4-Cyano-4-{[(ethylthio)carbonothioyl]thio}pentanoic Acid (CTA6)
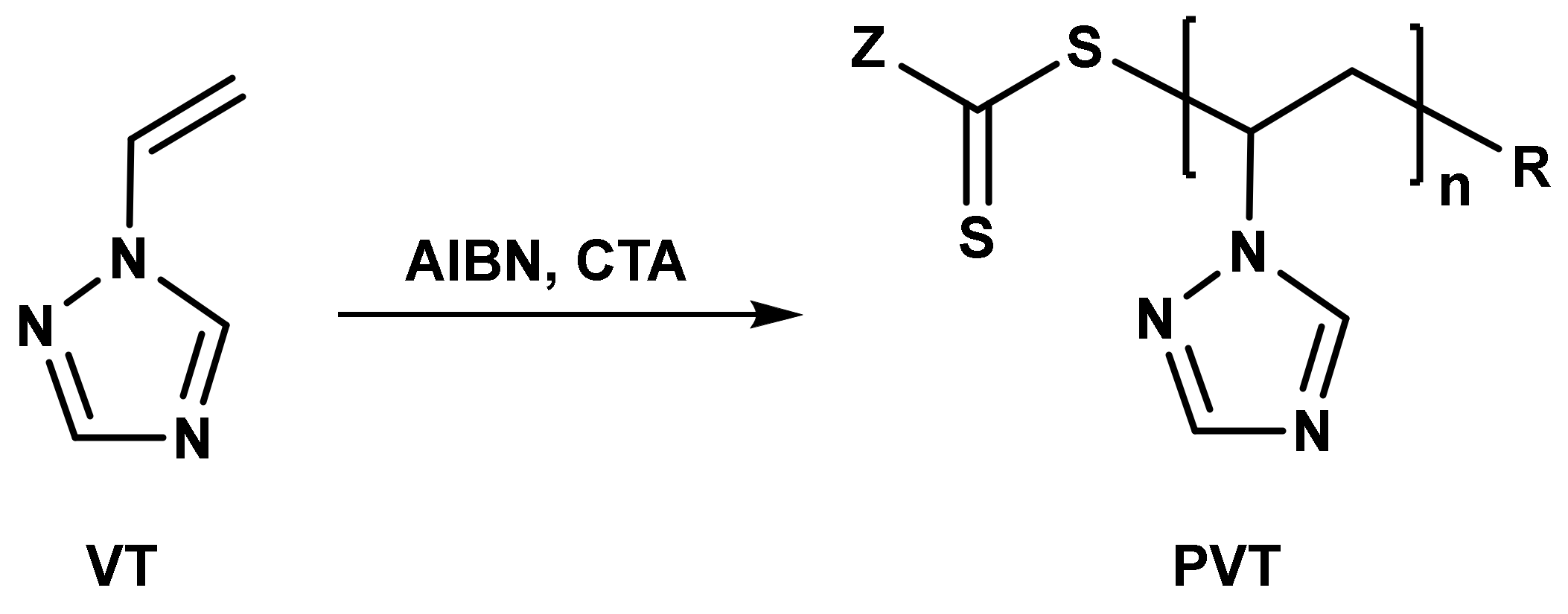
2.2.6. Free Radical Polymerization of 1-Vinyl-1,2,4-Triazole
2.2.7. Controlled Radical Polymerization of 1-Vinyl-1,2,4-Triazole
2.3. Characterization
3. Results and Discussion
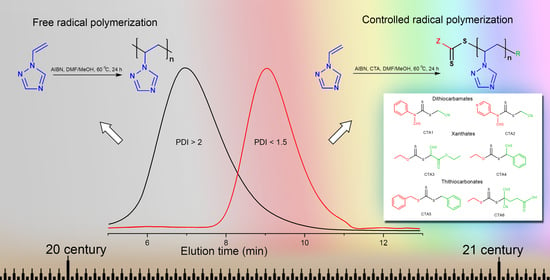
3.1. Free-Radical Polymerization of 1-Vinyl-1,2,4-Triazole
3.2. Controlled Radical Polymerization of 1-Vinyl-1,2,4-Triazole
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prozorova, G.F.; Pozdnyakov, A.S.; Korzhova, S.A.; Ermakova, T.G.; Novikov, M.A.; Titov, E.A.; Sosedova, L.M. Toxicity evaluation of polyvinyltriazole and a related silver-containing nanocomposite. Russ. Chem. Bull. 2014, 63, 2126–2129. [Google Scholar] [CrossRef]
- Sosedova, L.M.; Novikov, M.A.; Titov, E.A.; Pozdnyakov, A.S.; Korzhova, S.A.; Ermakova, T.G.; Prozorova, G.F. Synthesis, Antimicrobial Properties, and Toxicity of a Nanobiocomposite Based on Ag(0) Particles and Poly(1-Vinyl-1,2,4-Triazole). Pharm. Chem. J. 2019, 52, 912–916. [Google Scholar] [CrossRef]
- Prozorova, G.F.; Pozdnyakov, A.S. Synthesis, Properties, and Biological Activity of Poly(1-vinyl-1,2,4-triazole) and Silver Nanocomposites Based on It. Polym. Sci. Ser. C 2022, 1–11. [Google Scholar] [CrossRef]
- Prozorova, G.; Pozdnyakov, A.; Kuznetsova, N.; Korzhova, S.; Emel’yanov, A.; Ermakova, T.; Fadeeva, T.; Sosedova, L. Green synthesis of water-soluble nontoxic polymeric nanocomposites containing silver nanoparticles. Int. J. Nanomed. 2014, 9, 1883–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tikhonov, N.I.; Khutsishvili, S.S.; Larina, L.I.; Pozdnyakov, A.S.; Emel’yanov, A.I.; Prozorova, G.F.; Vashchenko, A.V.; Vakul’skaya, T.I. Silver polymer complexes as precursors of nanocomposites based on polymers of 1-vinyl-1,2,4-triazole. J. Mol. Struct. 2019, 1180, 272–279. [Google Scholar] [CrossRef]
