A Phosphorus-Nitrogen-Carbon Synergistic Nanolayered Flame Retardant for Polystyrene
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Nano-Sheeted BA-MA
2.3. Preparation of In Situ Dispersed PS/BA-MA
2.4. PS Blend Preparation
2.5. Material Characterization
3. Results and Discussion
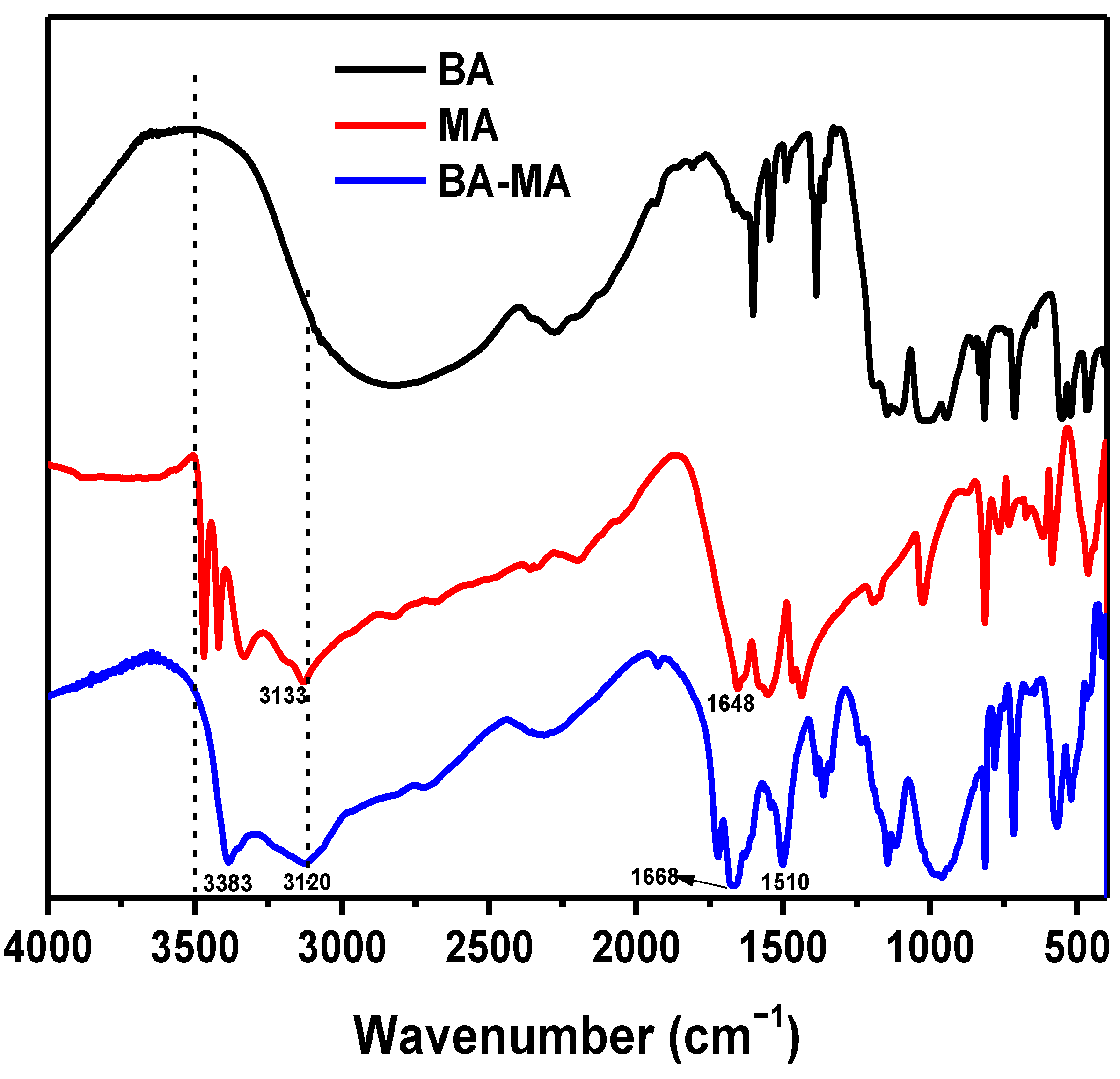
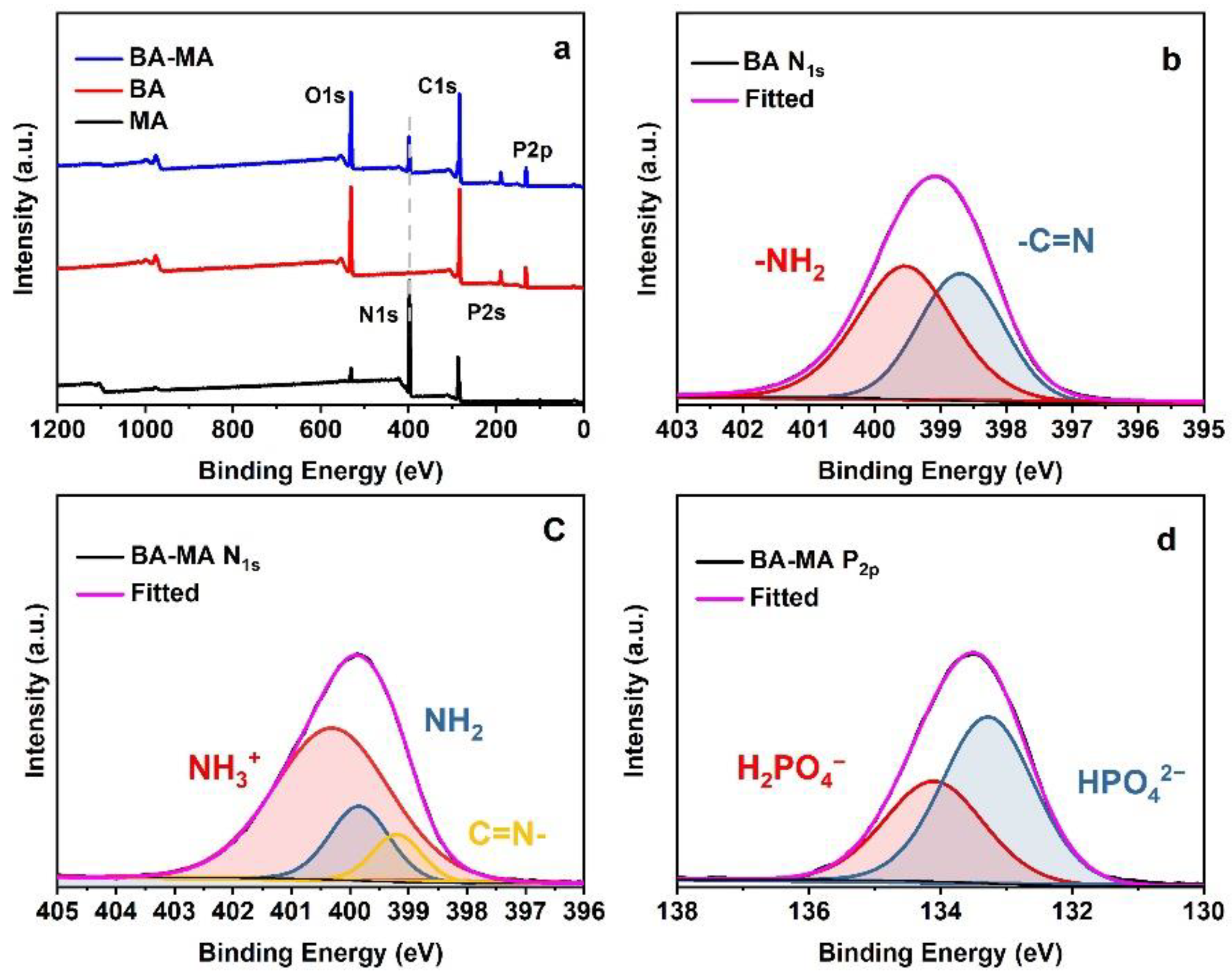
3.1. Fabrication and Morphology
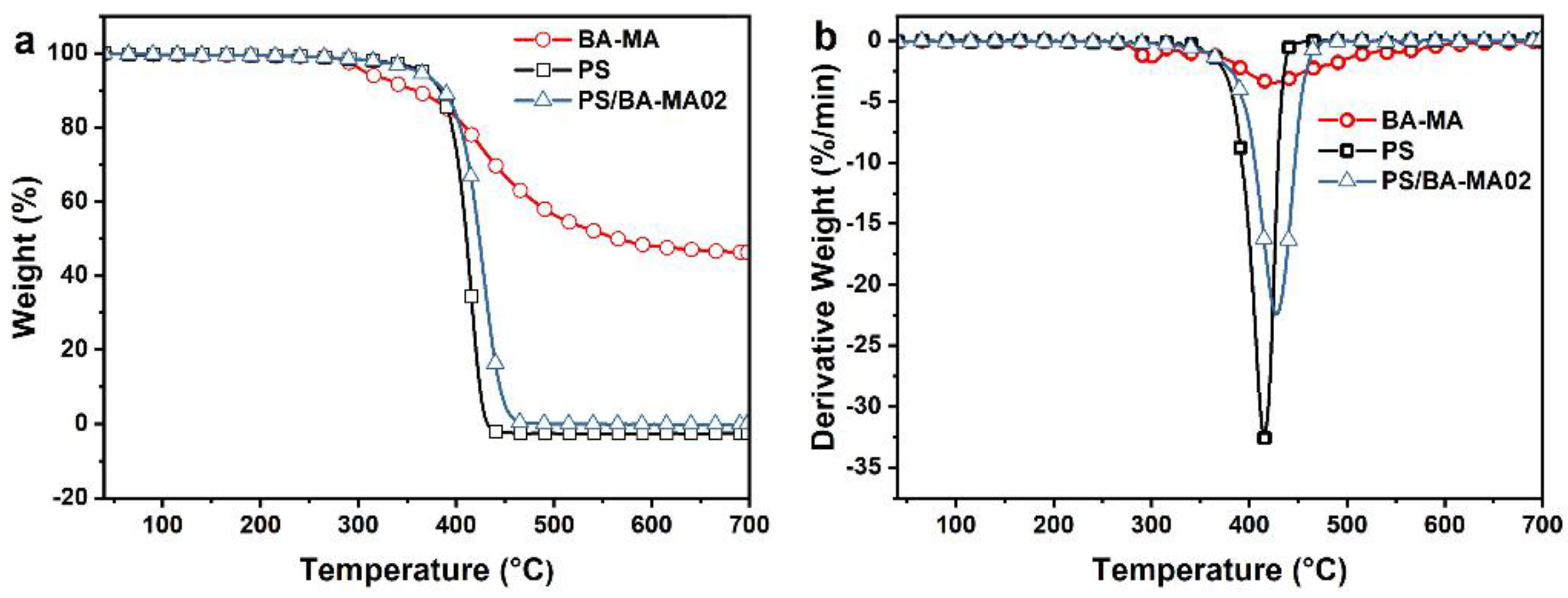
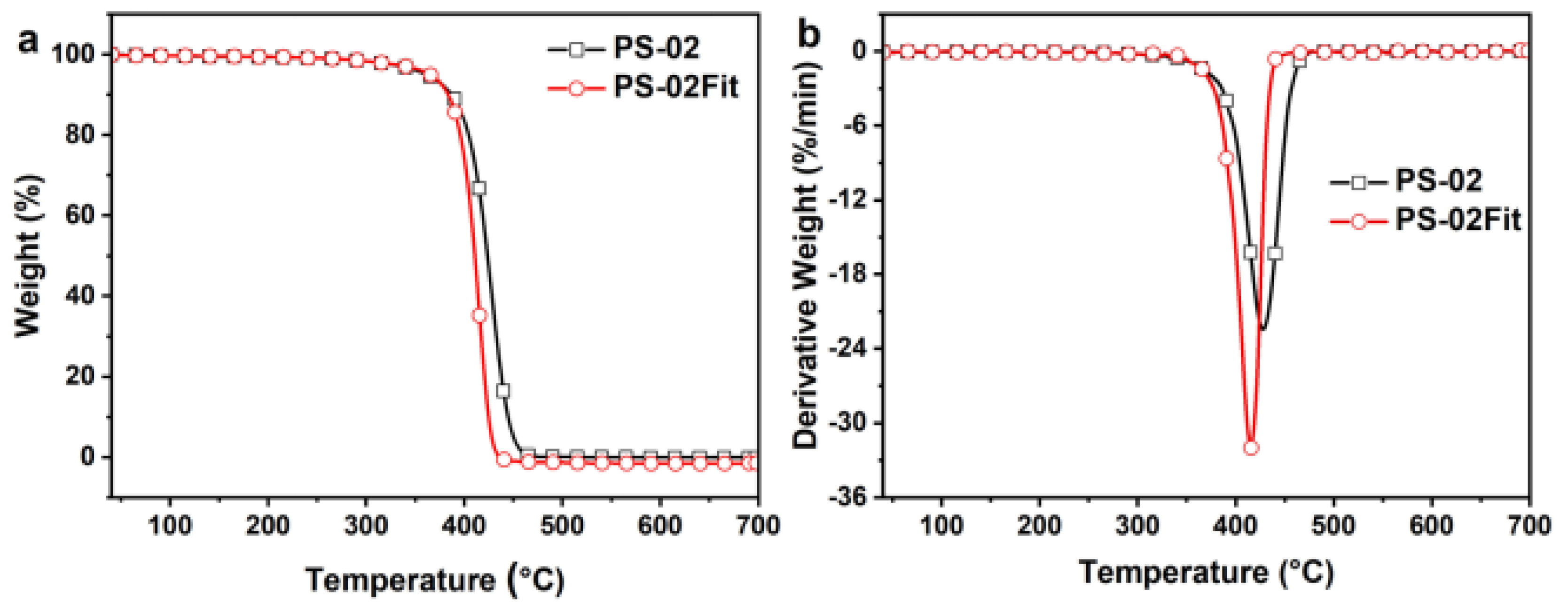
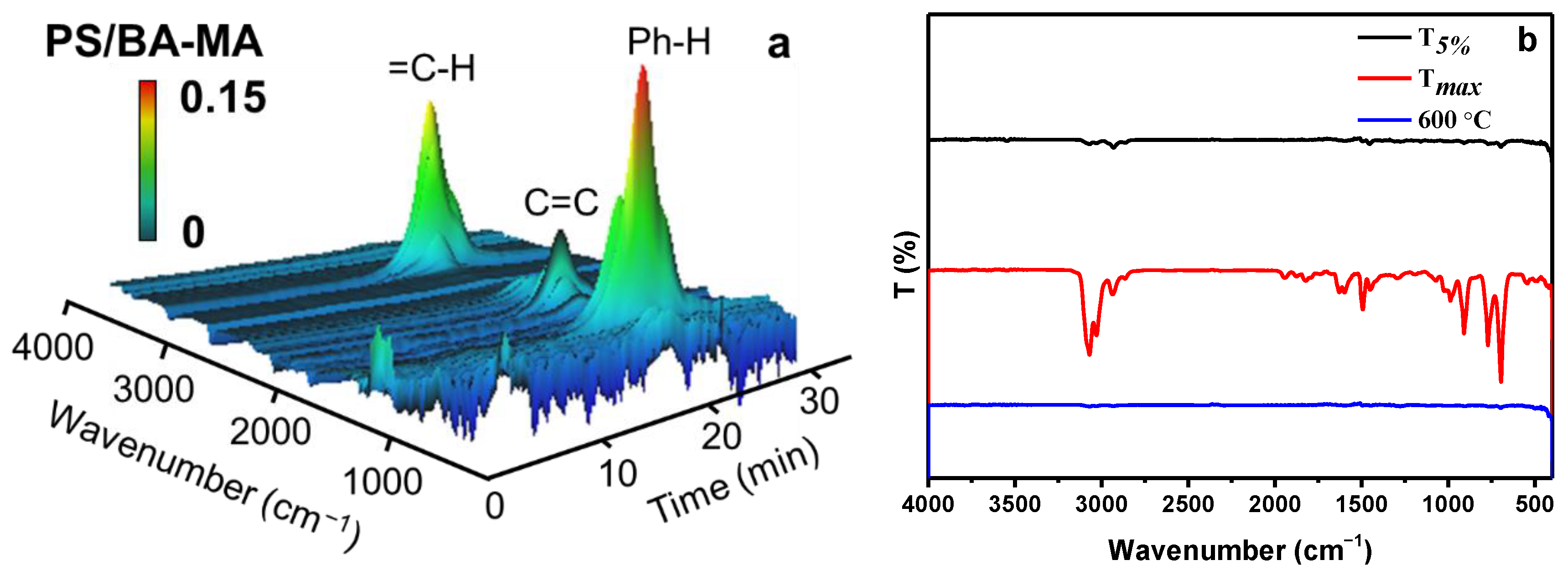
3.2. Thermal Stability
3.3. Flammability
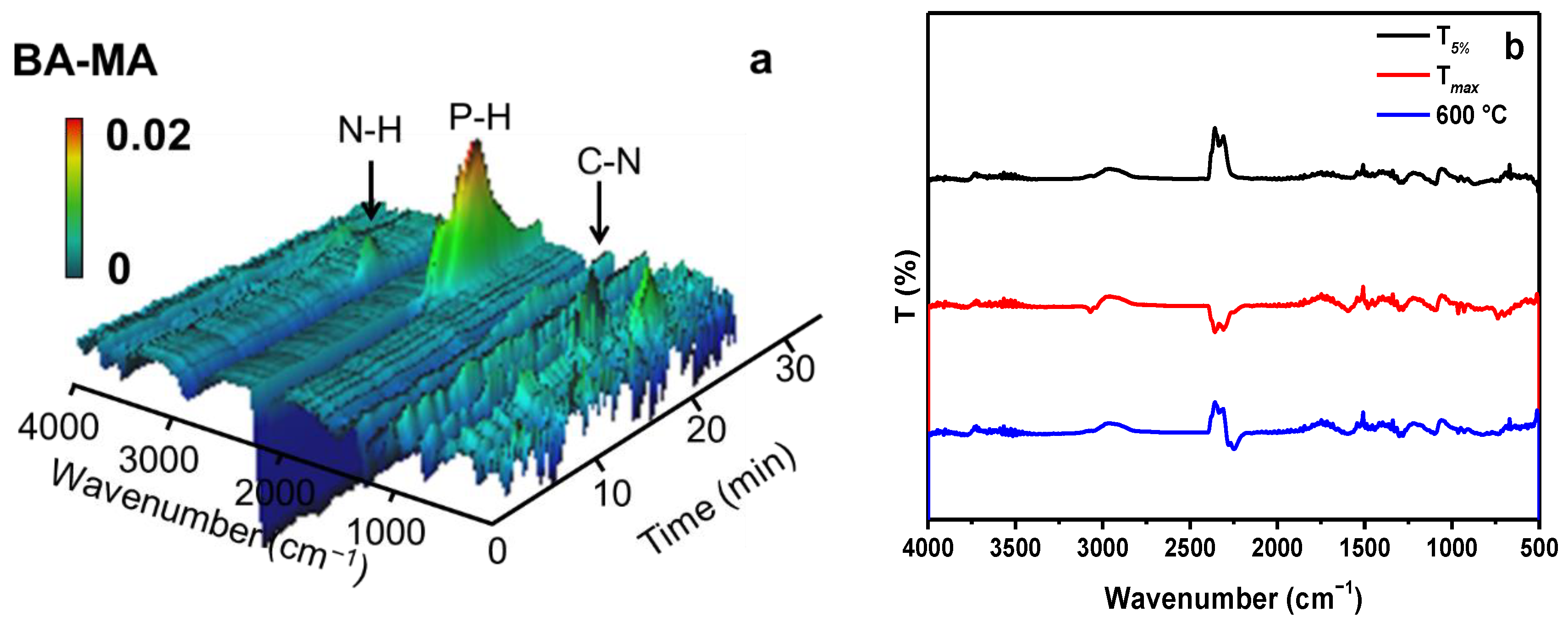
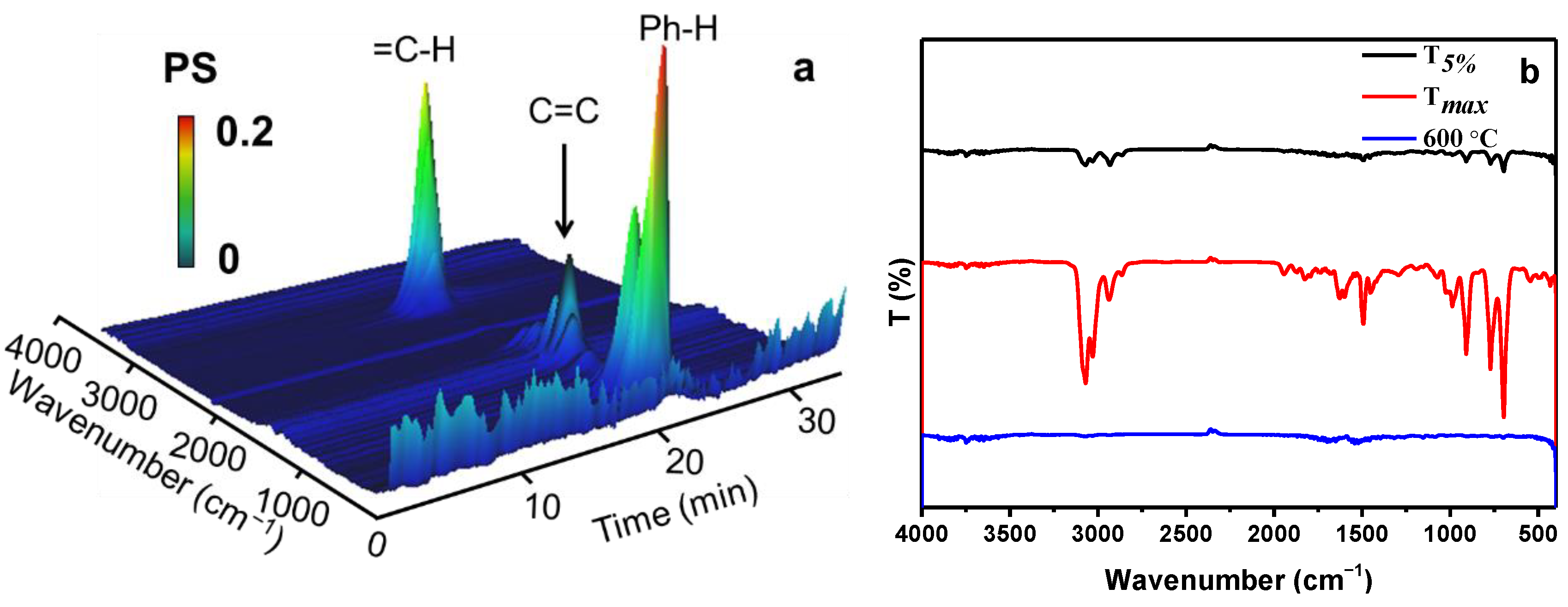
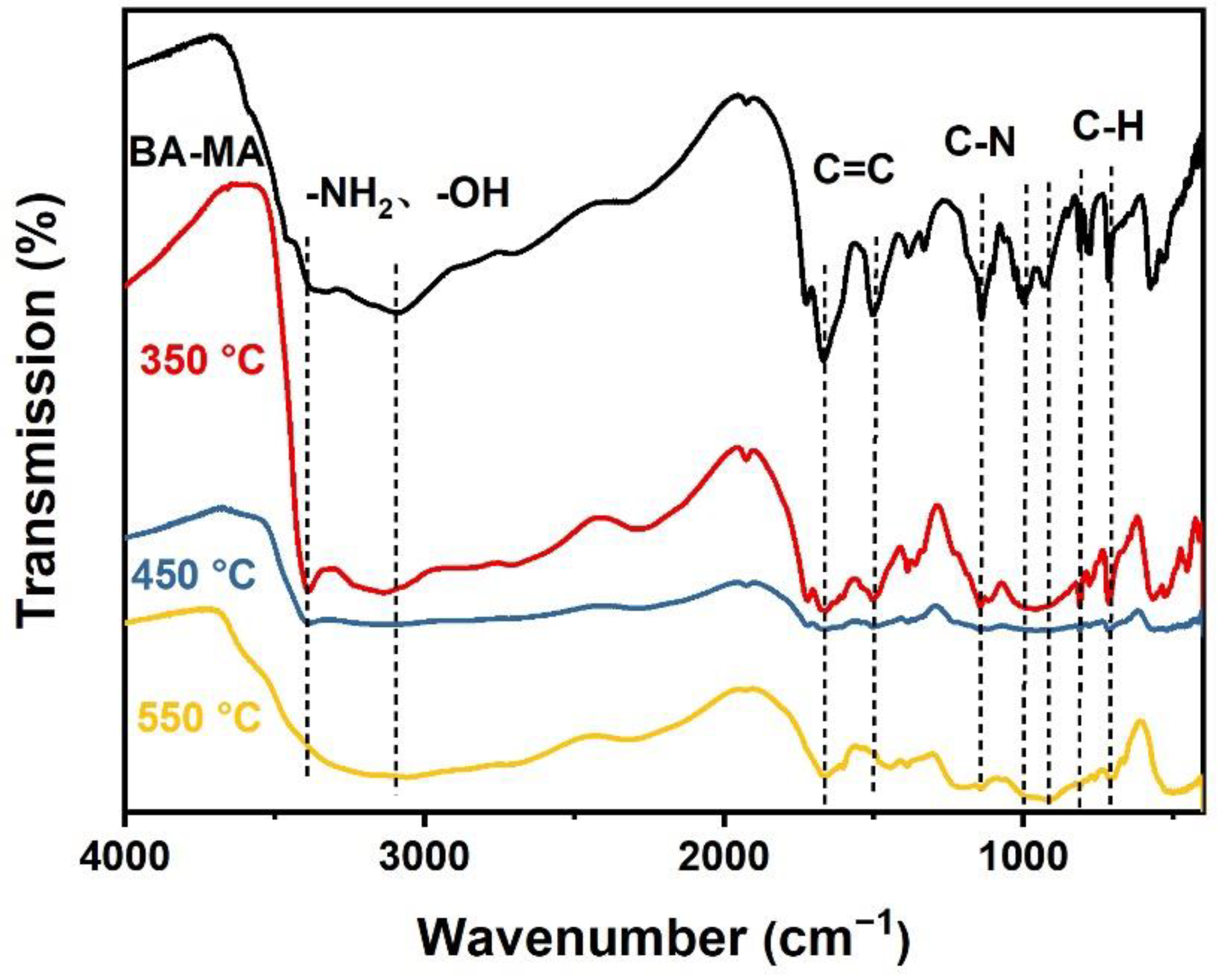
3.4. Flame Retardant and Smoke Suppression Mechanism
3.5. Mechanical Property
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, J.-B.; Zhao, H.-B.; Zhang, A.-N.; Wang, Y.-Q.; Wang, Y.-Z. Porous carbon/Fe composites from waste fabric for high-efficiency electromagnetic wave absorption. J. Mater. Sci. Technol. 2022, 126, 266–274. [Google Scholar] [CrossRef]
- Shi, H.G.; Zhao, H.B.; Liu, B.W.; Wang, Y.Z. Multifunctional Flame-Retardant Melamine-Based Hybrid Foam for Infrared Stealth, Thermal Insulation, and Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2021, 13, 26505–26514. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Long, M.-C.; Zhao, H.-B.; An, W.-L.; Xu, S.; Deng, C.; Wang, Y.-Z. Temperature-Responsive Intumescent Chemistry toward Fire Resistance and Super Thermal Insulation under Extremely Harsh Conditions. Chem. Mater. 2021, 33, 6018–6028. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Zhao, H.-B.; Cheng, J.-B.; Liu, B.-W.; Fu, Q.; Wang, Y.-Z. Hierarchical Ti3C2Tx@ZnO Hollow Spheres with Excellent Microwave Absorption Inspired by the Visual Phenomenon of Eyeless Urchins. Nano-Micro Lett. 2022, 14, 76–91. [Google Scholar] [CrossRef]
