Morphology-Controlled Synthesis of Polyphosphazene-Based Micro- and Nano-Materials and Their Application as Flame Retardants
Abstract
:1. Introduction
2. Experimental
2.1. Materials
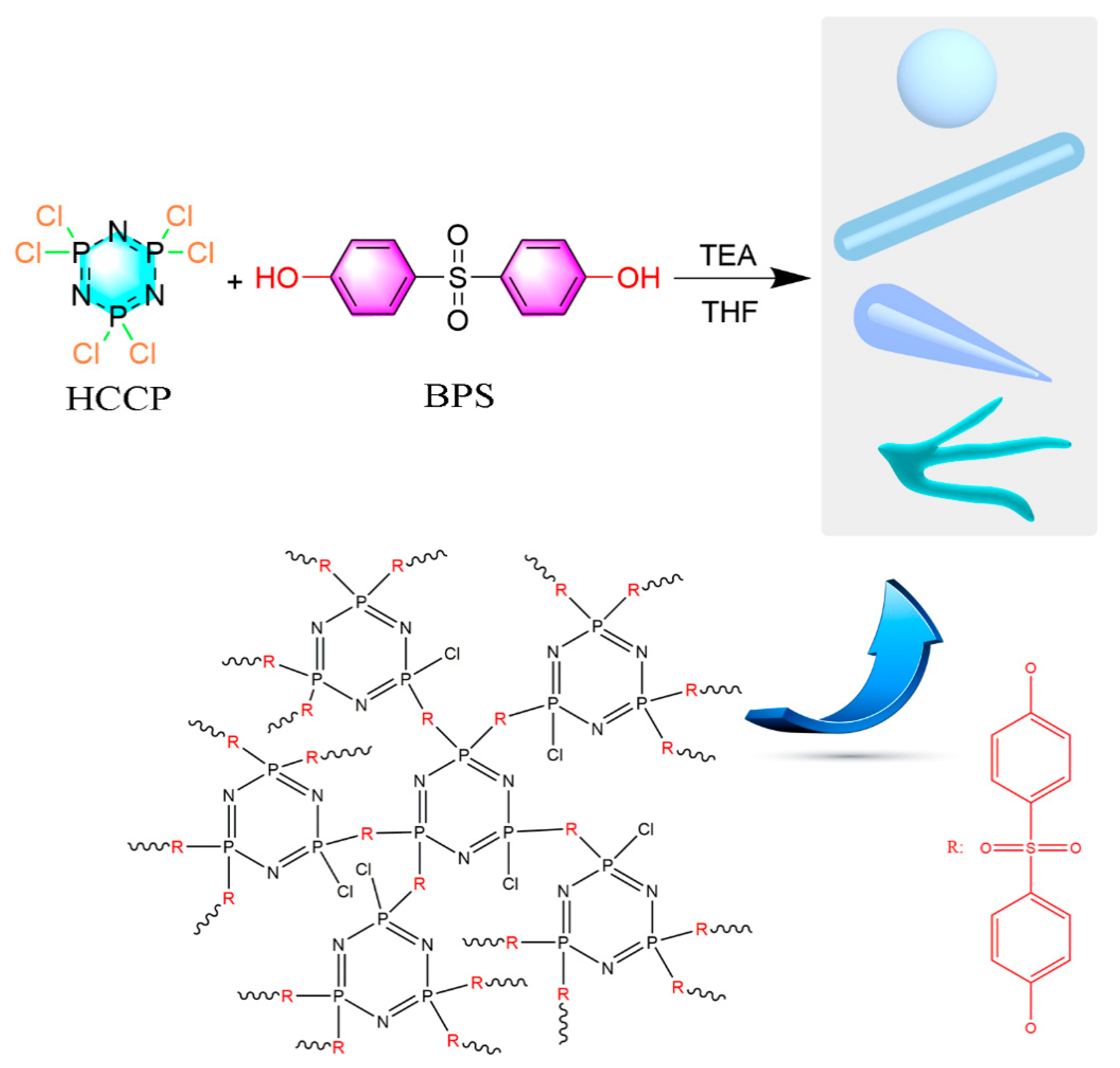
2.2. Synthesis of Micro/Nanoscale PZSs
2.2.1. Preparation of PZS Nanotubes (PZS_NT)
2.2.2. Preparation of PZS Microspheres (PZS_SP)
2.2.3. Preparation of PZS Capsicum-like Nanotubes (PZS_CLNT)
2.2.4. Preparation of PZS Branched Nanotubes (PZS_BNT)
2.3. Preparation of PET/PZS Composites
2.4. Characterization Techniques
3. Results and Discussion
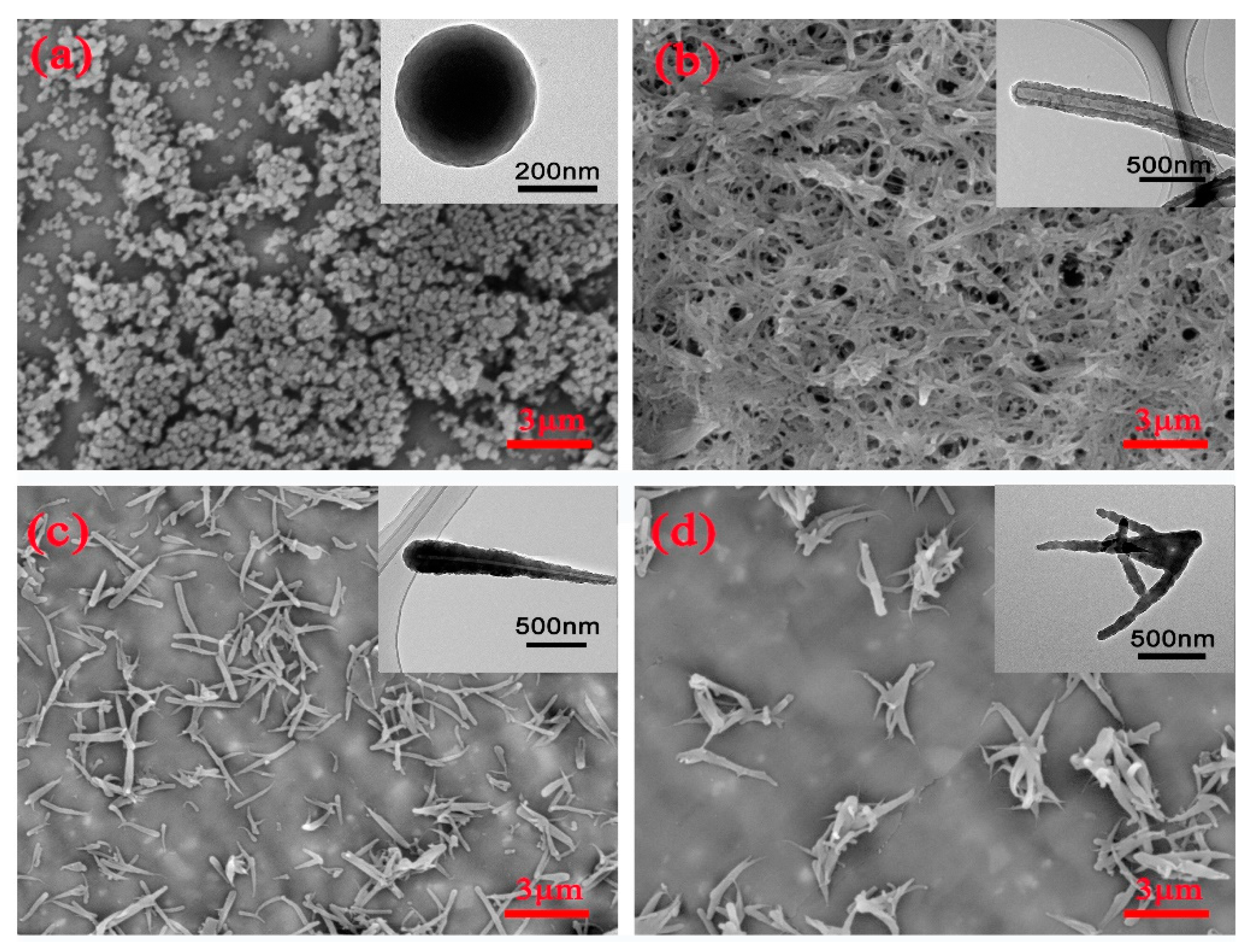
3.1. Characterization of PZSs
3.2. Thermal Stability Analysis
3.3. Analysis of Flame Retardancy and Burning Behavior
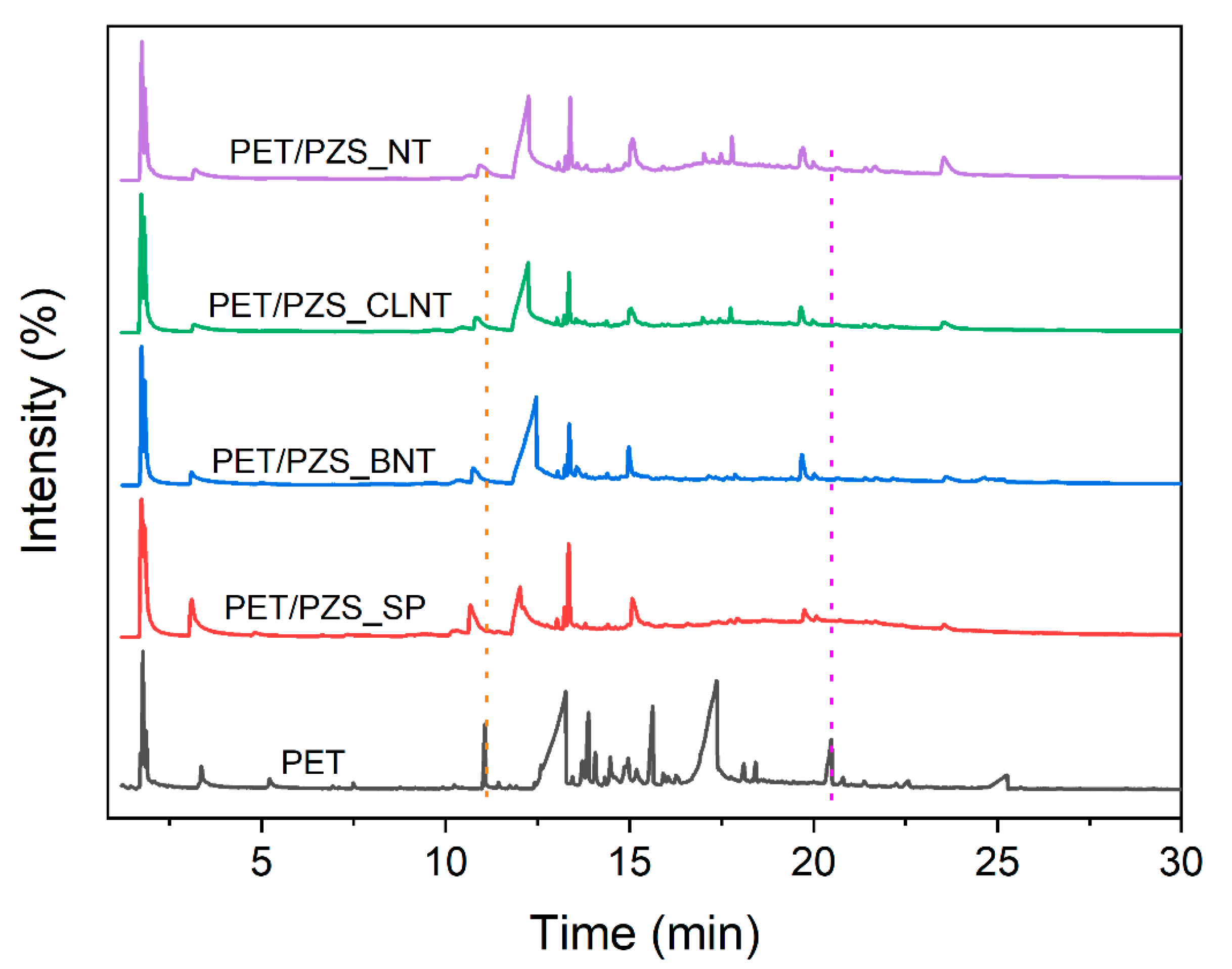
3.4. Pyrolysis Products Analysis
3.5. Condensed and Gas Phase Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, B.; Zhu, Y.; Wang, X.; Xu, H.; Zhong, Y.; Zhang, L.; Ma, Y.; Sui, X.; Wang, B.; Feng, X.; et al. Synthesis and application of poly(cyclotriphosphazene-resveratrol) microspheres for enhancing flame retardancy of poly(ethylene terephthalate). Polym. Adv. Technol. 2021, 33, 658–671. [Google Scholar] [CrossRef]
- Li, S.; Li, T.; Wang, X.; Zhong, Y.; Zhang, L.; Wang, B.; Feng, X.; Sui, X.; Xu, H.; Chen, Z.; et al. Polyphosphazene microspheres modified with transition metal hydroxystannate for enhancing the flame retardancy of polyethylene terephthalate. Polym. Adv. Technol. 2020, 31, 1194–1207. [Google Scholar] [CrossRef]
- Hou, S.; Chen, S.; Dong, Y.; Gao, S.; Zhu, B.; Lu, Q. Biodegradable Cyclomatrix Polyphosphazene Nanoparticles: A Novel pH-Responsive Drug Self-Framed Delivery System. ACS Appl. Mater. Interfaces 2018, 10, 25983–25993. [Google Scholar] [CrossRef] [PubMed]
