Study on a Hydrogel for Adsorption of Chloride Ions in Cementitious Materials
Abstract
:1. Introduction
2. Experimental
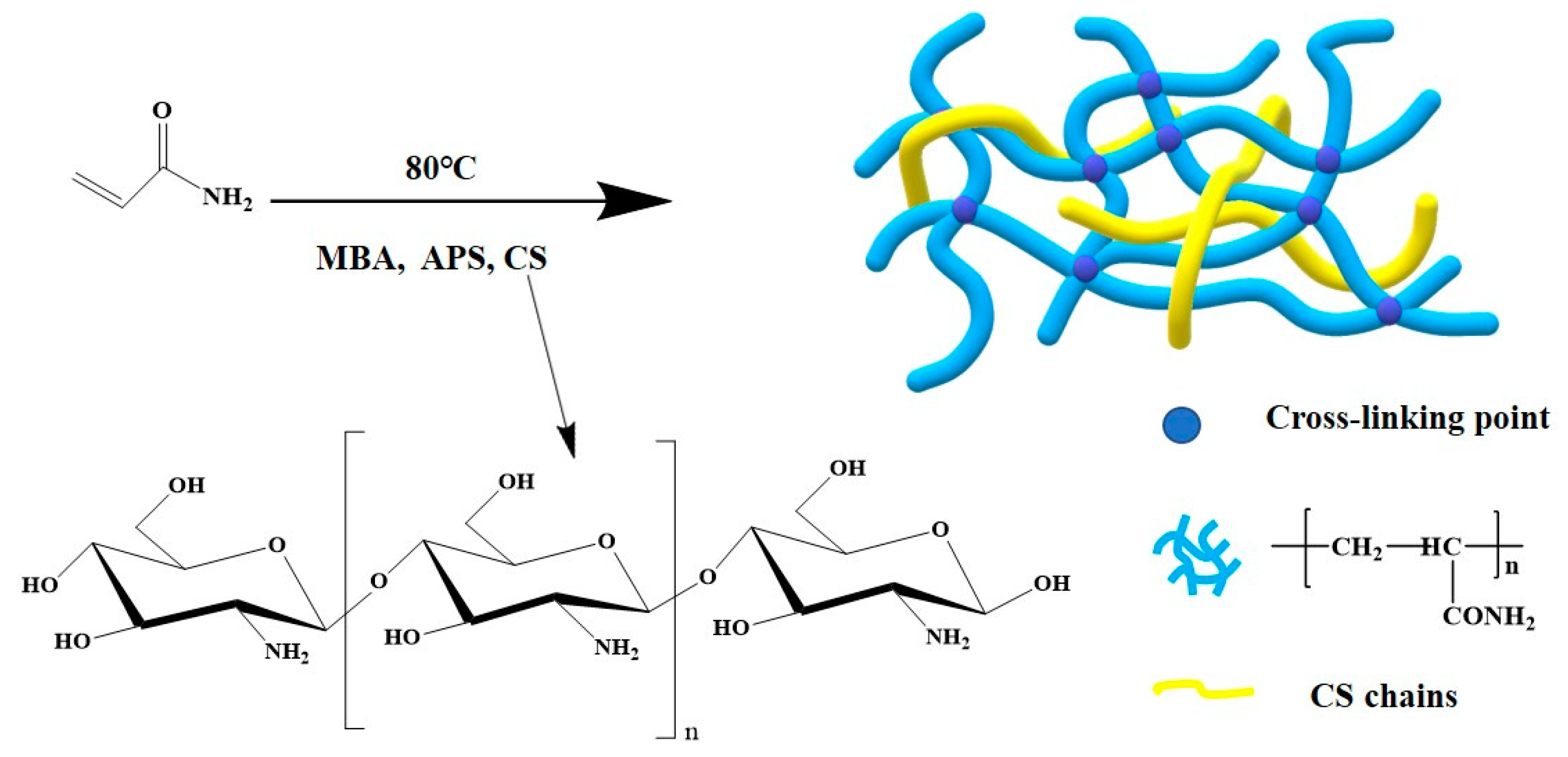
2.1. Materials and Preparation
2.2. Characterization of PAMC Gel
2.3. Isothermal Adsorption Model
2.4. Adsorption Behavior of PAMC in Solution
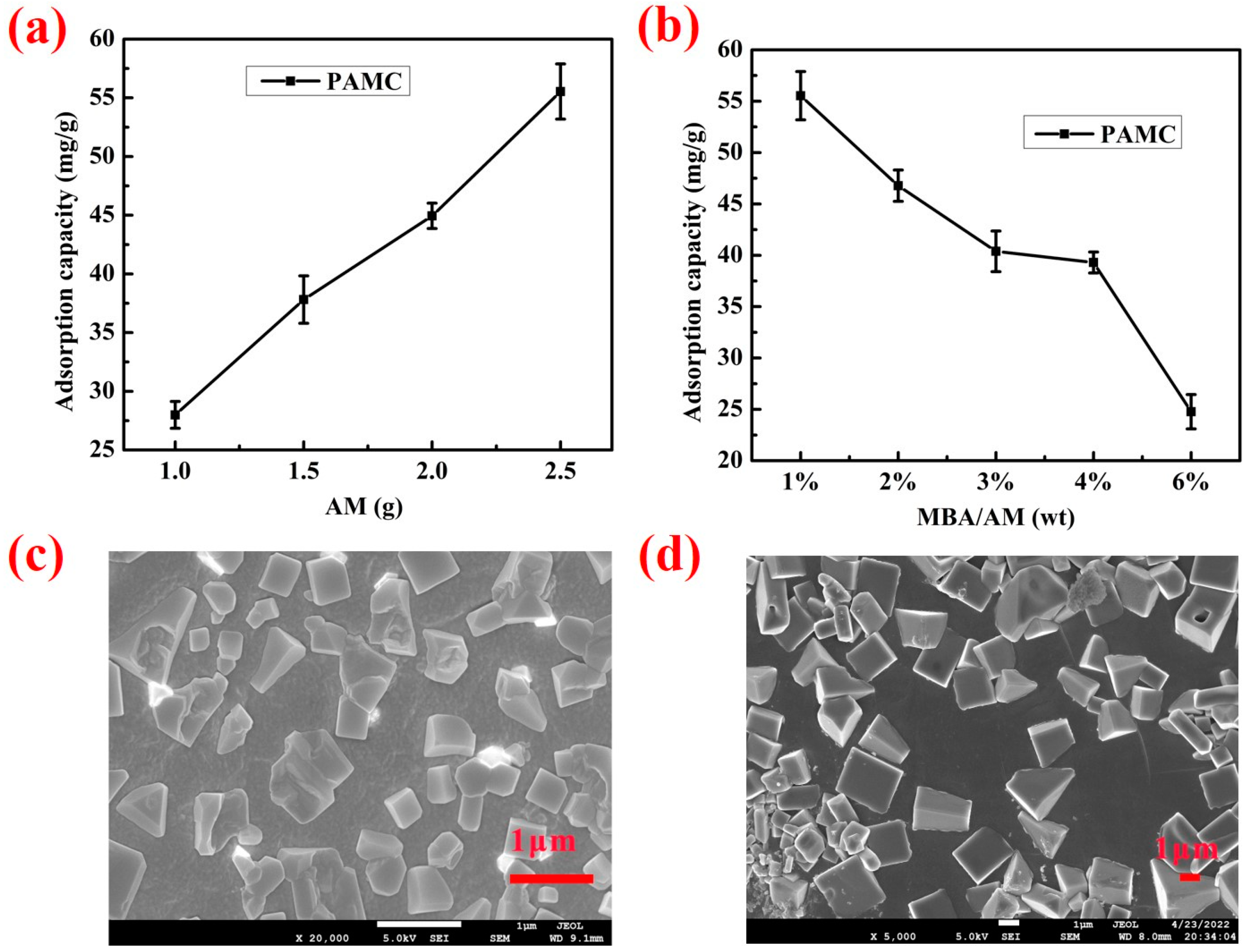
2.4.1. Effects of AM and MBA Contents on the Adsorption Performance of PAMC
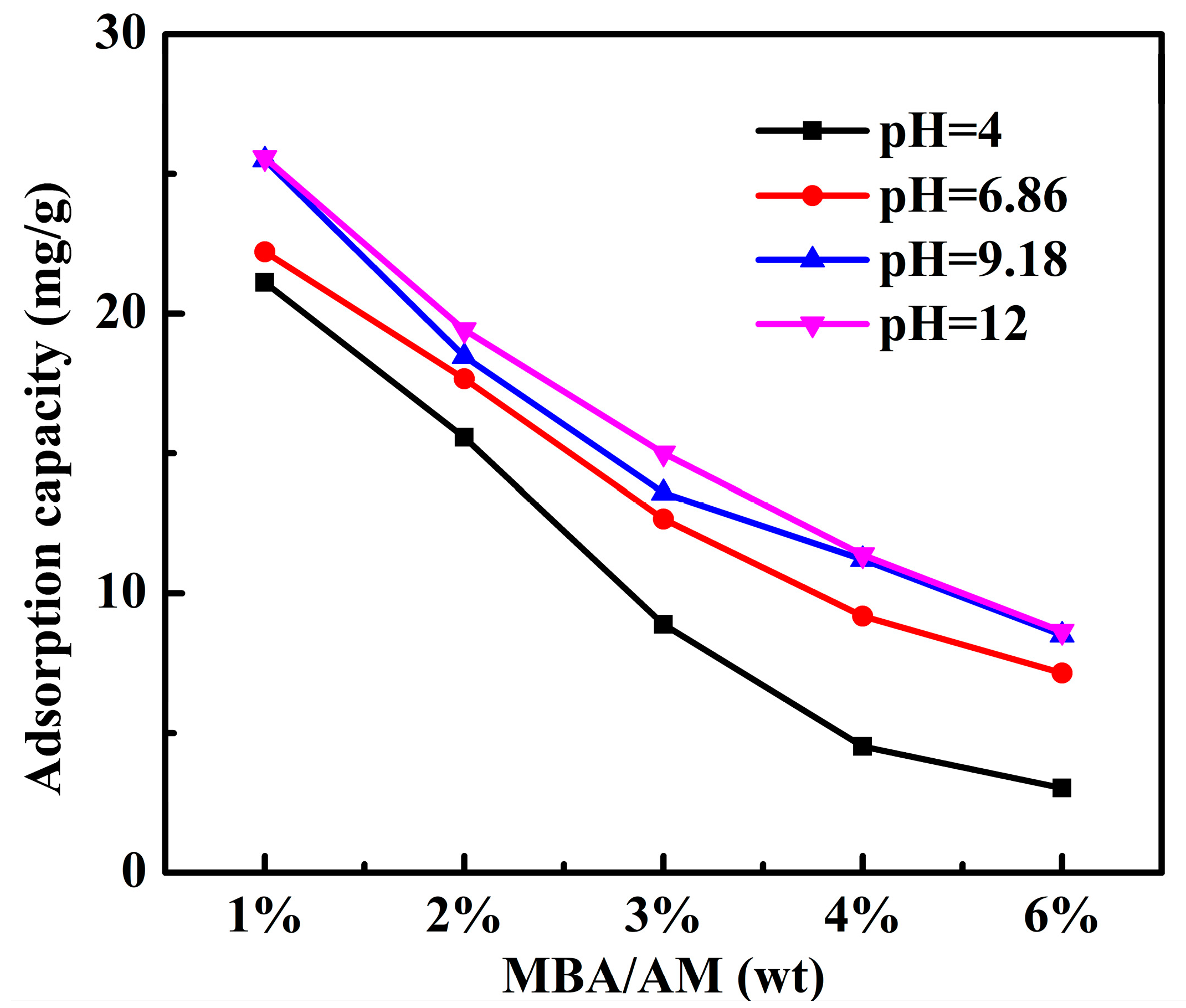
2.4.2. Effect of pH on Adsorption Performance
2.5. Adsorption Behavior of PAMC in Simulated Sea Sand
2.6. Chloride Penetration Resistance in Cement Paste
3. Results and Discussion
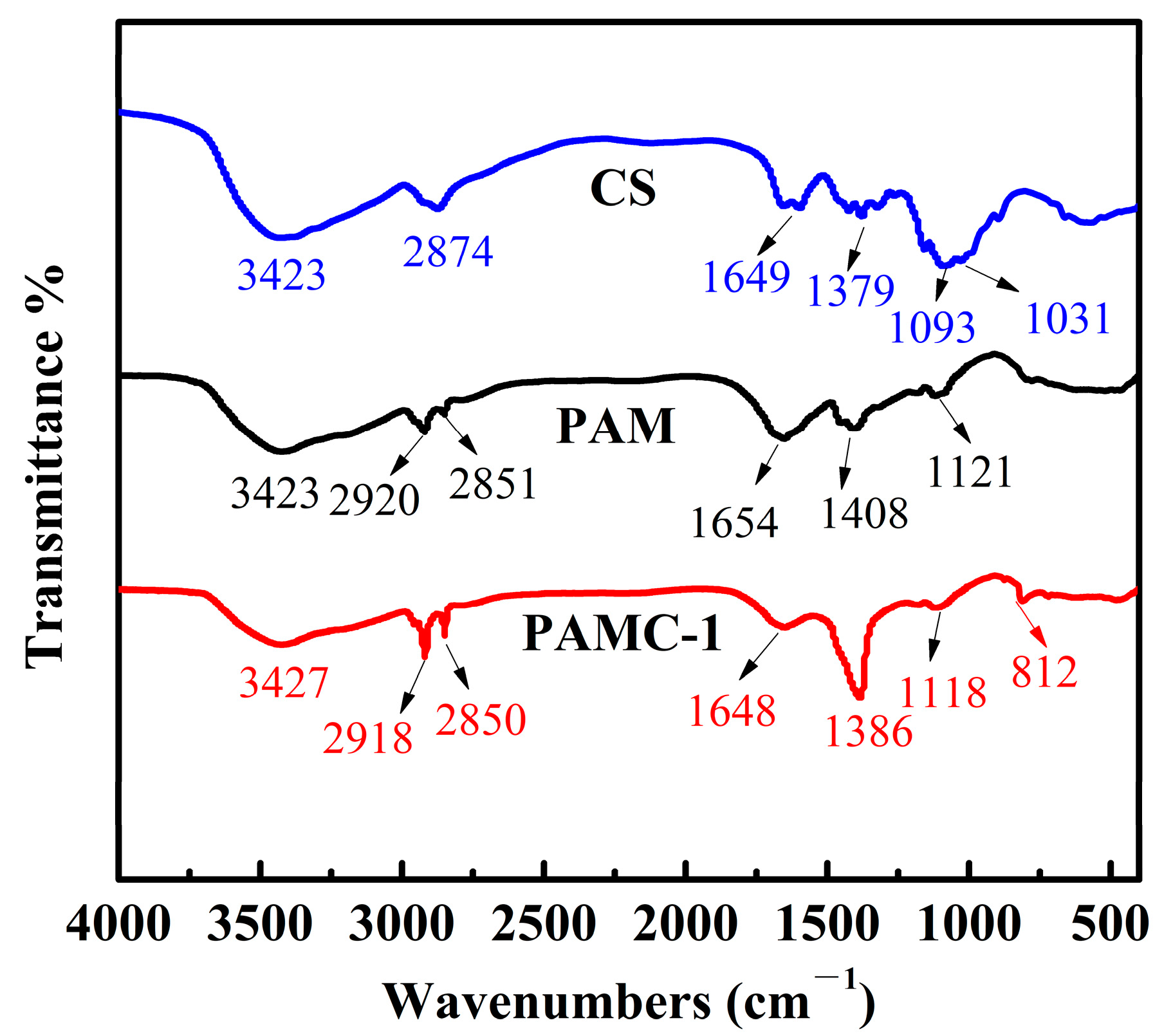
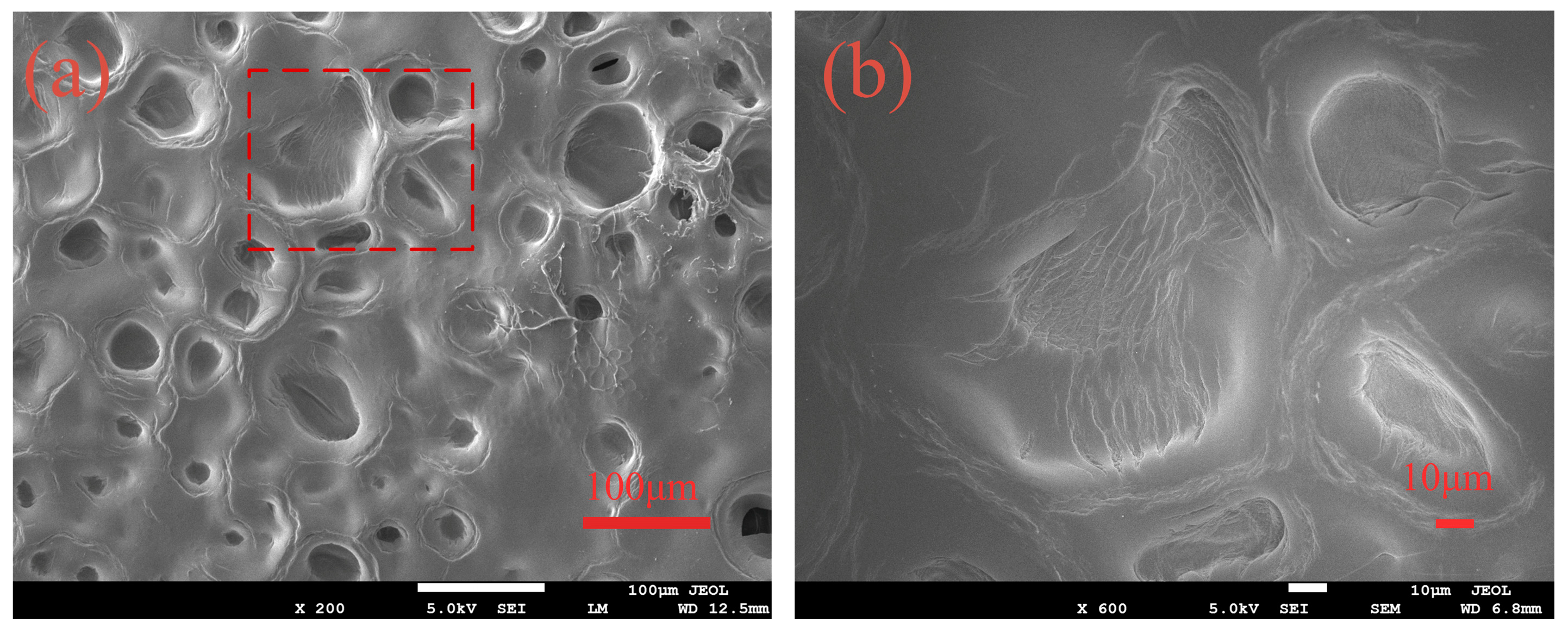
3.1. Composition and Morphology of PAMC
3.2. Adsorption in Solution
3.2.1. Adsorption in Different Concentrations of NaCl Solution
3.2.2. Adsorption in Simulated Seawater
3.2.3. Effect of pH on the Adsorption of PAMC
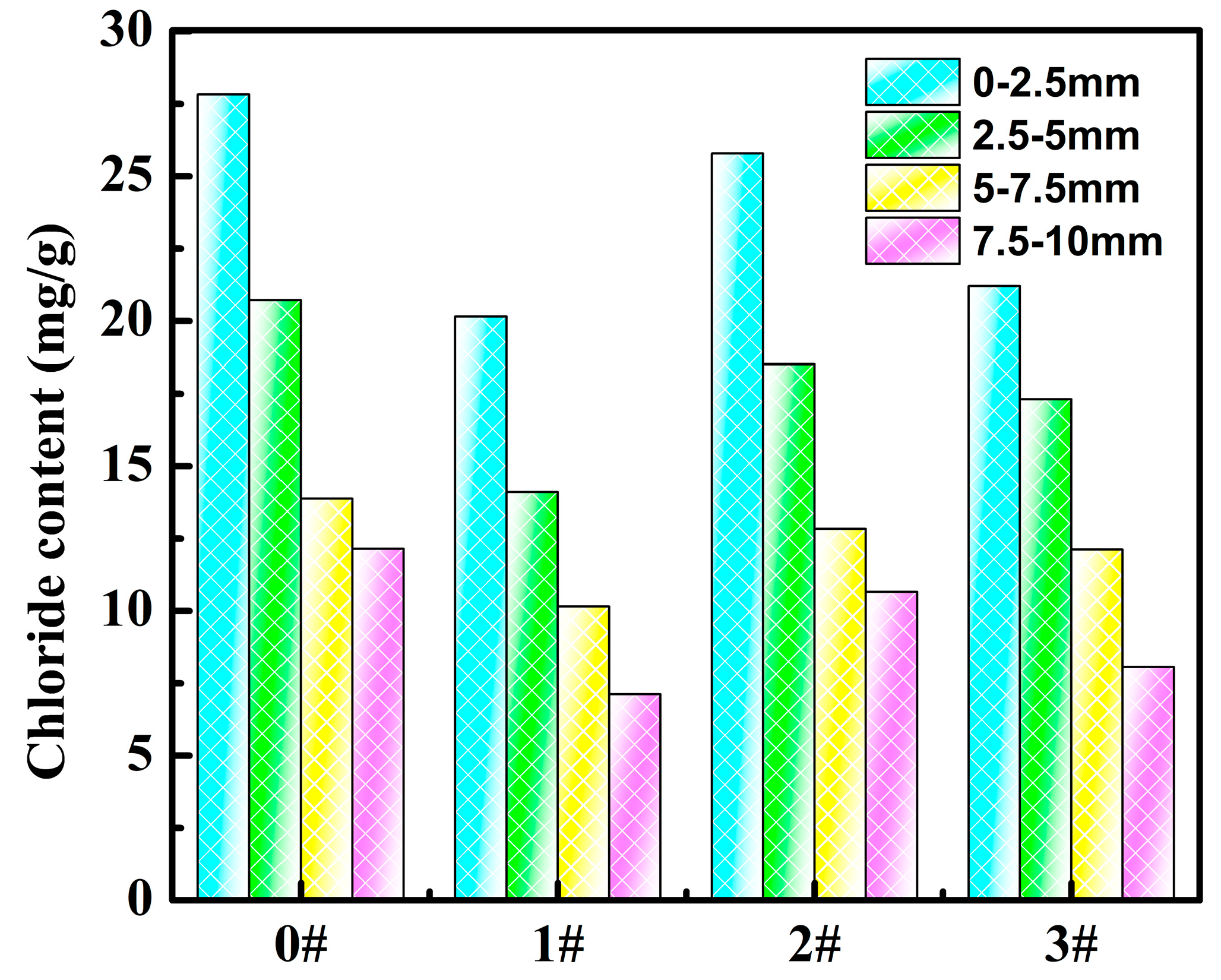
3.3. Adsorption in Sea Sand
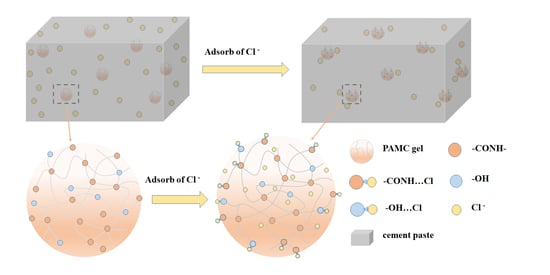
3.4. Chloride Penetration Resistance in Cement Paste
