Bone Mineralization in Electrospun-Based Bone Tissue Engineering
Abstract
:1. Introduction
2. Bone: Dynamic and Biphasic Tissue
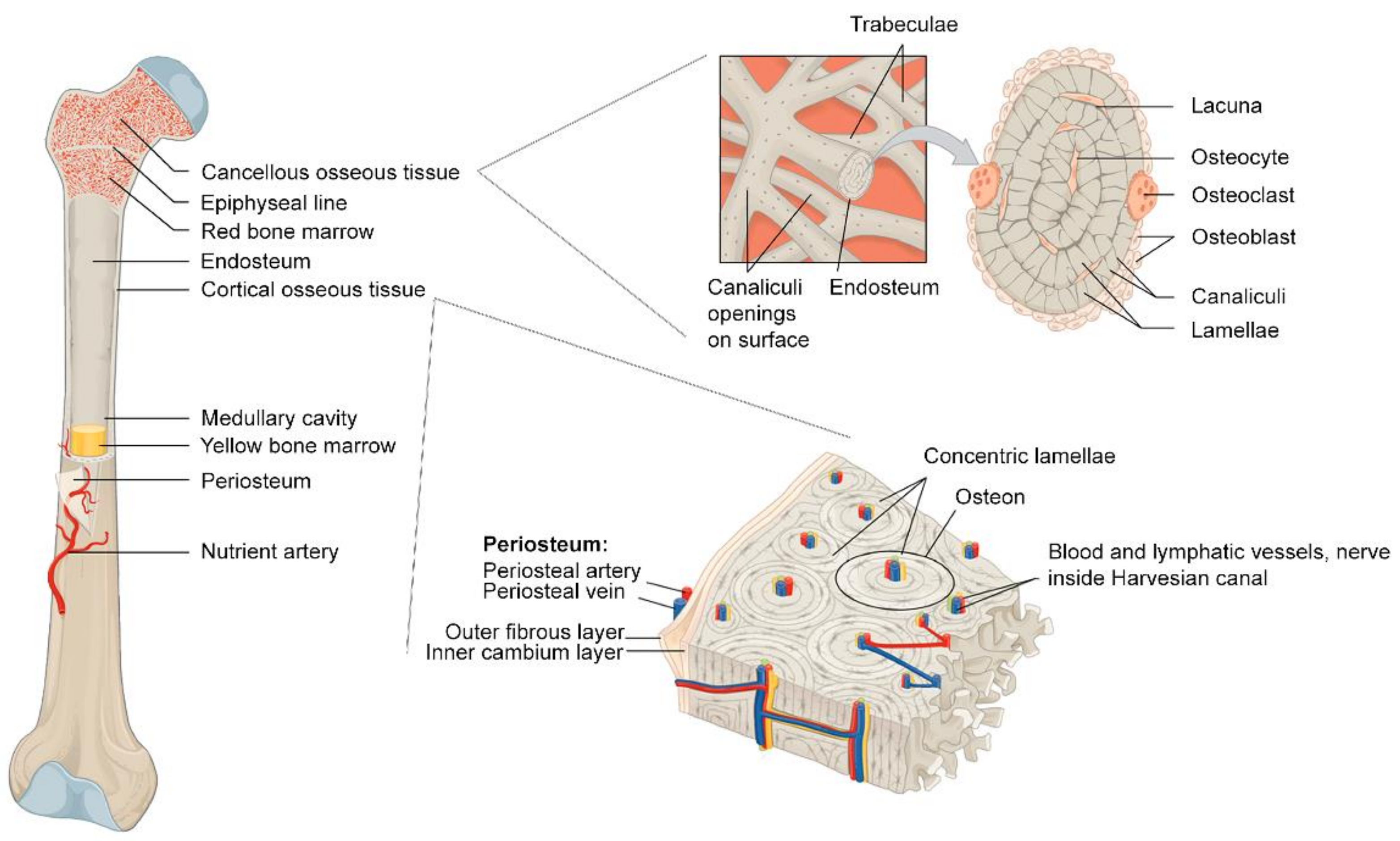
2.1. Microstructural Bone Formation: Biphasic Aspects of Bone
2.2. Macrostructural Bone Formation: Vascularization and Ossifications
2.3. Bone Remodeling and Bone Healing
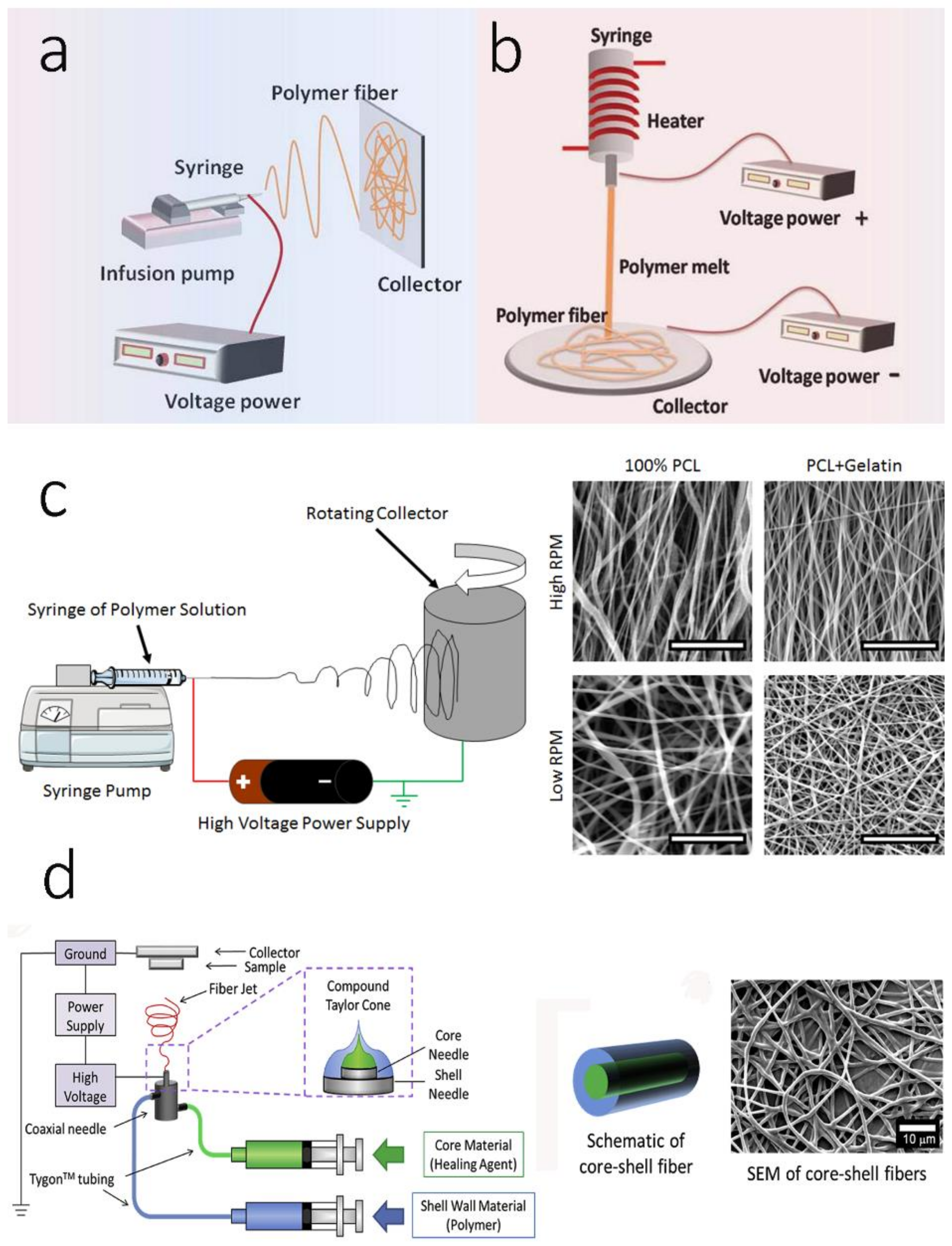
3. Electrospinning Technologies: Electrospun Scaffolds in Bone Mineralization
3.1. Monoaxicial Electrospinning
3.2. Melt Electrospinning
3.3. Aligned/Oriented Electrospinning
3.4. Multi-Axial Electrospinning
4. Simulated Body Fluid for Bone Scaffold Mineralization
5. Simulated Body Fluids for Electrospun-Based Bone Scaffolds
| Type of Electrospun Scaffold | Treated SBF Protocol | Descriptions | Ref. |
|---|---|---|---|
| PLGA/collagen/gelatin (2:1:1 weight ratio) | 10× m-SBF | The mineralized PCG nanofibers were fragmented and loaded with BMP-2 mimicry peptides 1 for alveolar bone regeneration in vivo. | [142] |
| Liginin/PCL | 1.5× SBF | The fibrous liginin/PCL films were completely coated by HA within 5 days. | [143] |
| Alginate/PLA | 1.5× SBF | The alginate/PLA composite was crosslinked by Ca2+ and mineralized. Anionic alginate assists with the nucleation and growth of calcium phosphate apatites. | [144] |
| Polysilsesquioxane (POSS)-loaded PLA | 1× SBF | The POSS-PLA showed acceleration in HA mineralization. | [145] |
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greenwald, A.S.; Boden, S.D.; Goldberg, V.M.; Khan, Y.; Laurencin, C.T.; Rosier, R.N. Bone-Graft Substitutes: Facts, Fictions, and Applications. JBJS 2001, 83, S98–S103. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J. A Not-So-New Treatment for Old Bones. N. Engl. J. Med. 2018, 379, 2465–2466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis Prevention, Diagnosis, and Therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Föger-Samwald, U.; Dovjak, P.; Azizi-Semrad, U.; Kerschan-Schindl, K.; Pietschmann, P. Osteoporosis: Pathophysiology and therapeutic options. EXCLI J. 2020, 19, 1017–1037. [Google Scholar] [CrossRef]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. P T 2018, 43, 92–104. [Google Scholar]