- Zinchenko, V.I.; Makarov, A.S.; Lopyrev, V.A.; Ermakova, T.G.; Tatarova, L.A. Flocculant for reducing copper content in juices and wine materials. Storage Process. Farm Prod. 2000, 3, 29–31. [Google Scholar]
- Aslan, A.; Bozkurt, A. Development and characterization of polymer electrolyte membranes based on ionical cross-linked poly(1-vinyl-1,2,4 triazole) and poly(vinylphosphonic acid). J. Power Sources 2009, 191, 442–447. [Google Scholar] [CrossRef]
- Lebedeva, O.; Pozhidaev, Y.; Raskulova, T.; Belkovich, A.; Ivanova, A.; Korzhova, S.; Emelyanov, A.; Pozdnyakov, A. Synthesis and characterization of new proton-exchange membranes based on poly-1-vinyl-1,2,4-triazole doped with phenol-2,4-disulfonic acid. Int. J. Energy Res. 2021, 45, 14547–14560. [Google Scholar] [CrossRef]
- Abbas, M.; Cakmak, G.; Tekin, N.; Kara, A.; Guney, H.Y.; Arici, E.; Sariciftci, N.S. Water soluble poly(1-vinyl-1,2,4-triazole) as novel dielectric layer for organic field effect transistors. Org. Electron. 2011, 12, 497–503. [Google Scholar] [CrossRef]
- Mansurova, L.A.; Skornyakova, A.B.; Sevast’yanova, N.A.; Tatarova, L.A.; Ermakova, T.G.; Polyrev, V.A.; Kazimirovskaya, V.B.; Slutskii, L.I. Influence of poly-1-vinyl-1,2,4-triazole on the biochemical induces of connective tissue. Pharm. Chem. J. 1991, 25, 531–533. [Google Scholar] [CrossRef]
- Ermakova, T.G.; Kuznetsova, N.P. Promising Fields of Application of Homopolymer and Copolymers of 1-vinyl-1,2,4-triazole. Nauka-Proizv 2003, 6, 55–59. [Google Scholar]
- Shurygina, I.A.; Prozorova, G.F.; Trukhan, I.S.; Korzhova, S.A.; Fadeeva, T.V.; Pozdnyakov, A.S.; Dremina, N.N.; Emel’yanov, A.I.; Kuznetsova, N.P.; Shurygin, M.G. NonToxic Silver/Poly-1-Vinyl-1,2,4-Triazole Nanocomposite Materials with Antibacterial Activity. Nanomaterials 2020, 10, 1477. [Google Scholar] [CrossRef]
- Pozdnyakov, A.S.; Ivanova, A.A.; Emel’yanov, A.I.; Bolgova, Y.I.; Trofimova, O.M.; Prozorova, G.F. Water-soluble stable polymer nanocomposites with AuNPs based on the functional poly(1-vinyl-1,2,4-triazole-co-N-vinylpyrrolidone). J. Organomet. Chem. 2020, 922, 121352. [Google Scholar] [CrossRef]