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Khan, N.; Pu, C.S. A new method of plugging the fracture to enhance oil production for fractured oil reservoir using gel particles and the HPAM/Cr3+ system. Polymers 2019, 11, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.M.; Li, T.; Shen, J.L.; Meng, Y.; Tong, S.H.; Guan, Q.F.; Xia, X.X. Core-shell structured magnetic carboxymethyl cellulose-based hydrogel nanosorbents for effective adsorption of methylene blue from aqueous solution. Polymers 2021, 13, 3054. [Google Scholar] [CrossRef] [PubMed]
- Quezada, G.R.; Rozas, R.E.; Toledo, P.G. Polyacrylamide adsorption on (101) quartz surfaces in saltwater for a range of pH values by molecular dynamics simulations. Miner. Eng. 2021, 162, 106741. [Google Scholar] [CrossRef]
- Zou, W.J.; Gong, L.; Huang, J.; Pan, M.F.; Lu, Z.Z.; Sun, C.B.; Zeng, H.B. Probing the adsorption and interaction mechanisms of hydrophobically modified polyacrylamide P(AM-NaAA-C16DMAAC) on model coal surface: Impact of salinity. Miner. Eng. 2019, 141, 105841. [Google Scholar] [CrossRef]
- Binaeian, E.; Zadvarzi, S.B.; Yuan, D. Anionic dye uptake via composite using chitosan-polyacrylamide hydrogel as matrix containing TiO2 nanoparticles; comprehensive adsorption studies. Int. J. Biol. Macromol. 2020, 162, 150–162. [Google Scholar] [CrossRef]
- Hong, T.T.; Okabe, H.; Hidaka, Y.; Omondi, B.A.; Hara, K. Radiation induced modified CMC-based hydrogel with enhanced reusability for heavy metal ions adsorption. Polymer 2019, 181, 121772. [Google Scholar] [CrossRef]
- Moon, S.J.; Jang, S.W.; Kim, Y.R.; Gil, M.; Lee, K.J. Prussian blue decorated hydrogel particles for effective removal of cesium ion from aqueous media. Polymer 2020, 186, 122029. [Google Scholar] [CrossRef]
- Zou, X.Q.; Zhang, H.; Chen, T.; Li, H.T.; Meng, C.H.; Xia, Y.; Guo, J. Preparation and characterization of polyacrylamide-e/sodium alginate microspheres and its adsorption of MB dye. Colloids Surf. 2019, 567, 184–192. [Google Scholar] [CrossRef]
- Feng, L.H.; Zhang, Q.; Ji, F.Y.; Jiang, L.; Liu, C.C.; Shen, Q.S.; Liu, Q. Phosphate removal performances of layered double hydroxides (LDH) embedded polyvinyl alcohol/lanthanum alginate hydrogels. Chem. Eng. J. 2020, 430, 132754. [Google Scholar] [CrossRef]