- Blume, S.W.; Curtis, J.R. Medical costs of osteoporosis in the elderly Medicare population. Osteoporos. Int. 2011, 22, 1835–1844. [Google Scholar] [CrossRef] [Green Version]
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The epidemiology of osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.h.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef]
- Robinson, P.G.; Abrams, G.D.; Sherman, S.L.; Safran, M.R.; Murray, I.R. Autologous Bone Grafting. Oper. Tech. Sports Med. 2020, 28, 150780. [Google Scholar] [CrossRef]
- Betz, R.R. Limitations of autograft and allograft: New synthetic solutions. Orthopedics 2002, 25, s561–s570. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Fishman, J.A.; Greenwald, M.A.; Grossi, P.A. Transmission of Infection With Human Allografts: Essential Considerations in Donor Screening. Clin. Infect. Dis. 2012, 55, 720–727. [Google Scholar] [CrossRef] [Green Version]
- Deijkers, R.L.; Bloem, R.M.; Petit, P.L.; Brand, R.; Vehmeyer, S.B.; Veen, M.R. Contamination of bone allografts: Analysis of incidence and predisposing factors. J. Bone Jt. Surgery. Br. Vol. 1997, 79, 161–166. [Google Scholar] [CrossRef]
- Ng, V.Y. Risk of disease transmission with bone allograft. Orthopedics 2012, 35, 679–681. [Google Scholar] [CrossRef] [Green Version]
- Shegarfi, H.; Reikeras, O. Review article: Bone transplantation and immune response. J. Orthop. Surg. 2009, 17, 206–211. [Google Scholar] [CrossRef]
- Shibuya, N.; Jupiter, D.C. Bone graft substitute: Allograft and xenograft. Clin. Podiatr. Med. Surg. 2015, 32, 21–34. [Google Scholar] [CrossRef]
- Nappe, C.E.; Rezuc, A.B.; Montecinos, A.; Donoso, F.A.; Vergara, A.J.; Martinez, B. Histological comparison of an allograft, a xenograft and alloplastic graft as bone substitute materials. J. Osseointegr. 2016, 8, 20–26. [Google Scholar]
- Gruskin, E.; Doll, B.A.; Futrell, F.W.; Schmitz, J.P.; Hollinger, J.O. Demineralized bone matrix in bone repair: History and use. Adv. Drug Deliv. Rev. 2012, 64, 1063–1077. [Google Scholar] [CrossRef]
