The Effect of a Green Smoothie on Microhardness, Profile Roughness and Color Change of Dental Restorative Materials
Abstract
:1. Introduction
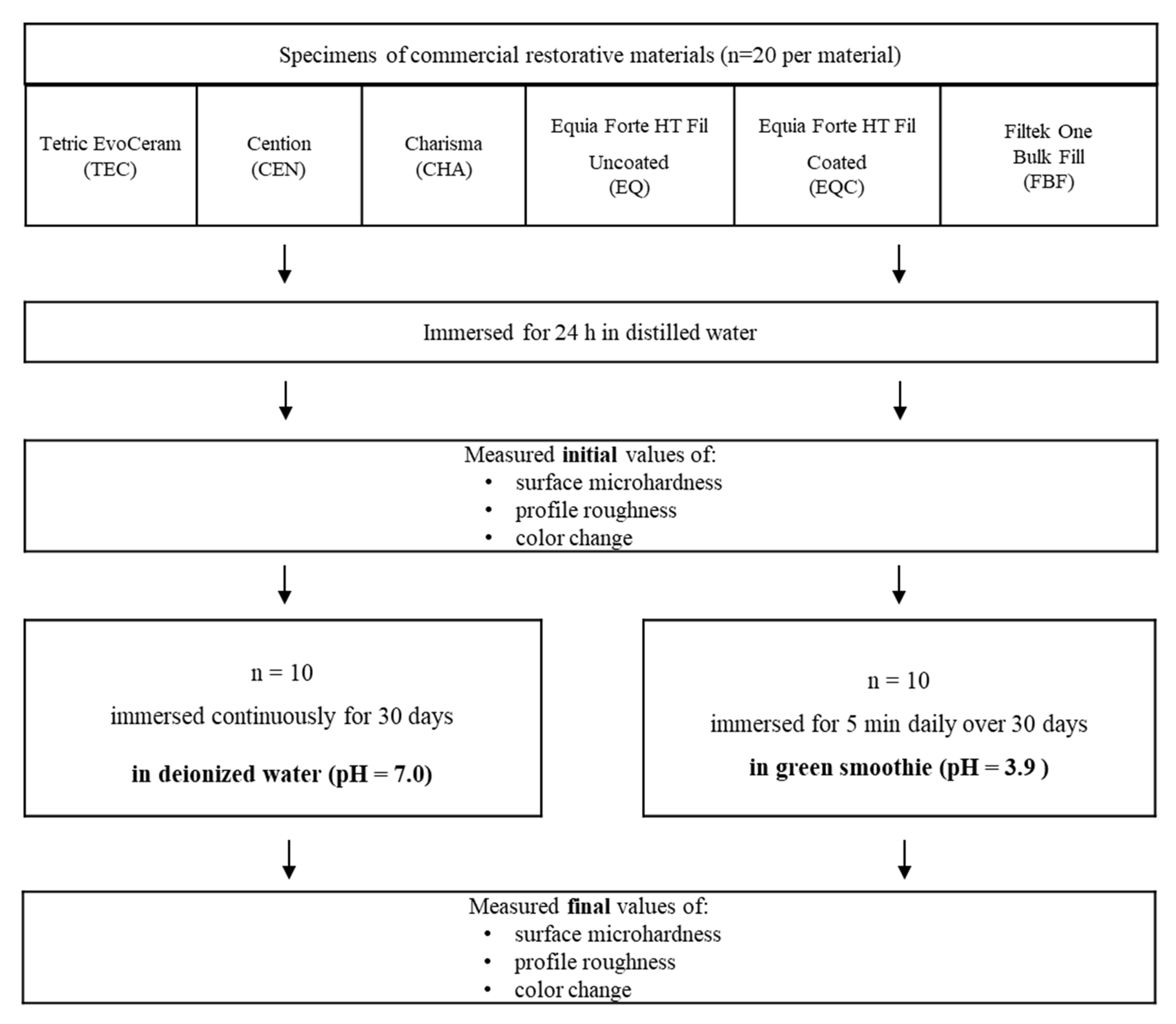
2. Materials and Methods
2.1. Specimen Preparation
2.2. Surface Microhardness Measurements
2.3. Profile Roughness Measurements
2.4. Color Measurements
2.5. Statistical Analysis
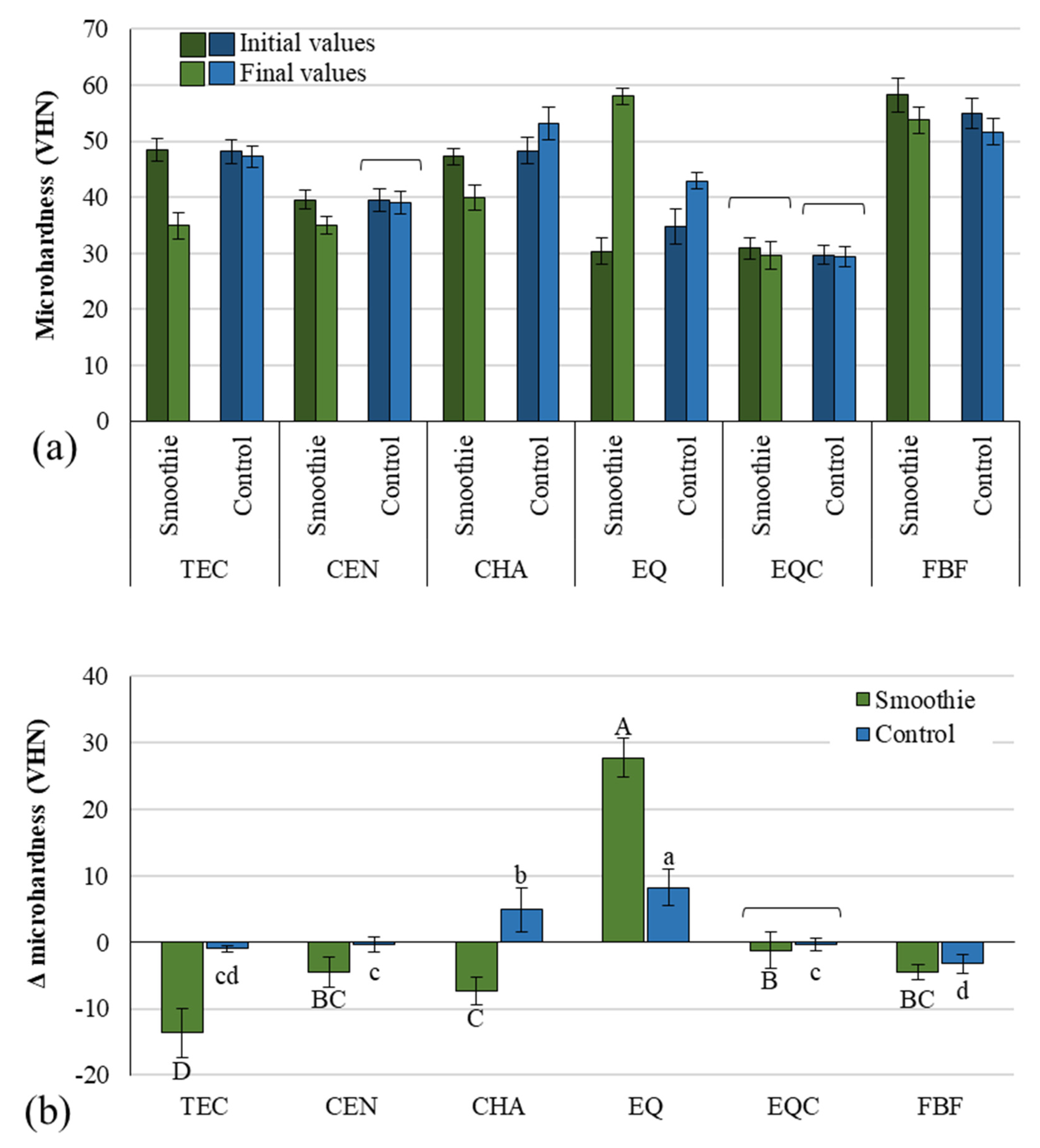
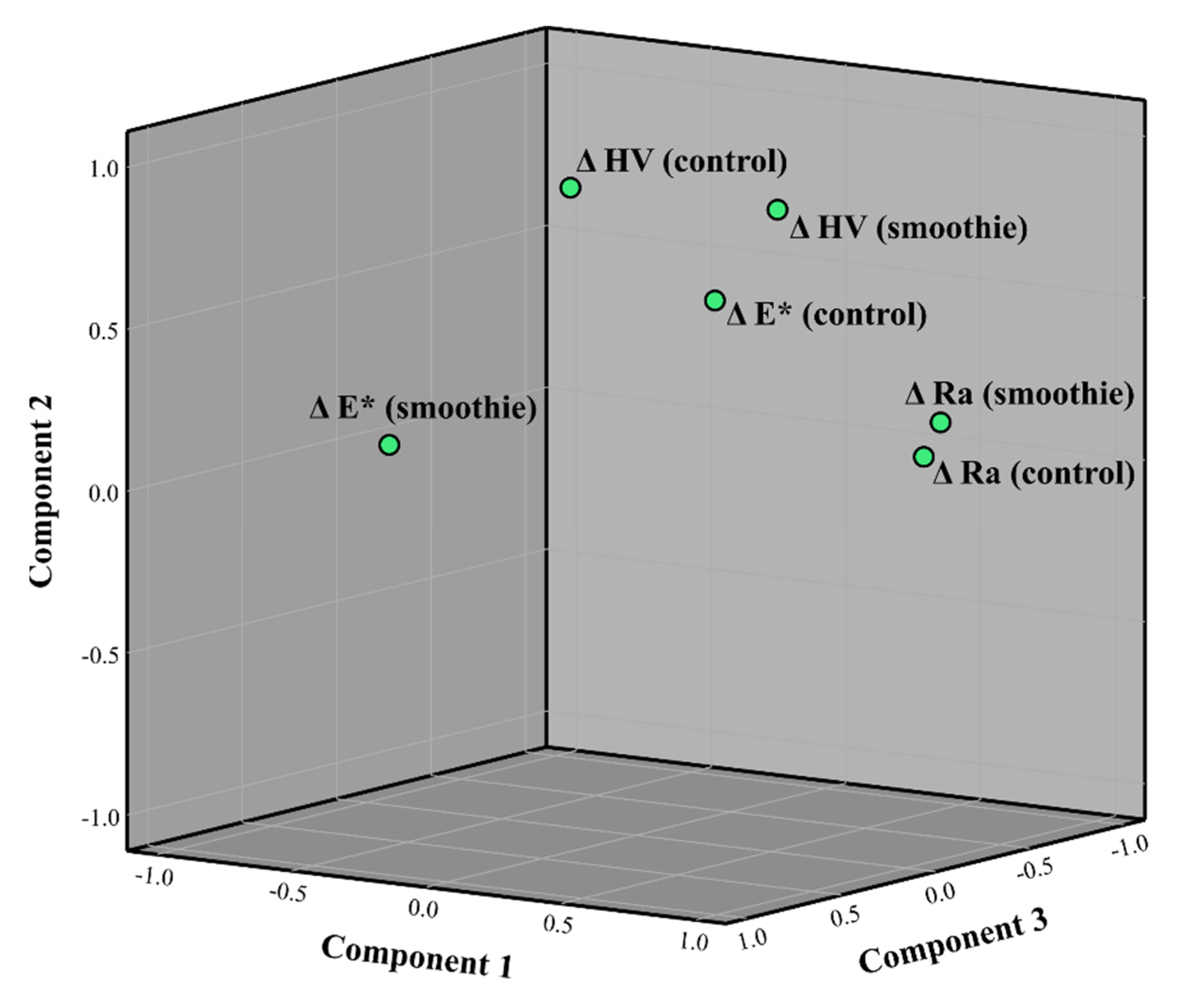
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic Aspects of Restorative Dentistry Biomaterials. Biomimetics 2020, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S. Wear Behavior of Various Dental Restorative Materials. Mater. Technol. 2019, 34, 25–31. [Google Scholar] [CrossRef]
- Ilie, N.; Furtos, G. A Comparative Study of Light Transmission by Various Dental Restorative Materials and the Tooth Structure. Oper. Dent. 2020, 45, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Huang, X.; Li, M.; Peng, X.; Wang, S.; Zhou, X.; Cheng, L. Development and Status of Resin Composite as Dental Restorative Materials. J. Appl. Polym. Sci. 2019, 136, 48180. [Google Scholar] [CrossRef] [Green Version]
- Aminoroaya, A.; Neisiany, R.E.; Khorasani, S.N.; Panahi, P.; Das, O.; Madry, H.; Cucchiarini, M.; Ramakrishna, S. A Review of Dental Composites: Challenges, Chemistry Aspects, Filler Influences, and Future Insights. Compos. Part B Eng. 2021, 216, 108852. [Google Scholar] [CrossRef]
- Ilie, N. Fracture and Viscoelastic Behavior of Novel Self-Adhesive Materials for Simplified Restoration Concepts. J. Mech. Behav. Biomed. Mater. 2022, 125, 104970. [Google Scholar] [CrossRef]
- Par, M.; Gubler, A.; Attin, T.; Tarle, Z.; Tauböck, T.T. Anti-Demineralizing Protective Effects on Enamel Identified in Experimental and Commercial Restorative Materials with Functional Fillers. Sci. Rep. 2021, 11, 11806. [Google Scholar] [CrossRef]