- Xi, H.; Li, Q.Q.; Yang, Y.; Zhang, J.F.; Guo, F.; Wang, X.G.; Xu, S.K.; Ruan, S.P. Highly effective removal of phosphate from complex water environment with porous Zr-bentonite alginate hydrogel beads: Facile synthesis and adsorption behavior study. Appl. Clay Sci. 2021, 201, 105919. [Google Scholar] [CrossRef]
- Yu, X.L.; Zhang, J.; Zheng, Y. Perchlorate adsorption onto epichlorohydrin crosslinked chitosan hydrogel beads. Sci. Total Environ. 2021, 761, 143236. [Google Scholar] [CrossRef]
- Qing, Z.L.; Wang, L.J.; Liu, X.Y.; Song, Z.W.; Qian, F.; Song, Y.H. Simply synthesized sodium alginate/zirconium hydrogel as adsorbent for phosphate adsorption from aqueous solution: Performance and mechanisms. Chemosphere 2022, 291, 133103. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Zhong, J.; Ding, W.J.; Ou, J.P. Strength, hydration, and microstructure of seawater sea-sand concrete using high-ferrite Portland cement. Constr. Build. Mater. 2021, 295, 123703. [Google Scholar] [CrossRef]
- Costa, A.; Júlio, A. Case studies of concrete deterioration in a marine environment in Portugal. Cem. Concr. Compos. 2002, 24, 169–179. [Google Scholar] [CrossRef]
- Wang, X.X.; Liu, J.P.; Jin, Z.Q.; Chen, F.X.; Zhong, P.H.; Zhang, L. Real-time strain monitoring of reinforced concrete under the attacks of sulphate and chloride ions based on XCT and DIC methods. Cem. Concr. Compos. 2022, 125, 104314. [Google Scholar] [CrossRef]
- Kenny, A.; Katz, A. Steel-concrete interface influence on chloride threshold for corrosion—Empirical reinforcement to theory. Constr. Build. Mater. 2020, 244, 118376. [Google Scholar] [CrossRef]
- Xiao, L.F.; Chen, D.Q.; Jiang, M.J.; Xiao, L.; Mei, G.X. Experimental and numerical analysis of chloride transport in finite concrete under reverse water pressure. Constr. Build. Mater. 2021, 304, 124576. [Google Scholar] [CrossRef]
- Li, W.J.; Guo, L. Peridynamic investigation of chloride diffusion in concrete under typical environmental factors. Ocean Eng. 2021, 239, 109770. [Google Scholar] [CrossRef]