- Li, M.E.; Wang, S.X.; Han, L.X.; Yuan, W.J.; Cheng, J.B.; Zhang, A.N.; Zhao, H.B.; Wang, Y.Z. Hierarchically porous SiO2/polyurethane foam composites towards excellent thermal insulating, flame-retardant and smoke-suppressant performances. J. Hazard. Mater. 2019, 375, 61–69. [Google Scholar] [CrossRef]
- Li, M.-E.; Yan, Y.-W.; Zhao, H.-B.; Jian, R.-K.; Wang, Y.-Z. A facile and efficient flame-retardant and smoke-suppressant resin coating for expanded polystyrene foams. Compos. Part B Eng. 2020, 185, 107797–107804. [Google Scholar] [CrossRef]
- Liu, B.-W.; Zhao, H.-B.; Chen, L.; Chen, L.; Wang, X.-L.; Wang, Y.-Z. Eco-friendly synergistic cross-linking flame-retardant strategy with smoke and melt-dripping suppression for condensation polymers. Compos. Part B Eng. 2021, 211, 108664–108676. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, H.-B.; Cheng, J.-B.; Li, W.; Zhang, J.; Wang, Y.-Z. A green, durable and effective flame-retardant coating for expandable polystyrene foams. Chem. Eng. J. 2022, 440, 135807–135817. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.-Z. A review on flame retardant technology in China. Part 1: Development of flame retardants. Polym. Adv. Technol. 2010, 21, 1–26. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.-Z.; Cai, G.-P.; Mai, Y.-W. Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Liu, B.W.; Zhao, H.B.; Wang, Y.Z. Advanced Flame-Retardant Methods for Polymeric Materials. Adv. Mater. 2021, 2107905. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Xie, T.; Yang, G. Effects of interfacial modification on the thermal, mechanical, and fire properties of high-impact polystyrene/microencapsulated red phosphorous. J. Appl. Polym. Sci. 2008, 110, 2139–2144. [Google Scholar] [CrossRef]