- Nicholson, J.W.; Sidhu, S.K.; Czarnecka, B. Enhancing the Mechanical Properties of Glass-Ionomer Dental Cements: A Review. Materials 2020, 13, 2510. [Google Scholar] [CrossRef]
- Drummond, J.L. Degradation, Fatigue, and Failure of Resin Dental Composite Materials. J. Dent. Res. 2008, 87, 710–719. [Google Scholar] [CrossRef] [Green Version]
- Delaviz, Y.; Finer, Y.; Santerre, J.P. Biodegradation of Resin Composites and Adhesives by Oral Bacteria and Saliva: A Rationale for New Material Designs That Consider the Clinical Environment and Treatment Challenges. Dent. Mater. 2014, 30, 16–32. [Google Scholar] [CrossRef]
- Xavier, A.; Sunny, S.; Rai, K.; Hegde, A. Repeated Exposure of Acidic Beverages on Esthetic Restorative Materials: An In-Vitro Surface Microhardness Study. J. Clin. Exp. Dent. 2016, 8, e312–e317. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, R.; Colombo, M.; Bitonti, G.; Chiesa, M.; Poggio, C.; Pietrocola, G. Restorative Materials Exposed to Acid Challenge: Influence of Temperature on In Vitro Weight Loss. Biomimetics 2022, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.G.; Soares, C.J.; Maia, T.S.; Bicalho, A.A.; Barbosa, T.P.; Costa, H.L.; Menezes, M.S. Effect of Acidic Drinks on Shade Matching, Surface Topography, and Mechanical Properties of Conventional and Bulk-Fill Composite Resins. J. Prosthet. Dent. 2019, 121, 868.e1–868.e8. [Google Scholar] [CrossRef] [PubMed]
- Nagdev, P.; Kumar, N.N.; Moyin, S.; Lahiri, B. Evaluation of the Impact of Acidic Drink on the Microhardness of Different Esthetic Restorative Materials: An In Vitro Study. J. Contemp. Dent. Pract. 2020, 21, 233–237. [Google Scholar] [CrossRef]
- Gurdogan Guler, E.B.; Bayrak, G.D.; Unsal, M.; Selvi Kuvvetli, S. Effect of Pediatric Multivitamin Syrups and Effervescent Tablets on the Surface Microhardness and Roughness of Restorative Materials: An In Vitro Study. J. Dent. Sci. 2021, 16, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Yazkan, B. Surface Degradation Evaluation of Different Self-adhesive Restorative Materials after Prolonged Energy Drinks Exposure. J. Esthet. Restor. Dent. 2020, 32, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-W.; Lee, M.