- Yang, C.Y.; Li, L.; Li, J.P. Service life of reinforced concrete seawalls suffering from chloride attack: Theoretical modelling and analysis. Constr. Build. Mater. 2020, 263, 120172. [Google Scholar] [CrossRef]
- Li, G.; Ding, Y.; Gao, T.Y.; Qin, T.M.; Lv, Y.J.; Wang, K.J. Chloride resistance of concrete containing nanoparticle-modified polymer cementitious coatings. Constr. Build. Mater. 2021, 299, 123736. [Google Scholar] [CrossRef]
- Bhogone, M.V.; Subramaniam, K.V.L. Enhanced concrete performance in cracking resistance and chloride penetration using hybrid fiber blends. Mater. Today Proc. 2022, 49, 1175–1179. [Google Scholar] [CrossRef]
- Carmona, J.; Garcés, P.; Climent, M.A. Efficiency of a conductive cement-based anodic system for the application of cathodic protection, cathodic prevention and electrochemical chloride extraction to control corrosion in reinforced concrete structures. Corros. Sci. 2015, 96, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Luján, V.A.F.; Rangel, J.M.M.; Quero, V.G.J.; García, P.M. Chloride-binding capacity of ternary concretes containing fly ash and untreated sugarcane bagasse ash. Cem. Concr. Compos. 2021, 120, 104040. [Google Scholar] [CrossRef]
- Hu, Q.L.; Liu, Y.; Feng, C.P.; Zhang, Z.Y.; Lei, Z.F.; Shimizu, K. Predicting equilibrium time by adsorption kinetic equations and modifying Langmuir isotherm by fractal-like approach. J. Mol. Liq. 2018, 268, 728–733. [Google Scholar] [CrossRef]
- Rosly, N.Z.; Ishak, S.; Abdullah, A.H.; Kamarudin, M.A.; Ashari, S.E.; Ahmad, S.A.A. Fabrication and Optimization Calix[8]arene-PbS Nanoadsorbents for the Adsorption of Methylene Blue: Isotherms, Kinetics and Thermodynamics Studies. J. Saudi Chem. Soc. 2021, 26, 101402. [Google Scholar] [CrossRef]
- Araújo, C.S.T.; Almeida, I.L.S.; Rezende, H.C.; Marcionilio, S.M.L.O.; Léon, J.J.L.; Matos, T.N. Elucidation of mechanism involved in adsorption of Pb(II) onto lobeira fruit (Solanum lycocarpum) using Langmuir, Freundlich and Temkin isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
- ASTM D1141-1998; Standard Practice for the Preparation of Substitute Ocean Water. ASTM International: West Conshohocken, PA, USA, 2003.