- Zezina, E.A.; Emel’yanov, A.I.; Pozdnyakov, A.S.; Prozorova, G.F.; Abramchuk, S.S.; Feldman, V.I.; Zezin, A.A. Radiation-induced synthesis of copper nanostructures in the films of interpolymer complexes. Radiat. Phys. Chem. 2019, 158, 115–121. [Google Scholar] [CrossRef]
- Zharikov, A.A.; Vinogradov, R.A.; Zezina, E.A.; Pozdnyakov, A.S.; Feldman, V.I.; Vasiliev, A.L.; Zezin, A.A. The radiation-induced preparation of ultrasmall gold nanoparticles in Au(III) complexes with units of poly(1-vinyl-1,2,4-triazole) and poly(1-vinyl-1,2,4-triazole)—Poly(acrylic acid). Colloid Interface Sci. Commun. 2022, 47, 100602. [Google Scholar] [CrossRef]
- Zezin, A.; Danelyan, G.; Emel’yanov, A.; Zharikov, A.; Prozorova, G.; Zezina, E.; Korzhova, S.; Fadeeva, T.; Abramchuk, S.; Shmakova, N.; et al. Synthesis of antibacterial polymer metal hybrids in irradiated poly-1-vinyl-1,2,4-triazole complexes with silver ions: pH tuning of nanoparticle sizes. Appl. Organomet. Chem. 2022, 36, e6581. [Google Scholar] [CrossRef]
- Barner-Kowollik, C. (Ed.) Handbook of RAFT Polymerization; Wiley: Hoboken, NJ, USA, 2008; ISBN 9783527319244. [Google Scholar]
- Chernikova, E.V.; Lysenko, E.A.; Serkhacheva, N.S.; Prokopov, N.I. Self-Assembly of Amphiphilic Block Copolymers during Reversible Addition-Fragmentation Chain Transfer Heterophase Polymerization: Problems, Achievements, and Outlook. Polym. Sci. Ser. C 2018, 60, 192–218. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Golubev, V.B.; Filippov, A.N.; Garina, E.S. The role of termination reactions of radical intermediates in reversible addition-fragmentation chain-transfer polymerization. Polym. Sci. Ser. C 2015, 57, 94–109. [Google Scholar] [CrossRef]