- Allcock, H.R.; Ravikiran, R.; Olshavsky, M.A. Synthesis and Characterization of Hindered Polyphosphazenes via Functionalized Intermediates: Exploratory Models for Electro-optical Materials. Macromolecules 1998, 31, 5206–5214. [Google Scholar] [CrossRef]
- Fushimi, T.; Allcock, H.R. Cyclotriphosphazenes with sulfur-containing side groups: Refractive index and optical dispersion. Dalton Trans. 2009, 14, 2477–2481. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fu, J.; Huang, D.; Zhang, C.; Xu, Q. Silver nanoparticles-decorated polyphosphazene nanotubes: Synthesis and applications. Nanoscale 2013, 5, 7913–7919. [Google Scholar] [CrossRef]
- Alongi, J.; Carletto, R.A.; Di Blasio, A.; Carosio, F.; Bosco, F.; Malucelli, G. DNA: A novel, green, natural flame retardant and suppressant for cotton. J. Mater. Chem. A 2013, 1, 4779–4785. [Google Scholar] [CrossRef]
- Jiang, J.; Cheng, Y.; Liu, Y.; Wang, Q.; He, Y.; Wang, B. Intergrowth charring for flame-retardant glass fabric-reinforced epoxy resin composites. J. Mater. Chem. A 2015, 3, 4284–4290. [Google Scholar] [CrossRef]
- Meng, L.; Lane, J.M.D.; Baca, L.; Tafoya, J.; Ao, T.; Stoltzfus, B.; Knudson, M.; Morgan, D.; Austin, K.; Park, C.; et al. Shape Dependence of Pressure-Induced Phase Transition in CdS Semiconductor Nanocrystals. J. Am. Chem. Soc. 2020, 142, 6505–6510. [Google Scholar] [CrossRef]
- Meng, L.; Yu, B.; Qin, Y. Templated interfacial synthesis of metal-organic framework (MOF) nano- and micro-structures with precisely controlled shapes and sizes. Commun. Chem. 2021, 4, 82. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, C.; Guo, C.; Li, Y.; Li, S.; Sun, L.; Li, H.; Xiang, D. Polybenzoxazine Resins with Polyphosphazene Microspheres: Synthesis, Flame Retardancy, Mechanisms, and Applications. ACS Omega 2019, 4, 20275–20284. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, W.; Xu, T.; Xu, H.; Zhong, Y.; Zhang, L.; Ma, Y.; Sui, X.; Wang, B.; Feng, X.; et al. Effect of weak intermolecular interactions in micro/nanoscale polyphosphazenes and polyethylene terephthalate composites on flame retardancy. Polym. Adv. Technol. 2022, 1–13. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, Y.; Yuan, W.; Xi, J.; Huang, X.; Tang, X.; Zheng, S. One-Pot Synthesis of Poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) Nanotubes via an In Situ Template Approach. Adv. Mater. 2006, 18, 2997–3000. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Huang, X.; Li, W.; Fu, J.; Tang, X. Preparation of novel hybrid inorganic–organic microspheres with active hydroxyl groups using ultrasonic irradiation via one-step precipitation polymerization. Mater. Lett. 2008, 62, 1389–1392. [Google Scholar] [CrossRef]
- Fu, J.; Huang, X.; Zhu, Y.; Zhu, L.; Tang, X. Facile Synthesis and Characterization of Novel Capsicum-like Polyphosphazene Nanotubes via an in situ Template Route. Macromol. Chem. Phys. 2008, 209, 1845–1850. [Google Scholar] [CrossRef]
- Fu, J.; Huang, X.; Huang, Y.; Zhu, L.; Zhu, Y.; Tang, X. Facile Preparation of Branched Phosphazene-Containing Nanotubes via an in situ Template Approach. Macromol. Mater. Eng. 2008, 293, 173–177. [Google Scholar] [CrossRef]
- Li, T.; Li, S.; Ma, T.; Zhong, Y.; Zhang, L.; Xu, H.; Wang, B.; Sui, X.; Feng, X.; Chen, Z.; et al. Flame-retardant poly(ethylene terephthalate) enabled by a novel melamine polyphosphate nanowire. Polym. Adv. Technol. 2019, 31, 795–806. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, W.; Xu, T.; Xu, H.; Zhong, Y.; Zhang, L.; Ma, Y.; Sui, X.; Wang, B.; Feng, X.; et al. Preparation and characterization of polyphosphazene-based flame retardants with different functional groups. Polym. Degrad. Stab. 2022, 196, 109815. [Google Scholar] [CrossRef]