- Saha, S.; Sarkar, P. Arsenic remediation from drinking water by synthesized nano-alumina dispersed in chitosan-grafted polyacrylamide. J. Hazard. Mater. 2012, 227–228, 68–78. [Google Scholar] [CrossRef]
- Li, X.; Zheng, H.L.; Wang, Y.L.; Sun, Y.J.; Xu, B.C.; Zhao, C.L. Fabricating an enhanced sterilization chitosan-based flocculants: Synthesis, characterization, evaluation of sterilization and flocculation. Chem. Eng. J. 2017, 319, 119–130. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.W.; Huang, M.; Yan, H.; Yang, H.; Xiao, S.J.; Li, A.M. Preparation of chitosan-graft-polyacrylamide magnetic composite microspheres for enhanced selective removal of mercury ions from water. J. Colloid Interface Sci. 2015, 455, 261–270. [Google Scholar] [CrossRef]
- Viswanathan, N.; Sundaram, C.S.; Meenakshi, S. Removal of fluoride from aqueous solution using protonated chitosan beads. J. Hazard. Mater. 2009, 161, 423–430. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, K.; Hu, G.X.; Zeng, F.M.; Li, X.; Li, C.; Zhang, Y. Underwater super elastic MOF/polyacrylamide/chitosan composite aerogel for efficient 2, 4-dichlorophenoxyacetic acid adsorption. Colloids Surf. A 2022, 635, 127970. [Google Scholar] [CrossRef]
- Zhang, L.; Sellaoui, L.; Franco, D.; Dotto, G.L.; Bajahzar, A.; Belmabrouk, H.; Petriciolet, A.B.; Oliveira, M.L.S.; Li, Z.C. Adsorption of dyes brilliant blue, sunset yellow and tartrazine from aqueous solution on chitosan: Analytical interpretation via multilayer statistical physics model. Chem. Eng. J. 2020, 382, 122952. [Google Scholar] [CrossRef]
- Biswas, S.; Mallik, B.S. Solvent-mediated dynamics and stretching profile of amide modes: QM/MM simulations of N-methylacetamide in ionic and various molecular liquids. J. Mol. Liq. 2020, 317, 114202. [Google Scholar] [CrossRef]
- Stoney, D.A.; Stoney, P.L. Identification of factors affecting SEM/EDS analysis for discrimination and classification among common items of evidence using particle combination profiles. Forensic Sci. Int. 2022, 330, 111125. [Google Scholar] [CrossRef]




| Samples | AM | MBA/AM | CS | APS | Gc | Sd |
|---|---|---|---|---|---|---|
| (g) | (wt%) | (g) | (g) | (wt%) | (wt%) | |
| PAM1C | 1 | 1 | 0.5 | 0.01 | 91 ± 3.6 | 188.1 |
| PAM1.5C | 1.5 | 1 | 0.5 | 0.01 | 87 ± 2.3 | 186.0 |
| PAM2C | 2 | 1 | 0.5 | 0.01 | 87 ± 1.5 | 178.4 |
| PAMC-1 | 2.5 | 1 | 0.5 | 0.01 | 84 ± 2.6 | 176.5 |
| PAMC-2 | 2.5 | 2 | 0.5 | 0.01 | 90 ± 1.5 | 121.8 |
| PAMC-3 | 2.5 | 3 | 0.5 | 0.01 | 88 ± 1 | 92.7 |
| PAMC-4 | 2.5 | 4 | 0.5 | 0.01 | 90 ± 1.5 | 70.9 |
| PAMC-6 | 2.5 | 6 | 0.5 | 0.01 | 93 ± 0.6 | 46.5 |
| Sample | Content (g) | ||||
|---|---|---|---|---|---|
| Water | P.O.52.5 | PAMC-1 | PAMC-3 | PAMC-6 | |
| 0 | 80 | 200 | - | - | - |
| 1 | 80 | 200 | 3 | - | - |
| 2 | 80 | 200 | - | 3 | - |
| 3 | 80 | 200 | - | - | 3 |
| PAMC-1 | pH = 4 | pH = 6.86 | pH = 9.18 | pH = 12 |
|---|---|---|---|---|
| Swelling degree (%) | 174 | 165 | 170 | 178 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, M.; Wu, L.; Zhang, G.; Yang, Y.; Chen, W.; Li, Q.; Tang, P.; Chen, W. Study on a Hydrogel for Adsorption of Chloride Ions in Cementitious Materials. Polymers 2022, 14, 2081. https://doi.org/10.3390/polym14102081
Cao M, Wu L, Zhang G, Yang Y, Chen W, Li Q, Tang P, Chen W. Study on a Hydrogel for Adsorption of Chloride Ions in Cementitious Materials. Polymers. 2022; 14(10):2081. https://doi.org/10.3390/polym14102081
Chicago/Turabian StyleCao, Meng, Lili Wu, Guixia Zhang, Ying Yang, Wei Chen, Qiu Li, Pei Tang, and Wanyu Chen. 2022. "Study on a Hydrogel for Adsorption of Chloride Ions in Cementitious Materials" Polymers 14, no. 10: 2081. https://doi.org/10.3390/polym14102081
APA StyleCao, M., Wu, L., Zhang, G., Yang, Y., Chen, W., Li, Q., Tang, P., & Chen, W. (2022). Study on a Hydrogel for Adsorption of Chloride Ions in Cementitious Materials. Polymers, 14(10), 2081. https://doi.org/10.3390/polym14102081