- Kadam, A.; Millhouse, P.W.; Kepler, C.K.; Radcliff, K.E.; Fehlings, M.G.; Janssen, M.E.; Sasso, R.C.; Benedict, J.J.; Vaccaro, A.R. Bone substitutes and expanders in Spine Surgery: A review of their fusion efficacies. Int. J. Spine Surg. 2016, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Pang, S.; Su, F.Y.; Green, A.; Salim, J.; McKittrick, J.; Jasiuk, I. Comparison of different protocols for demineralization of cortical bone. Sci. Rep. 2021, 11, 7012. [Google Scholar] [CrossRef]
- Figueiredo, M.; Cunha, S.; Martins, G.; Freitas, J.; Judas, F.; Figueiredo, H. Influence of hydrochloric acid concentration on the demineralization of cortical bone. Chem. Eng. Res. Des. 2011, 89, 116–124. [Google Scholar] [CrossRef]
- Russell, J.L.; Block, J.E. Clinical utility of demineralized bone matrix for osseous defects, arthrodesis, and reconstruction: Impact of processing techniques and study methodology. Orthopedics 1999, 22, 524–531. [Google Scholar]
- Han, B.; Tang, B.; Nimni, M.E. Quantitative and sensitive in vitro assay for osteoinductive activity of demineralized bone matrix. J. Orthop. Res. 2003, 21, 648–654. [Google Scholar] [CrossRef]
- Russell, N.; Walsh, W.R.; Lovric, V.; Kim, P.; Chen, J.H.; Larson, M.J.; Vizesi, F. In-vivo Performance of Seven Commercially Available Demineralized Bone Matrix Fiber and Putty Products in a Rat Posterolateral Fusion Model. Front. Surg. 2020, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [Green Version]
- Raic, A.; Friedrich, F.; Kratzer, D.; Bieback, K.; Lahann, J.; Lee-Thedieck, C. Potential of electrospun cationic BSA fibers to guide osteogenic MSC differentiation via surface charge and fibrous topography. Sci. Rep. 2019, 9, 20003. [Google Scholar] [CrossRef]
- Hashemi, J.; Barati, G.; Enderami, S.E.; Safdari, M. Osteogenic Differentiation of Induced Pluripotent Stem Cells on Electrospun Nanofibers: A Review of Literature. Mater. Today Commun. 2020, 25, 101561. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. S2), S96–S101. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, K.; Gouma, P.I. Electrospinning for bone tissue engineering. Recent Pat. Nanotechnol. 2008, 2, 1–7. [Google Scholar] [CrossRef]