- Toms, R.V.; Prokopov, N.I.; Mineev, K.O.; Plutalova, A.V.; Chernikova, E.V. Controlling monomer sequence distribution in RAFT polymerization of styrene and acrylic acid. Mendeleev Commun. 2022, 32, 238–240. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Gao, H.; Sumerlin, B.S.; Tsarevsky, N.V. (Eds.) Reversible Deactivation Radical Polymerization: Mechanisms and Synthetic Methodologies; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2018; Volume 1284, ISBN 9780841233188. [Google Scholar]
- Nakabayashi, K.; Mori, H. Recent progress in controlled radical polymerization of N-vinyl monomers. Eur. Polym. J. 2013, 49, 2808–2838. [Google Scholar] [CrossRef] [Green Version]
- Bekanova, M.Z.; Neumolotov, N.K.; Jablanovic, A.D.; Plutalova, A.V.; Chernikova, E.V. Radical Substitution of the Dithiocarbonyl Group of Poly(methyl methacrylate) Obtained by Reversible Addition–Fragmentation Chain Transfer Polymerization. Polym. Sci. Ser. C 2019, 61, 186–197. [Google Scholar] [CrossRef]
- Barner-Kowollik, C.; Quinn, J.F.; Morsley, D.R.; Davis, T.P. Modeling the reversible addition-fragmentation chain transfer process in cumyl dithiobenzoate-mediated styrene homopolymerizations: Assessing rate coefficients for the addition-fragmentation equilibrium. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 1353–1365. [Google Scholar] [CrossRef]
- Mori, H.; Ishikawa, K.; Abiko, Y.; Maki, Y.; Onuma, A.; Morishima, M. Synthesis of Triazole-Based Amphiphilic Block Copolymers Containing Carbazole Moiety By RAFT Polymerization. Macromol. Chem. Phys. 2012, 213, 1803–1814. [Google Scholar] [CrossRef]
- Coote, M.L.; Radom, L. Substituent Effects in Xanthate-Mediated Polymerization of Vinyl Acetate: Ab Initio Evidence for an Alternative Fragmentation Pathway. Macromolecules 2004, 37, 590–596. [Google Scholar] [CrossRef]
- Nguyen, T.L.U.; Eagles, K.; Davis, T.P.; Barner-Kowollik, C.; Stenzel, M.H. Investigation of the influence of the architectures of poly(vinyl pyrrolidone) polymers made via the reversible addition–fragmentation chain transfer/macromolecular design via the interchange of xanthates mechanism on the stabilization of suspension polym. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 4372–4383. [Google Scholar] [CrossRef]