-J.; Oh, S.-H.; Kim, K.-M. Changes in the Physical Properties and Color Stability of Aesthetic Restorative Materials Caused by Various Beverages. Dent. Mater. J. 2019, 38, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Gawriołek, M.; Sikorska, E.; Ferreira, L.F.V.; Costa, A.I.; Khmelinskii, I.; Krawczyk, A.; Sikorski, M.; Koczorowski, R. Color and Luminescence Stability of Selected Dental Materials In Vitro: Color and Luminescence Stability. J. Prosthodont. 2012, 21, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Ertas, E.; Güler, A.U.; Yücel, A.Ç.; Köprülü, H.; Güler, E. Color Stability of Resin Composites after Immersion in Different Drinks. Dent. Mater. J. 2006, 25, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Lepri, C.P.; Palma-Dibb, R.G. Surface Roughness and Color Change of a Composite: Influence of Beverages and Brushing. Dent. Mater. J. 2012, 31, 689–696. [Google Scholar] [CrossRef] [Green Version]
- Hengtrakool, C.; Kukiattrakoon, B.; Kedjarune-Leggat, U. Effect of Naturally Acidic Agents on Microhardness and Surface Micromorphology of Restorative Materials. Eur. J. Dent. 2011, 05, 089–100. [Google Scholar] [CrossRef] [Green Version]
- Blacker, S.M.; Creanor, S.L.; Creanor, S. An in Vitro Investigation of the Initial Ph and Titratable Acidity of a Selection of Fruit Smoothies. Dent. Update 2011, 38, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Nayagam, J.R.; Rajan, R. Calcium Oxalate Crystals as Raw Food Antinutrient: A Review. J. Pharm. Res. Int. 2021, 33, 295–301. [Google Scholar] [CrossRef]
- Hever, J.; Cronise, R.J. Plant-Based Nutrition for Healthcare Professionals: Implementing Diet as a Primary Modality in the Prevention and Treatment of Chronic Disease. J. Geriatr. Cardiol. 2017, 14, 355–368. [Google Scholar] [CrossRef]