- Sai, T.; Ran, S.; Guo, Z.; Fang, Z. A Zr-based metal organic frameworks towards improving fire safety and thermal stability of polycarbonate. Compos. Part B Eng. 2019, 176, 107198. [Google Scholar] [CrossRef]
- Yu, B.; Tawiah, B.; Wang, L.-Q.; Yuen, A.C.Y.; Zhang, Z.-C.; Shen, L.-L.; Lin, B.; Fei, B.; Yang, W.; Li, A.; et al. Interface decoration of exfoliated MXene ultra-thin nanosheets for fire and smoke suppressions of thermoplastic polyurethane elastomer. J. Hazard. Mater. 2019, 374, 110–119. [Google Scholar] [CrossRef]
- Qiu, S.; Xing, W.; Mu, X.; Feng, X.; Ma, C.; Yuen, K.K.R.; Hu, Y. A 3D Nanostructure Based on Transition-Metal Phosphide Decorated Heteroatom-Doped Mesoporous Nanospheres Interconnected with Graphene: Synthesis and Applications. ACS Appl. Mater. Interfaces 2016, 8, 32528–32540. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wu, W.-H.; Wang, Y.-H.; Jiao, Y.-H.; Lu, L.-Y.; Qu, H.-Q.; Qin, X.-Y. Synthesis of a novel phosphazene-based flame retardant with active amine groups and its application in reducing the fire hazard of Epoxy Resin. J. Hazard. Mater. 2019, 366, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.-K.; Wang, X.-S.; Guo, D.-M.; Zhang, Y.; Zhai, F.-Y.; Wang, X.-L.; Chen, L.; Wang, Y.-Z. The high-temperature self-crosslinking contribution of azobenzene groups to the flame retardance and anti-dripping of copolyesters. J. Mater. Chem. A 2013, 1, 9264–9272. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, P.; Qian, Y.; Yu, H.; Liu, J. Effect of talc on thermal stability and flame retardancy of polycarbonate/PSBPBP composite. J. Appl. Polym. Sci. 2012, 125, 3167–3174. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Delobel, R.; Amoureux, J.-P. Synergistic effect of zeolite in an intumescence process: Study of the carbonaceous structures using solid-state NMR. J. Chem. Soc. Faraday Trans. 1996, 92, 149–158. [Google Scholar] [CrossRef]