- Yan, Y.-W.; Huang, J.-Q.; Guan, Y.-H.; Shang, K.; Jian, R.-K.; Wang, Y.-Z. Flame retardance and thermal degradation mechanism of polystyrene modified with aluminum hypophosphite. Polym. Degrad. Stab. 2014, 99, 35–42. [Google Scholar] [CrossRef]
- Lyu, W.; Cui, Y.; Zhang, X.; Yuan, J.; Zhang, W. Fire and thermal properties of PA 66 resin treated with poly-N-aniline-phenyl phosphamide as a flame retardant. Fire Mater. 2017, 41, 349–361. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Duquesne, S.; Rochery, M. Recent Advances for Intumescent Polymers. Macromol. Mater. Eng. 2004, 289, 499–511. [Google Scholar] [CrossRef]
- Feng, C.; Liang, M.; Jiang, J.; Huang, J.; Liu, H.J.T.A. Synergistic effect of a novel triazine charring agent and ammonium polyphosphate on the flame retardant properties of halogen-free flame retardant polypropylene composites. Thermochim. Acta 2016, 627–629, 83–90. [Google Scholar] [CrossRef]
- Gu, J.-W.; Zhang, G.-C.; Dong, S.-L.; Zhang, Q.-Y.; Kong, J. Study on preparation and fire-retardant mechanism analysis of intumescent flame-retardant coatings. Surf. Coat. Technol. 2007, 201, 7835–7841. [Google Scholar] [CrossRef]
- Ai, Y.-F.; Xia, L.; Pang, F.-Q.; Xu, Y.-L.; Zhao, H.-B.; Jian, R.-K. Mechanically strong and flame-retardant epoxy resins with anti-corrosion performance. Compos. Part B Eng. 2020, 193, 108019–108031. [Google Scholar] [CrossRef]
- Cao, Z.-J.; Liao, W.; Wang, S.-X.; Zhao, H.-B.; Wang, Y.-Z. Polyurethane foams with functionalized graphene towards high fire-resistance, low smoke release, superior thermal insulation. Chem. Eng. J. 2019, 361, 1245–1254. [Google Scholar] [CrossRef]
- Rao, W.; Shi, J.; Yu, C.; Zhao, H.-B.; Wang, Y.-Z. Highly efficient, transparent, and environment-friendly flame-retardant coating for cotton fabric. Chem. Eng. J. 2021, 424, 130556–130568. [Google Scholar] [CrossRef]