- Bociong, K.; Szczesio, A.; Sokolowski, K.; Domarecka, M.; Sokolowski, J.; Krasowski, M.; Lukomska-Szymanska, M. The Influence of Water Sorption of Dental Light-Cured Composites on Shrinkage Stress. Materials 2017, 10, 1142. [Google Scholar] [CrossRef]
- Borges, A.L.S.; Dal Piva, A.M.d.O.; Moecke, S.E.; de Morais, R.C.; Tribst, J.P.M. Polymerization Shrinkage, Hygroscopic Expansion, Elastic Modulus and Degree of Conversion of Different Composites for Dental Application. J. Compos. Sci. 2021, 5, 322. [Google Scholar] [CrossRef]
- Francois, P.; Fouquet, V.; Attal, J.-P.; Dursun, E. Commercially Available Fluoride-Releasing Restorative Materials: A Review and a Proposal for Classification. Materials 2020, 13, 2313. [Google Scholar] [CrossRef]
- Yeo, H.W.; Loo, M.Y.; Alkhabaz, M.; Li, K.C.; Choi, J.J.E.; Barazanchi, A. Bulk-Fill Direct Restorative Materials: An In Vitro Assessment of Their Physio-Mechanical Properties. Oral 2021, 1, 75–87. [Google Scholar] [CrossRef]
- Par, M.; Lapas-Barisic, M.; Gamulin, O.; Panduric, V.; Spanovic, N.; Tarle, Z. Long Term Degree of Conversion of Two Bulk-Fill Composites. Acta Stomatol. Croat. 2016, 50, 292–300. [Google Scholar] [CrossRef]
- Aliping-McKenzie, M.; Linden, R.W.A.; Nicholson, J.W. The Effect of Coca-Cola and Fruit Juices on the Surface Hardness of Glass-Ionomers and “Compomers”. J. Oral Rehabil. 2004, 31, 1046–1052. [Google Scholar] [CrossRef]
- Szczesio-Wlodarczyk, A.; Sokolowski, J.; Kleczewska, J.; Bociong, K. Ageing of Dental Composites Based on Methacrylate Resins—A Critical Review of the Causes and Method of Assessment. Polymers 2020, 12, 882. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Gubler, A.; Attin, T.; Tarle, Z.; Tarle, A.; Tauböck, T.T. Ion Release and Hydroxyapatite Precipitation of Resin Composites Functionalized with Two Types of Bioactive Glass. J. Dent. 2022, 118, 103950. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.W. Maturation Processes in Glass-Ionomer Dental Cements. Acta Biomater. Odontol. Scand. 2018, 4, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bueno, L.S.; Silva, R.M.; Magalhães, A.P.R.; Navarro, M.F.L.; Pascotto, R.C.; Buzalaf, M.A.R.; Nicholson, J.W.; Sidhu, S.K.; Borges, A.F.S. Positive Correlation between Fluoride Release and Acid Erosion of Restorative Glass-Ionomer Cements. Dent. Mater. 2019, 35, 135–143. [Google Scholar] [CrossRef]
- Hahnel, S.; Henrich, A.; Bürgers, R.; Handel, G.; Rosentritt, M. Investigation of Mechanical Properties of Modern Dental Composites after Artificial Aging for One Year. Oper. Dent. 2010, 35, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Badra, V.V.; Faraoni, J.J.; Ramos, R.P.; Palma-Dibb, R.G. Influence of Different Beverages on the Microhardness and Surface Roughness of Resin Composites. Oper. Dent. 2005, 30, 213–219. [Google Scholar]
- Zhou, X.; Wang, S.; Peng, X.; Hu, Y.; Ren, B.; Li, M.; Hao, L.; Feng, M.; Cheng, L.; Zhou, X. Effects of Water and Microbial-Based Aging on the Performance of Three Dental Restorative Materials. J. Mech. Behav. Biomed. Mater. 2018, 80, 42–50. [Google Scholar] [CrossRef]
- Wang, L.; Cefaly, D.F.G.; dos Santos, J.L.; dos Santos, J.R.; Lauris, J.R.P.; Mondelli, R.F.L.; Atta, M.T. In Vitro Interactions between Lactic Acid Solution and Art Glass-Ionomer Cements. J. Appl. Oral Sci. 2009, 17, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Par, M.; Gubler, A.; Attin, T.; Tarle, Z.; Tarle, A.; Tauböck, T.T. Experimental Bioactive Glass-Containing Composites and Commercial Restorative Materials: Anti-Demineralizing Protection of Dentin. Biomedicines 2021, 9, 1616. [Google Scholar] [CrossRef]
- Sarac, D.; Sarac, Y.S.; Kulunk, S.; Ural, C.; Kulunk, T. The Effect of Polishing Techniques on the Surface Roughness and Color Change of Composite Resins. J. Prosthet. Dent. 2006, 96, 33–40. [Google Scholar] [CrossRef]