- Pound, G.; Eksteen, Z.; Pfukwa, R.; McKenzie, J.M.; Lange, R.F.M.; Klumperman, B. Unexpected reactions associated with the xanthate-mediated polymerization of N -vinylpyrrolidone. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6575–6593. [Google Scholar] [CrossRef]
- Mori, H.; Ookuma, H.; Nakano, S.; Endo, T. Xanthate-Mediated Controlled Radical Polymerization ofN-Vinylcarbazole. Macromol. Chem. Phys. 2006, 207, 1005–1017. [Google Scholar] [CrossRef]
- Mori, H.; Ookuma, H.; Endo, T. Poly( N -vinylcarbazole) Star Polymers and Amphiphilic Star Block Copolymers by Xanthate-Mediated Controlled Radical Polymerization. Macromolecules 2008, 41, 6925–6934. [Google Scholar] [CrossRef]
- Maki, Y.; Mori, H.; Endo, T. Xanthate-Mediated Controlled Radical Polymerization of N -Vinylindole Derivatives. Macromolecules 2007, 40, 6119–6130. [Google Scholar] [CrossRef]
- Rizzardo, E.; Chiefari, J.; Benaglia, M.; Thang, S.; Moad, G. Raft Polymerisation. WO2010083569A1, 22 January 2010. [Google Scholar]
- Destarac, M.; Brochon, C.; Catala, J.-M.; Wilczewska, A.; Zard, S.Z. Macromolecular Design via the Interchange of Xanthates (MADIX): Polymerization of Styrene with O-Ethyl Xanthates as Controlling Agents. Macromol. Chem. Phys. 2002, 203, 2281–2289. [Google Scholar] [CrossRef]
- Mori, H.; Yahagi, M.; Endo, T. RAFT Polymerization of N -Vinylimidazolium Salts and Synthesis of Thermoresponsive Ionic Liquid Block Copolymers. Macromolecules 2009, 42, 8082–8092. [Google Scholar] [CrossRef]
- Aoyagi, N.; Endo, T. Functional RAFT agents for radical-controlled polymerization: Quantitative synthesis of trithiocarbonates containing functional groups as RAFT agents using equivalent amount of CS 2. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 3702–3709. [Google Scholar] [CrossRef]