- Qiu, S.; Xing, W.; Feng, X.; Yu, B.; Mu, X.; Yuen, K.K.R.; Hu, Y. Self-standing cuprous oxide nanoparticles on silica@ polyphosphazene nanospheres: 3D nanostructure for enhancing the flame retardancy and toxic effluents elimination of epoxy resins via synergistic catalytic effect. Chem. Eng. J. 2017, 309, 802–814. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, B.; Zheng, Y.; Yang, J.; Duan, Z.; Hu, Y. Design of reduced graphene oxide decorated with DOPO-phosphanomidate for enhanced fire safety of epoxy resin. J. Colloid Interface Sci. 2018, 521, 160–171. [Google Scholar] [CrossRef]
- Wang, C.; Wu, L.; Dai, Y.; Zhu, Y.; Wang, B.; Zhong, Y.; Zhang, L.; Sui, X.; Xu, H.; Mao, Z. Application of self-templated PHMA sub-microtubes in enhancing flame-retardance and anti-dripping of PET. Polym. Degrad. Stab. 2018, 154, 239–247. [Google Scholar] [CrossRef]
- Liang, W.-J.; Zhao, B.; Zhao, P.-H.; Zhang, C.-Y.; Liu, Y.-Q. Bisphenol-S bridged penta(anilino)cyclotriphosphazene and its application in epoxy resins: Synthesis, thermal degradation, and flame retardancy. Polym. Degrad. Stab. 2017, 135, 140–151. [Google Scholar] [CrossRef]
- Wu, J.-N.; Chen, L.; Fu, T.; Zhao, H.-B.; Guo, D.-M.; Wang, X.-L.; Wang, Y.-Z. New application for aromatic Schiff base: High efficient flame-retardant and anti-dripping action for polyesters. Chem. Eng. J. 2018, 336, 622–632. [Google Scholar] [CrossRef]
- Sai, T.; Ran, S.; Guo, Z.; Yan, H.; Zhang, Y.; Wang, H.; Song, P.; Fang, Z. Transparent, highly thermostable and flame retardant polycarbonate enabled by rod-like phosphorous-containing metal complex aggregates. Chem. Eng. J. 2021, 409, 128223. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, Z.; Pan, Y.-T.; Li, D.; Wang, D.-Y.; Yang, R. Dry synthesis of mesoporous nanosheet assembly constructed by cyclomatrix polyphosphazene frameworks and its application in flame retardant polypropylene. Chem. Eng. J. 2020, 395, 125076. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Liu, J.; Fan, X.; Wang, B.; Wang, M.; Ren, W.; Wang, J.; Li, M.; Shi, J. Brand new P-doped g-C3N4: Enhanced photocatalytic activity for H2 evolution and Rhodamine B degradation under visible light. J. Mater. Chem. A 2015, 3, 3862–3867. [Google Scholar] [CrossRef]
- Chen, R.; Luo, Z.; Yu, X.; Tang, H.; Zhou, Y.; Zhou, H. Synthesis of chitosan-based flame retardant and its fire resistance in epoxy resin. Carbohydr. Polym. 2020, 245, 116530. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xia, L.; Jian, R.; Ai, Y.; Zheng, X.; Chen, G.; Wang, J. Flame-retarding epoxy resin with an efficient P/N/S-containing flame retardant: Preparation, thermal stability, and flame retardance. Polym. Degrad. Stab. 2018, 149, 69–77. [Google Scholar] [CrossRef]
- Malmgren, S.; Ciosek, K.; Hahlin, M.; Gustafsson, T.; Gorgoi, M.; Rensmo, H.; Edström, K. Comparing anode and cathode electrode/electrolyte interface composition and morphology using soft and hard X-ray photoelectron spectroscopy. Electrochimica Acta 2013, 97, 23–32. [Google Scholar] [CrossRef]
- Li, P.; Liu, C.; Xu, Y.-J.; Jiang, Z.-M.; Liu, Y.; Zhu, P. Novel and eco-friendly flame-retardant cotton fabrics with lignosulfonate and chitosan through LbL: Flame retardancy, smoke suppression and flame-retardant mechanism. Polym. Degrad. Stab. 2020, 181, 109302. [Google Scholar] [CrossRef]
- Peng, H.; Mao, Y.; Wang, D.; Fu, S. B-N-P-linked covalent organic frameworks for efficient flame retarding and toxic smoke suppression of polyacrylonitrile composite fiber. Chem. Eng. J. 2021, 430, 133120. [Google Scholar] [CrossRef]