- Sabatini, C.; Campillo, M.; Aref, J. Color Stability of Ten Resin-Based Restorative Materials. J. Esthet. Restor. Dent. 2012, 24, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Villalta, P.; Lu, H.; Okte, Z.; Garcia-Godoy, F.; Powers, J.M. Effects of Staining and Bleaching on Color Change of Dental Composite Resins. J. Prosthet. Dent. 2006, 95, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Barutcigil, Ç.; Yıldız, M. Intrinsic and Extrinsic Discoloration of Dimethacrylate and Silorane Based Composites. J. Dent. 2012, 40, e57–e63. [Google Scholar] [CrossRef] [PubMed]
- Marovic, D.; Par, M.; Macan, M.; Klarić, N.; Plazonić, I.; Tarle, Z. Aging-Dependent Changes in Mechanical Properties of the New Generation of Bulk-Fill Composites. Materials 2022, 15, 902. [Google Scholar] [CrossRef]
- Haugen, H.J.; Marovic, D.; Par, M.; Khai Le Thieu, M.; Reseland, J.E.; Johnsen, G.F. Bulk Fill Composites Have Similar Performance to Conventional Dental Composites. Int. J. Mol. Sci. 2020, 21, 5136. [Google Scholar] [CrossRef]



| Material (Abbreviation) /Manufacturer/Lot No. | Type | Composition |
|---|---|---|
| Tetric EvoCeram (TEC)/ Ivoclar Vivadent, Schaan, Lichtenstein/Z01KN4 | Conventional resin composite | Urethanedimethacrylate, Bis-GMA, ytterbium trifluoride, ethoxylated bisphenol A dimethacrylate, barium glass filler, ytterbium trifluoride, mixed oxide |
| Cention N (CEN)/ Ivoclar Vivadent, Schaan, Lichtenstein/Z00HCG | “Alkasite”(Resin composite with reactive glass fillers) | Powder: barium aluminum silicate glass, ytterbium trifluoride, isofiller, calcium barium aluminum fluorosilicate glass, calcium fluoro silicate glass; Liquid: urethane dimethacrylate, tricyclodecandimethanol dimethacrylate, tetramethyl-xylylene, diurethane dimethacrylate, polyethylene glycol 400, dimethacrylate, ivocerin, hydroxyperoxid |