- Rao, W.; Zhao, P.; Yu, C.; Zhao, H.-B.; Wang, Y.-Z. High strength, low flammability, and smoke suppression for epoxy thermoset enabled by a low-loading phosphorus-nitrogen-silicon compound. Compos. Part B Eng. 2021, 211, 108640–108652. [Google Scholar] [CrossRef]
- Yu, C.; Wu, T.; Yang, F.; Rao, W.; Zhao, H.-B.; Zhu, Z. Construction of hetero-structured nanohybrid relying on reactive phosphazene towards flame retardation and mechanical enhancement of epoxy resins. Eur. Polym. J. 2022, 167, 111075–111087. [Google Scholar] [CrossRef]
- Zhang, A.-N.; Zhao, H.-B.; Cheng, J.-B.; Li, M.-E.; Li, S.-L.; Cao, M.; Wang, Y.-Z. Construction of durable eco-friendly biomass-based flame-retardant coating for cotton fabrics. Chem. Eng. J. 2021, 410, 128361–128371. [Google Scholar] [CrossRef]
- Wang, J.S.; Wang, D.Y.; Yun, L.; Ge, X.G.; Wang, Y.-Z. Polyamide-enhanced flame retardancy of ammonium polyphosphate on epoxy resin. J. Appl. Polym. Sci. 2010, 108, 2644–2653. [Google Scholar] [CrossRef]
- Yin, X.; Mao, Z.; Jin, F.; Yong, G.; Zheng, A.J.P.D. Stability, Synthesis of 1-hydroxy ethylidene-1,1-diphosphonic ammonium and the promise of this ammonium salt as an intumescent flame retardant in polystyrene. Polym. Degrad. Stabil. 2014, 102, 186–194. [Google Scholar]
- Yan, Y.-W.; Chen, L.; Jian, R.-K.; Kong, S.; Wang, Y.-Z. Intumescence: An effect way to flame retardance and smoke suppression for polystryene. Polym. Degrad. Stabil. 2012, 97, 1423–1431. [Google Scholar] [CrossRef]
- Tai, Q.; Song, L.; Hao, F.; Tao, Y.; Yuen, R.; Hu, Y. Investigation of a combination of novel polyphosphoramide and boron-containing compounds on the thermal and flame-retardant properties of polystyrene. J. Polym. Res. 2012, 19, 9763. [Google Scholar] [CrossRef]