- Convertine, A.J.; Benoit, D.S.W.; Duvall, C.L.; Hoffman, A.S.; Stayton, P.S. Development of a novel endosomolytic diblock copolymer for siRNA delivery. J. Control. Release 2009, 133, 221–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopyrev, V.A.; Tatarova, L.A.; Vakulskaya, T.I.; Ermakova, T.G. Study of the mechanism of radical polymerization of 1-vinyl-1,2,4-triazole. Polym. Sci. Ser. B 1985, 27, 221–225. [Google Scholar]
- Tatarova, L.A.; Ermakova, T.G.; Lopyrev, V.A.; Kedrina, N.F.; Razvodovsky, E.F.; Berlin, A.A.; Enikolopyan, N.S. Method for obtaining water-soluble polymers of 1-vinyl-1,2,4-triazole. USSR Certif. Authorsh. 1979, 6, 85–88. [Google Scholar]
- Tatarova, L.A.; Ermakova, T.G.; Kedrina, N.F.; Kasaikin, V.A.; Novikov, D.D.; Lopyrev, V.A. Light scattering and viscosity of poly-1-vinyl-1,2,4-triazole solutions. Polym. Sci. Ser. B 1982, 24, 697–699. [Google Scholar]
- Smith, A.E.; Xu, X.; McCormick, C.L. Stimuli-responsive amphiphilic (co)polymers via RAFT polymerization. Prog. Polym. Sci. 2010, 35, 45–93. [Google Scholar] [CrossRef]
- Perrier, S.; Takolpuckdee, P. Macromolecular design via reversible addition-fragmentation chain transfer (RAFT)/xanthates (MADIX) polymerization. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5347–5393. [Google Scholar] [CrossRef]
- Favier, A.; Charreyre, M.-T. Experimental Requirements for an Efficient Control of Free-Radical Polymerizations via the Reversible Addition-Fragmentation Chain Transfer (RAFT) Process. Macromol. Rapid Commun. 2006, 27, 653–692. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living Radical Polymerization by the RAFT Process. Aust. J. Chem. 2005, 58, 379. [Google Scholar] [CrossRef]

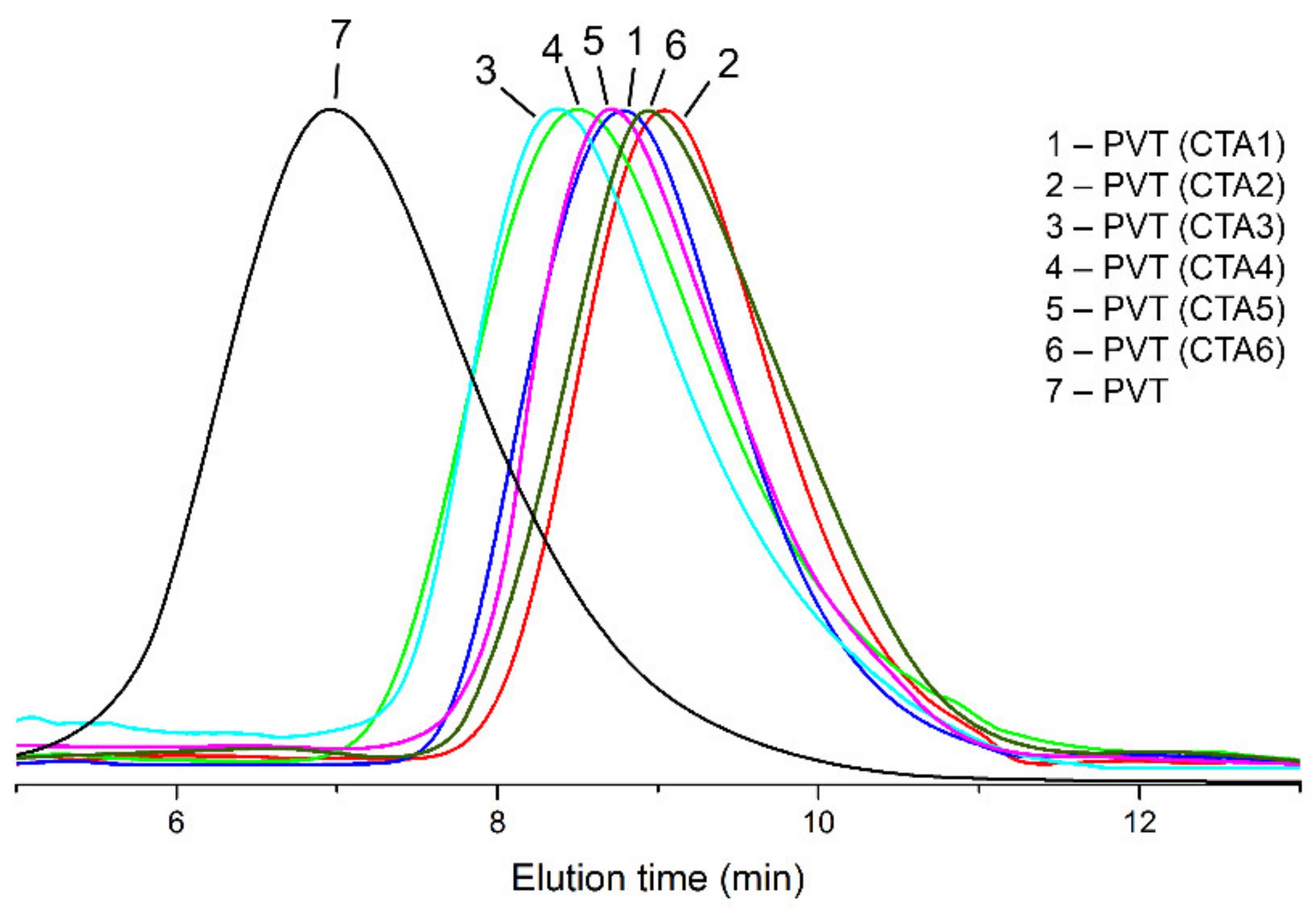
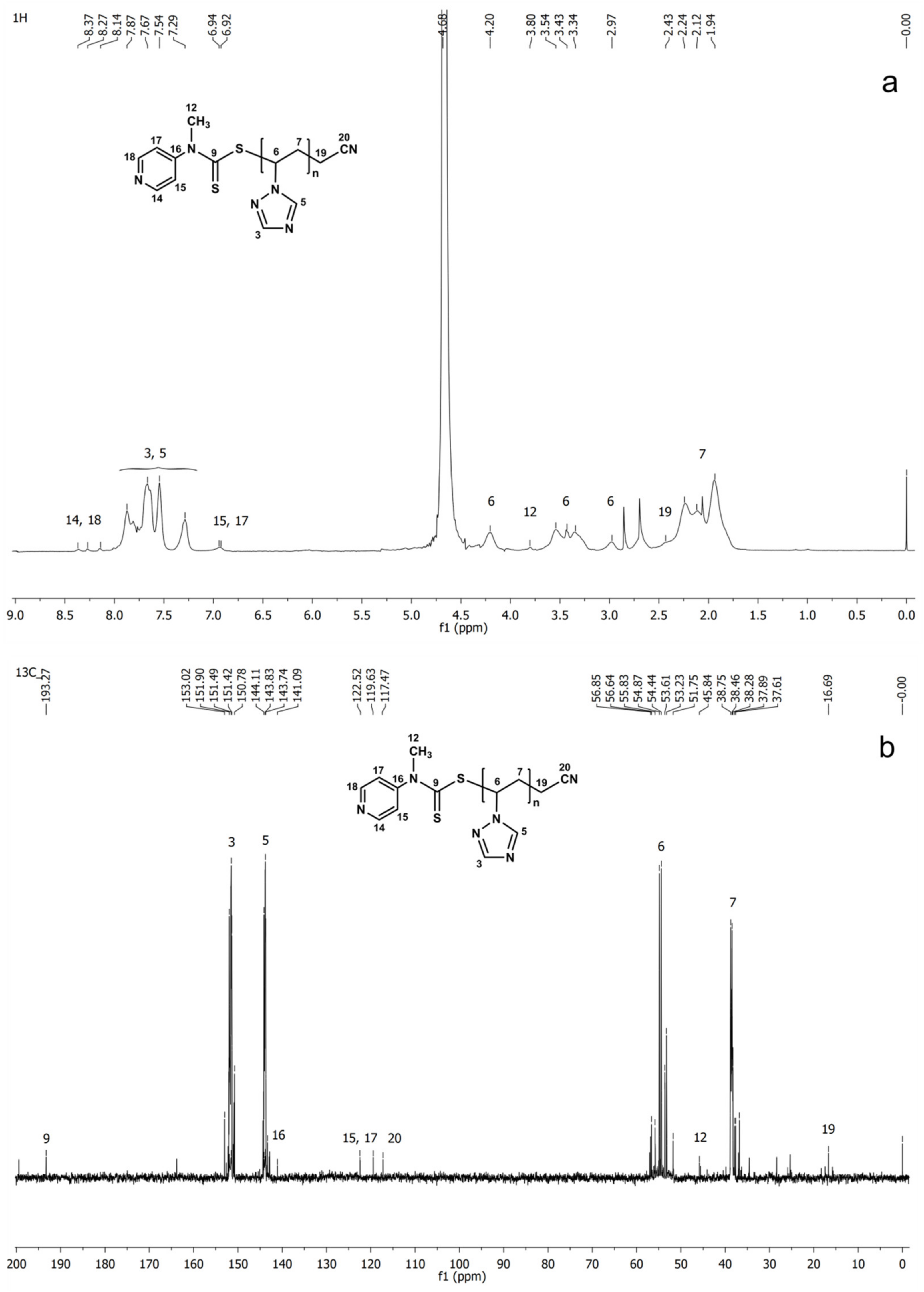



| Entry | CTA | Conversion, % ** | Mn, Da | PDI |
|---|---|---|---|---|
| 1 | CTA1 | 99 | 20,000 | 1.26 |
| 2 | CTA2 | >99 | 17,000 | 1.20 |