| Charisma Classic (CHA)/Kulzer, Hanau, Germany/KA10758 | Conventional resin composite | Bis-GMA, TEGDMA; Filler load: 61% by volume (60% inorganic filler by volume and pre-polymerized filler), particle size of 0.005–10 µm, barium aluminum fluoride glass. |
| Equia Forte HT Fil (EQ/EQC)/ GC, Tokyo, Japan/210201A | Glass hybrid | Powder: fluoroaluminosilicate glass, polyacrylic acid, iron oxide Liquid: polybasic carboxylic acid, water |
| Filtek One Bulk Fill Restorative (FBF)/ 3M, St. Paul, MN, USA/NF15156 | Bulk-fill resin composite | Nonagglomerated/nonaggregated 20 nm silica filler, nonagglomerated/nonaggregated 4 to 11 nm zirconia filler, aggregated zirconia/silica cluster filler, ytterbium trifluoride filler consisting of agglomerate 100 nm particles, ERGP-DMA, diurethane- DMA, 1,12-dodecane-DMA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veček, N.N.; Par, M.; Sever, E.K.; Miletić, I.; Krmek, S.J. The Effect of a Green Smoothie on Microhardness, Profile Roughness and Color Change of Dental Restorative Materials. Polymers 2022, 14, 2067. https://doi.org/10.3390/polym14102067
Veček NN, Par M, Sever EK, Miletić I, Krmek SJ. The Effect of a Green Smoothie on Microhardness, Profile Roughness and Color Change of Dental Restorative Materials. Polymers. 2022; 14(10):2067. https://doi.org/10.3390/polym14102067
Chicago/Turabian StyleVeček, Nikolina Nika, Matej Par, Eva Klarić Sever, Ivana Miletić, and Silvana Jukić Krmek. 2022. "The Effect of a Green Smoothie on Microhardness, Profile Roughness and Color Change of Dental Restorative Materials" Polymers 14, no. 10: 2067. https://doi.org/10.3390/polym14102067
APA StyleVeček, N. N., Par, M., Sever, E. K., Miletić, I., & Krmek, S. J. (2022). The Effect of a Green Smoothie on Microhardness, Profile Roughness and Color Change of Dental Restorative Materials. Polymers, 14(10), 2067. https://doi.org/10.3390/polym14102067