- Yuan, S.; Chen, W.; Liu, G. Synergistic effect of THEIC-Based charring agent on flame retardant properties of polylactide. J. Appl. Polym. Sci. 2015, 132, 41218–41223. [Google Scholar] [CrossRef]
- Hu, X.; Guo, Y.; Chen, L.; Wang, X.; Li, L.; Wang, Y. A novel polymeric intumescent flame retardant: Synthesis, thermal degradation mechanism and application in ABS copolymer. Polym. Degrad. Stabil. 2012, 97, 1772–1778. [Google Scholar] [CrossRef]
- Apaydin, K.; Laachachi, A.; Ball, V.; Jimenez, M.; Bourbigot, S.; Toniazzo, V.; Ruch, D. Polyallylamine-montmorillonite as super flame retardant coating assemblies by layer-by layer deposition on polyamide. Polym. Degrad. Stabil. 2013, 98, 627–634. [Google Scholar] [CrossRef]
- Yao, H.B.; Tan, Z.H.; Fang, H.Y.; Yu, S.-H. Artificial Nacre-like Bionanocomposite Films from the Self-Assembly of Chitosan-Montmorillonite Hybrid Building Blocks. Angew. Chem. Int. Ed. 2010, 49, 10127–10131. [Google Scholar] [CrossRef] [PubMed]
- Matusinovic, Z.; Wilkie, C.A. Fire retardancy and morphology of layered double hydroxide nanocomposites: A review. J. Mater. Chem. C 2012, 22, 18701–18704. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Wang, Q.; Wilkie, C.A.; O’Hare, D. Flame retardant polymer/layered double hydroxide nanocomposites. J. Mater. Chem. A 2014, 2, 10996–11016. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Lu, Q.; Chen, B.; Chen, J.; Huang, Y.; Ma, Q.; Tan, C.; Yang, J.; Cao, X.; et al. Self-Assembly of Single-Layer CoAl-Layered Double Hydroxide Nanosheets on 3D Graphene Network Used as Highly Efficient Electrocatalyst for Oxygen Evolution Reaction. Adv. Mater. 2016, 28, 7640–7645. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B.; Fichera, M.A.; Jaeger, C. Flame retardancy mechanisms of aluminium phosphinate in combination with melamine polyphosphate and zinc borate in glass-fibre reinforced polyamide 6,6. Polym. Degrad. Stabil. 