| 3 | CTA3 | 97 | 25,000 | 1.38 |
| 4 | CTA4 | 97 | 24,000 | 1.35 |
| 5 | CTA5 | 95 | 21,000 | 1.32 |
| 6 | CTA6 | 96 | 18,000 | 1.30 |
| Entry | CTA | Solvent | [AIBN]:[CTA] | Conversion, % ** | Mn, Da | PDI |
|---|---|---|---|---|---|---|
| 1 | CTA1 | DMF | 1:1 | 99 | 61,000 | 1.59 |
| 2 | 1:2.5 | 98 | 45,000 | 1.45 | ||
| 3 | 1:5 | 98 | 22,000 | 1.46 | ||
| 4 | 1:10 | 98 | 14,000 | 1.42 | ||
| 5 | MeOH | 1:1 | >99 | 49,000 | 1.53 | |
| 6 | 1:2.5 | 98 | 34,000 | 1.41 | ||
| 7 | 1:5 | 99 | 20,000 | 1.26 | ||
| 8 | 1:10 | 99 | 13,000 | 1.24 | ||
| 9 | CTA2 | DMF | 1:1 | >99 | 44,000 | 1.46 |
| 10 | 1:2.5 | >99 | 26,000 | 1.37 | ||
| 11 | 1:5 | >99 | 18,000 | 1.31 | ||
| 12 | 1:10 | 98 | 13,000 | 1.25 | ||
| 13 | MeOH | 1:1 | 99 | 27,000 | 1.37 | |
| 14 | 1:2.5 | >99 | 26,000 | 1.27 | ||
| 15 | 1:5 | >99 | 17,000 | 1.20 | ||
| 16 | 1:10 | >99 | 11,000 | 1.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozdnyakov, A.S.; Kuznetsova, N.P.; Semenova, T.A.; Bolgova, Y.I.; Ivanova, A.A.; Trofimova, O.M.; Emel’yanov, A.I. Dithiocarbamates as Effective Reversible Addition–Fragmentation Chain Transfer Agents for Controlled Radical Polymerization of 1-Vinyl-1,2,4-triazole. Polymers 2022, 14, 2029. https://doi.org/10.3390/polym14102029
Pozdnyakov AS, Kuznetsova NP, Semenova TA, Bolgova YI, Ivanova AA, Trofimova OM, Emel’yanov AI. Dithiocarbamates as Effective Reversible Addition–Fragmentation Chain Transfer Agents for Controlled Radical Polymerization of 1-Vinyl-1,2,4-triazole. Polymers. 2022; 14(10):2029. https://doi.org/10.3390/polym14102029
Chicago/Turabian StylePozdnyakov, Alexander S., Nadezhda P. Kuznetsova, Tatyana A. Semenova, Yuliya I. Bolgova, Anastasia A. Ivanova, Olga M. Trofimova, and Artem I. Emel’yanov. 2022. "Dithiocarbamates as Effective Reversible Addition–Fragmentation Chain Transfer Agents for Controlled Radical Polymerization of 1-Vinyl-1,2,4-triazole" Polymers 14, no. 10: 2029. https://doi.org/10.3390/polym14102029