- Li, J.; Pan, F.; Xu, H.; Zhang, L.; Zhong, Y.; Mao, Z. The flame-retardancy and anti-dripping properties of novel poly(ethylene terephalate)/cyclotriphosphazene/silicone composites. Polym. Degrad. Stabil. 2014, 110, 268–277. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, X.; Yu, B.; Feng, X.; Mu, X.; Yuen, K.K.R.; Hu, Y. Flame-retardant-wrapped polyphosphazene nanotubes: A novel strategy for enhancing the flame retardancy and smoke toxicity suppression of epoxy resins. J. Hazard. Mater. 2017, 325, 327–339. [Google Scholar] [CrossRef]
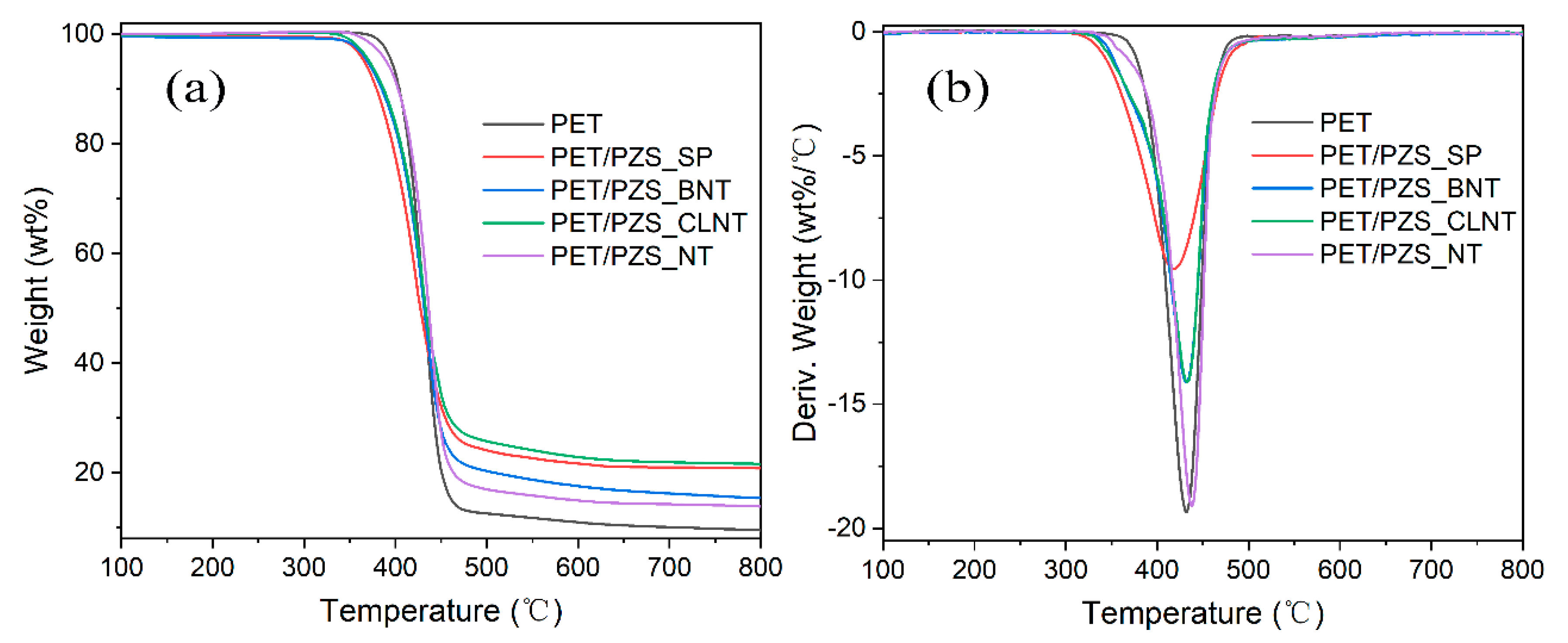

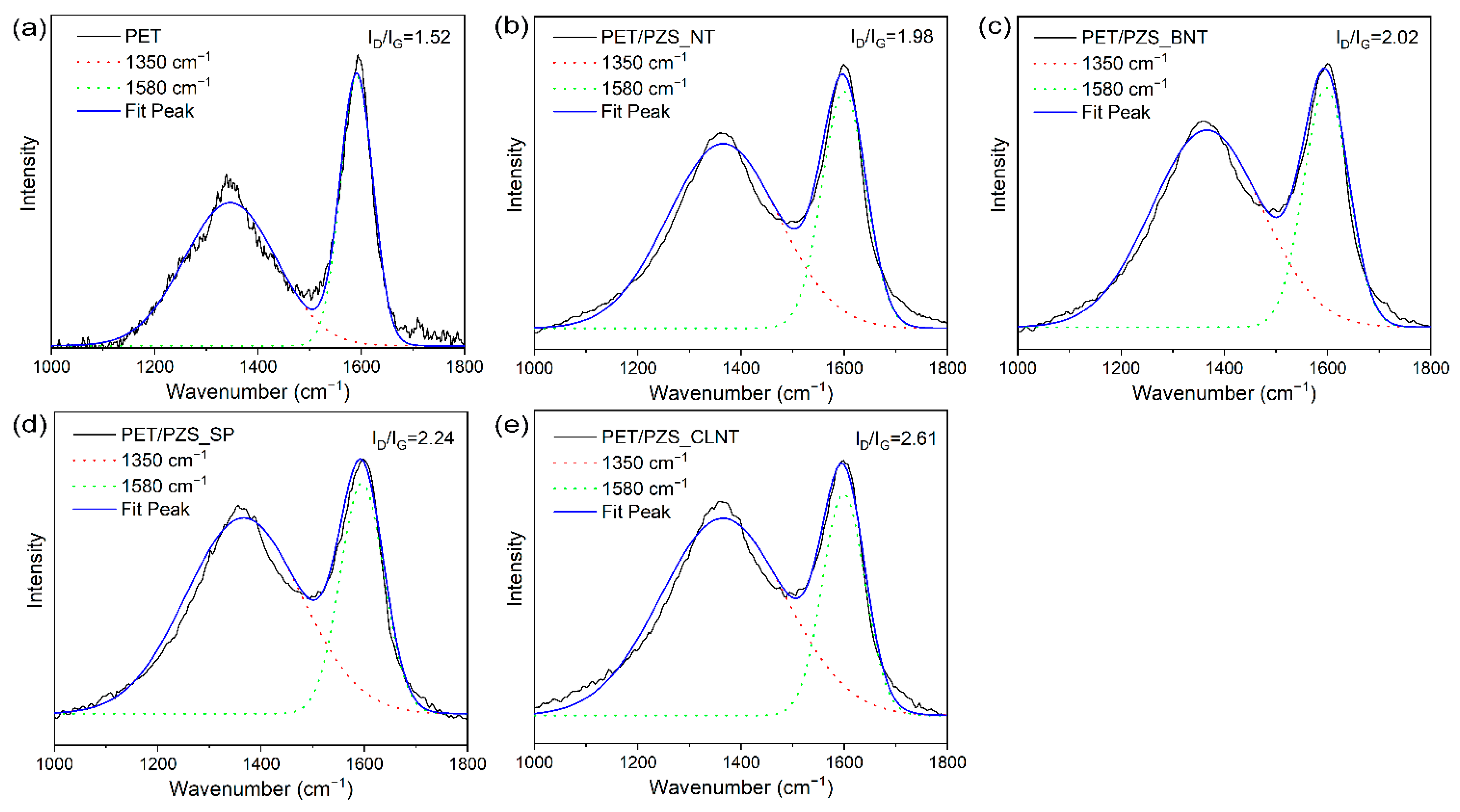
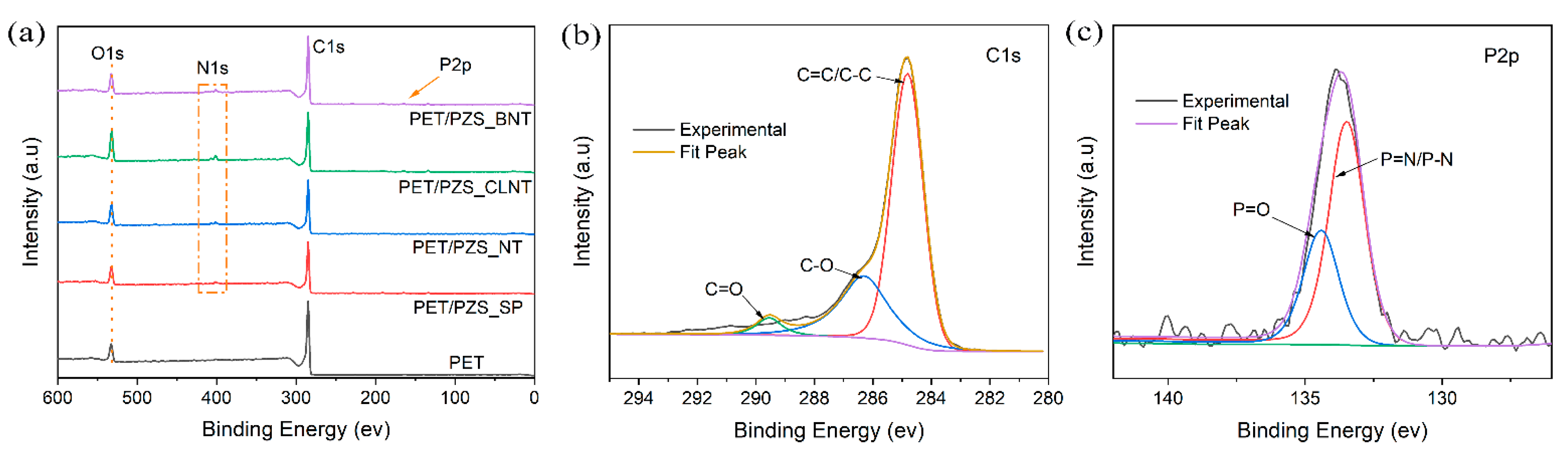

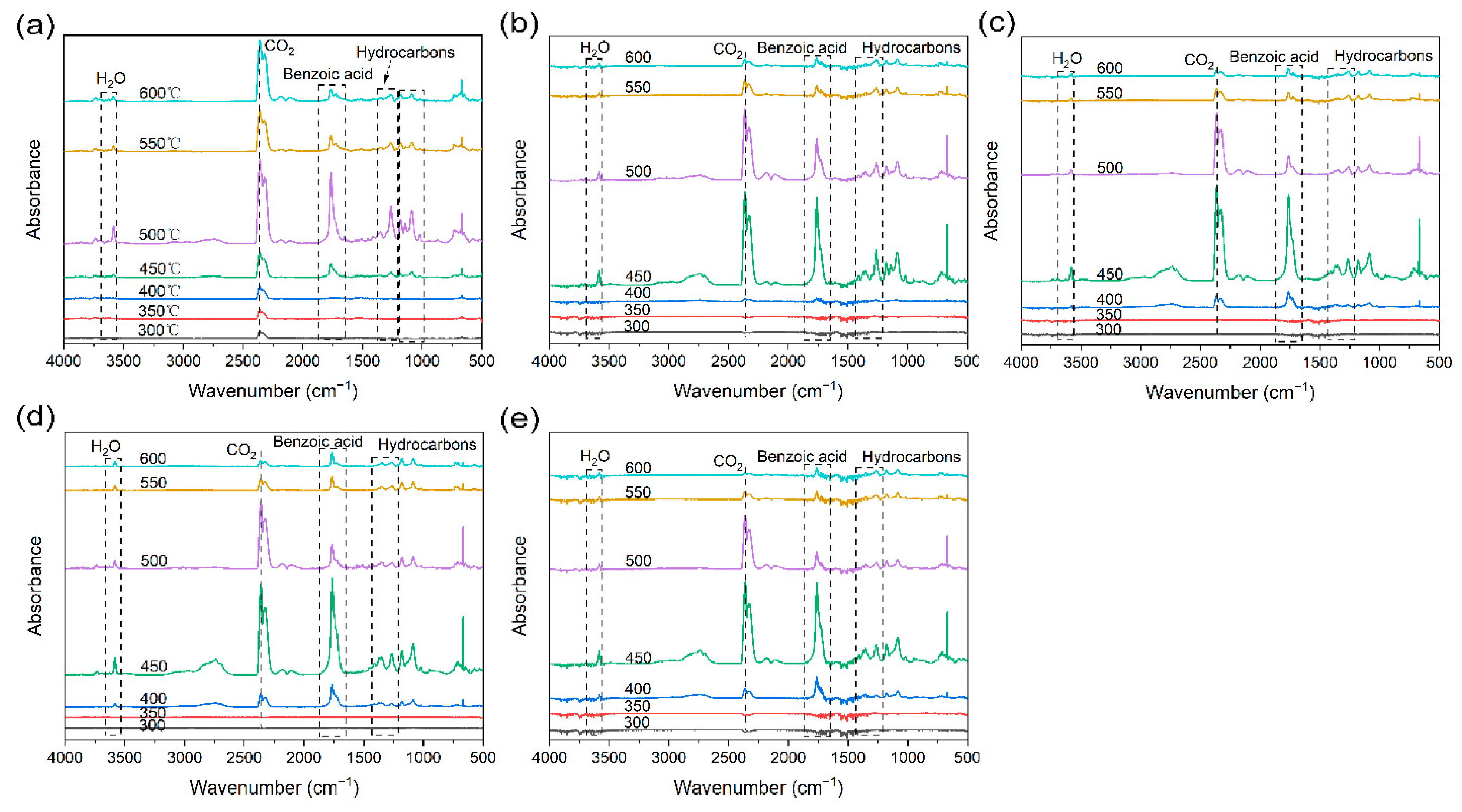
| Samples | T5 (°C) | Tmax (°C) | Char Residue at 800 °C (wt%) |
|---|---|---|---|
| PET | 395 | 433 | 9.5 |
| PET/PZS_SP | 366 | 416 | 20.8 |
| PET/PZS_NT | 390 | 438 | 13.8 |
| PET/PZS_CLNT | 372 | 432 | 21.5 |
| PET/PZS_BNT | 370 | 432 | 15.4 |
| Samples | LOI (vol%) | UL-94 | PHRR (kW/m2) | THR (MJ/m2) | TSP (m2) | |
|---|---|---|---|---|---|---|
| Rating | Dripping | |||||
| PET | 25.2 | V-2 | Severe | 715.94 | 120.3 | 15.9 |
| PET/PZS_SP | 33.1 | V-0 | Slow | 530.74 | 93.0 | 11.7 |
| PET/PZS_NT | 32.5 | V-0 | Slow | 525.68 | 101.3 | 12.7 |
| PET/PZS_CLNT | 34.4 | V-0 | Slow | 506.28 | 90.5 | 11.9 |
| PET/PZS_BNT | 32.8 | V-0 | Slow | 504.22 | 96.3 | 12.4 |
| Peak No. | Main Products | Pure PET | PET/PZS_SP | PET/PZS_BNT | PET/PZS_CLNT | PET/PZS_NT | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (min) | Intensity (%) | Time (min) | Intensity (%) | Time (min) | Intensity (%) | Time (min) | Intensity (%) | Time (min) | Intensity (%) | ||
| 1 | CO2 | 1.772 | 5.27 | 1.733 | 18.06 | 1.734 | 12.09 | 1.724 | 16.38 | 1.743 | 13.89 |
| 2 | CH3CHO | 1.866 | 1.61 | 1.807 | 18.77 | 1.817 | 12.16 | 1.807 | 16.51 | 1.831 | 8.05 |
| 3 | C6H6 | 3.365 | 1.78 | 3.1 | 12.71 | 3.098 | 6.3 | 3.174 | 5.09 | 3.193 | 6.45 |
| 6 |  | 11.068 | 2.6 | 10.676 | 8.38 | 10.746 | 4.5 | 10.825 | 5.32 | 10.946 | 5.31 |