2007, 92, 1528–1545. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liu, Y.; Wang, Q. Stability, Flame retardant polyoxymethylene with aluminium hydroxide/melamine/novolac resin synergistic system. Polym. Degrad. Stabil. 2010, 95, 945–954. [Google Scholar] [CrossRef]
- Wu, C.S.; Liu, Y.L.; Chiu, Y.S. Epoxy resins possessing flame retardant elements from silicon incorporated epoxy compounds cured with phosphorus or nitrogen containing curing agents. Polymer 2002, 43, 4277–4284. [Google Scholar] [CrossRef]
- Xing, W.; Yang, W.; Yang, W.; Hu, Q.; Si, J.; Lu, H.; Yang, B.; Song, L.; Hu, Y.; Yuen, R.K.K. Functionalized Carbon Nanotubes with Phosphorus- and Nitrogen-Containing Agents: Effective Reinforcer for Thermal, Mechanical, and Flame-Retardant Properties of Polystyrene Nanocomposites. Acs Appl. Mater. Interfaces 2016, 8, 26266–26274. [Google Scholar] [CrossRef]
- Wang, P.J.; Liao, D.J.; Hu, X.P.; Pan, N.; Li, W.X.; Wang, D.Y.; Yao, Y. Facile fabrication of biobased P-N-C-containing nano-layered hybrid: Preparation, growth mechanism and its efficient fire retardancy in epoxy. Polym. Degrad. Stabil. 2019, 159, 153–162. [Google Scholar] [CrossRef]
- Wang, P.; Xia, L.; Jian, R.; Ai, Y.; Zheng, X.; Chen, G.; Wang, J. Flame-retarding epoxy resin with an efficient P/N/S-containing flame retardant: Preparation, thermal stability, and flame retardance. Polym. Degrad. Stabil. 2018, 149, 69–77. [Google Scholar] [CrossRef]
- Wang, M.; Xiao, G.; Chen, C.; Chen, C.; Yang, Z.; Zhong, F.; Liu, Y.; Zou, R. Highly thermally stable zirconium oxide deposited layered double hydroxide for enhancing flame retardancy of waterborne epoxy coatings. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127368–127382. [Google Scholar] [CrossRef]
- Rao, W.-H.; Zhu, Z.-M.; Wang, S.-X.; Wang, T.; Tan, Y.; Liao, W.; Zhao, H.-B.; Wang, Y.-Z. A reactive phosphorus-containing polyol incorporated into flexible polyurethane foam: Self-extinguishing behavior and mechanism. Polym. Degrad. Stab. 2018, 153, 192–200. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Zhao, H.-B.; Degracia, K.; Han, L.-X.; Sun, H.; Sun, M.; Wang, Y.-Z.; Schiraldi, D.A. Green Approach to Improving the Strength and Flame Retardancy of Poly(vinyl alcohol)/Clay Aerogels: Incorporating Biobased Gelatin. Acs Appl. Mater. Interfaces 2017, 9, 42258–42265. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-W.; Chen, L.; Guo, D.-M.; Liu, X.-F.; Lei, Y.-F.; Ding, X.-M.; Wang, Y.-Z. Fire-Safe Polyesters Enabled by End-Group Capturing Chemistry. Angew. Chem. Int. Ed. 2019, 58, 9188–9193. [Google Scholar] [CrossRef]
- Fu, T.; Zhao, X.; Chen, L.; Wu, W.-S.; Zhao, Q.; Wang, X.-L.; Guo, D.-M.; Wang, Y.-Z. Bioinspired Color Changing Molecular Sensor toward Early Fire Detection Based on Transformation of Phthalonitrile to Phthalocyanine. Adv. Funct. Mater. 2019, 29, 1806586–1806594. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Liu, B.-W.; Wang, Y.-Z.; Zhao, H.-B. Recent advances in halogen-free flame-retardant polyurethane foams. Acta Polym. Sin. 2022, 53, 1–14. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, B.-W.; Wang, Y.-Z.; Fu, T.; Zhao, H.-B. P-doped PANI/AgMWs nano/micro coating towards high-efficiency flame retardancy and electromagnetic interference shielding. Compos. Part B Eng. 2022, 238, 109944–109955. [Google Scholar] [CrossRef]




| Element | Weight Conc. | Atomic Conc. |
|---|---|---|
| C | 60.26 | 68.41 |
| N | 11.33 | 11.03 |
| O | 19.55 | 16.66 |
| P | 8.85 | 3.90 |
| Total | 100.00 | 100.00 |
| Sample | T5% (°C) | Tmax (°C) | Loss Ratemax (%/min) | Residue at 600 °C (%) |