| 9 | C6H5COOH | 13.263 | 28.36 | 12.021 | 21.45 | 12.463 | 43.16 | 12.248 | 36.11 | 12.253 | 37.92 |
| 11 | C6H5CHCHCOOCHCH2 | 13.711 | 1.65 | 13.227 | 1.41 | 13.245 | 0.82 | 13.222 | 0.82 | 13.257 | 0.88 |
| 12 | C6H5-C6H5 | 13.881 | 3.98 | 13.345 | 7.74 | 13.364 | 5.26 | 13.349 | 4.76 | 13.385 | 4.95 |
| 13 | C6H5(COOCHCH2)2 | 15.628 | 5.17 | 15.079 | 5.96 | 14.977 | 3.39 | 15.036 | 4.24 | 15.081 | 6.56 |
| 19 | C15H12O3 | 18.427 | 0.93 | 17.939 | 0.81 | 17.87 | 0.64 | 17.745 | 1.37 | 17.786 | 2.03 |
| 31 | 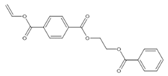 | 25.255 | 3.51 | 23.558 | 1.16 | 23.602 | 1.69 | 23.539 | 3.07 | 23.544 | 5.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Wu, W.; Xu, T.; Xu, H.; Zhong, Y.; Zhang, L.; Ma, Y.; Sui, X.; Wang, B.; Feng, X.; et al. Morphology-Controlled Synthesis of Polyphosphazene-Based Micro- and Nano-Materials and Their Application as Flame Retardants. Polymers 2022, 14, 2072. https://doi.org/10.3390/polym14102072
Zhu Y, Wu W, Xu T, Xu H, Zhong Y, Zhang L, Ma Y, Sui X, Wang B, Feng X, et al. Morphology-Controlled Synthesis of Polyphosphazene-Based Micro- and Nano-Materials and Their Application as Flame Retardants. Polymers. 2022; 14(10):2072. https://doi.org/10.3390/polym14102072
Chicago/Turabian StyleZhu, Yuanzhao, Wei Wu, Tong Xu, Hong Xu, Yi Zhong, Linping Zhang, Yimeng Ma, Xiaofeng Sui, Bijia Wang, Xueling Feng, and et al. 2022. "Morphology-Controlled Synthesis of Polyphosphazene-Based Micro- and Nano-Materials and Their Application as Flame Retardants" Polymers 14, no. 10: 2072. https://doi.org/10.3390/polym14102072
APA StyleZhu, Y., Wu, W., Xu, T., Xu, H., Zhong, Y., Zhang, L., Ma, Y., Sui, X., Wang, B., Feng, X., & Mao, Z. (2022). Morphology-Controlled Synthesis of Polyphosphazene-Based Micro- and Nano-Materials and Their Application as Flame Retardants. Polymers, 14(10), 2072. https://doi.org/10.3390/polym14102072