|---|---|---|---|---|
| BA-MA | 307 | 423 | −3.51 | 48.07 |
| PS | 366 | 415 | −32.60 | / |
| PS-02 | 362 | 428 | −22.53 | / |
| PS-02Fit | 362 | 416 | −31.99 | / |
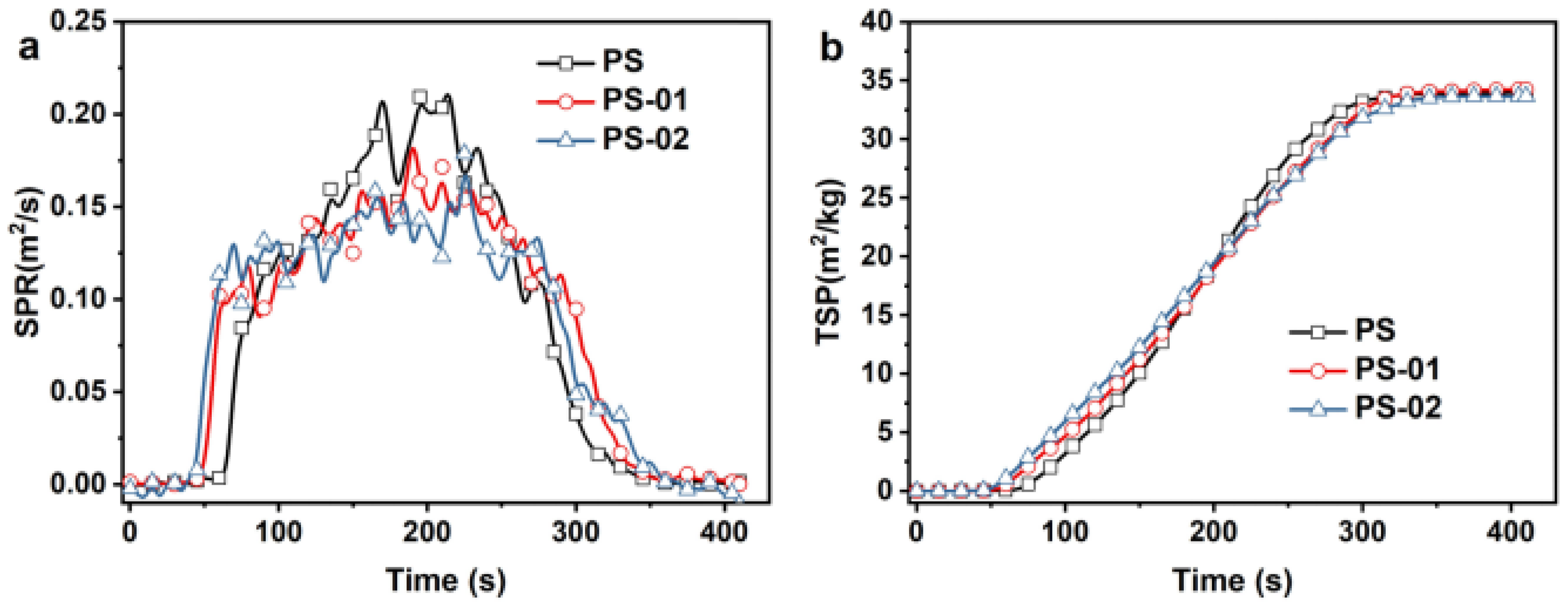
| Sample | TTI (s) | PHRR (kW/m2) | THR (MJ/m2) | Time to PHRR (s) | FGR (kW/m2/s) | PSPR (m2/s) | TSP (m2/kg) |
|---|---|---|---|---|---|---|---|
| PS | 55 | 702.38 | 113.06 | 200 | 3.5 | 0.22 | 33.96 |
| PS-01 | 51 | 593.97 | 112.65 | 205 | 2.9 | 0.19 | 34.25 |
| PS-02 | 45 | 563.45 | 111.80 | 225 | 2.5 | 0.18 | 33.66 |
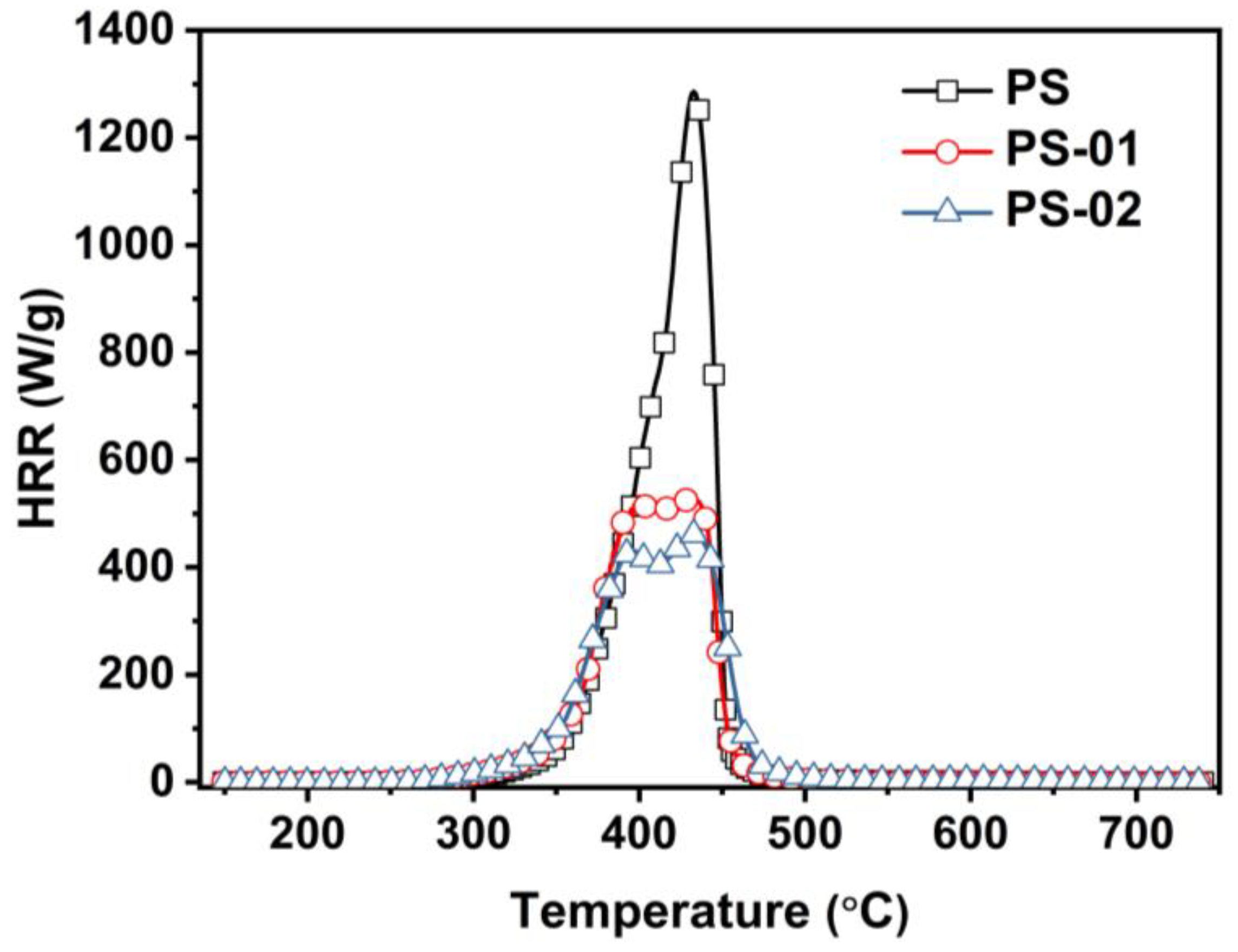
| Sample | HRC (J/g/K) | PHRR (W/g) | THR (kJ/g) | Tp (°C) |
|---|---|---|---|---|
| PS | 1120 | 1286 | 39.2 | 433 |
| PS-01 | 524 | 406 | 38.9 | 383 |
| PS-02 | 384 | 431 | 34.7 | 397 |
| Sample | Tensile Strength/MPa | Flexural Strength/MPa |
|---|---|---|
| PS | 95 ± 5 | 247 ± 8 |
| PS-01 | 92 ± 3 | 237 ± 15 |
| PS-02 | 94 ± 4 | 239 ± 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, W.-J.; Zhao, W.; Wu, G.; Zhao, H.-B. A Phosphorus-Nitrogen-Carbon Synergistic Nanolayered Flame Retardant for Polystyrene. Polymers 2022, 14, 2055. https://doi.org/10.3390/polym14102055
Yuan W-J, Zhao W, Wu G, Zhao H-B. A Phosphorus-Nitrogen-Carbon Synergistic Nanolayered Flame Retardant for Polystyrene. Polymers. 2022; 14(10):2055. https://doi.org/10.3390/polym14102055
Chicago/Turabian StyleYuan, Wen-Jie, Wei Zhao, Gang Wu, and Hai-Bo Zhao. 2022. "A Phosphorus-Nitrogen-Carbon Synergistic Nanolayered Flame Retardant for Polystyrene" Polymers 14, no. 10: 2055. https://doi.org/10.3390/polym14102055
APA StyleYuan, W.-J., Zhao, W., Wu, G., & Zhao, H.-B. (2022). A Phosphorus-Nitrogen-Carbon Synergistic Nanolayered Flame Retardant for Polystyrene. Polymers, 14(10), 2055. https://doi.org/10.3390/polym14102055