- Rodrigues, M.T.; Martins, A.; Dias, I.R.; Viegas, C.A.; Neves, N.M.; Gomes, M.E.; Reis, R.L. Synergistic effect of scaffold composition and dynamic culturing environment in multilayered systems for bone tissue engineering. J. Tissue Eng. Regen. Med. 2012, 6, e24–e30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, C.; Ren, Z. Bone Tissue Engineering: Scaffolds with Osteoinductivity for Bone Regeneration. BioMed Res. Int. 2017, 2017, 1038476. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Lim, D.-J. Cross-Linking Agents for Electrospinning-Based Bone Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 5444. [Google Scholar] [CrossRef]
- Ingavle, G.C.; Leach, J.K. Advancements in electrospinning of polymeric nanofibrous scaffolds for tissue engineering. Tissue Eng. Part B Rev. 2013, 20, 277–293. [Google Scholar] [CrossRef]
- Shin, K.; Acri, T.; Geary, S.; Salem, A.K. Biomimetic Mineralization of Biomaterials Using Simulated Body Fluids for Bone Tissue Engineering and Regenerative Medicine. Tissue Eng. Part A 2017, 23, 1169–1180. [Google Scholar] [CrossRef]
- Müller, L.; Müller, F.A. Preparation of SBF with different HCO3- content and its influence on the composition of biomimetic apatites. Acta Biomater. 2006, 2, 181–189. [Google Scholar] [CrossRef]
- Yilmaz, B.; Evis, Z.; Tezcaner, A.; Banerjee, S. Surface Characterization and Biocompatibility of Selenium-Doped Hydroxyapatite Coating on Titanium Alloy. Int. J. Appl. Ceram. Technol. 2016, 13, 1059–1068. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, S.-J.; Park, Y.-J.; Song, H.-J. Calcium phosphate compound formed on electrospun chitosan nanofibers using modified simulated body fluid. Polym. Bull. 2019, 76, 4205–4214. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, S.J.; Hwang, M.J.; Song, H.J.; Park, Y.J. Biomimetic fabrication of calcium phosphate/chitosan nanohybrid composite in modified simulated body fluids. Express Polym. Lett. 2017, 11, 14–20. [Google Scholar] [CrossRef]
- Ghorbani, F.; Zamanian, A.; Behnamghader, A.; Joupari, M.D. Microwave-induced rapid formation of biomimetic hydroxyapatite coating on gelatin-siloxane hybrid microspheres in 10X-SBF solution. e-Polymers 2018, 18, 247–255. [Google Scholar] [CrossRef]
- Yilmaz, B.; Pazarceviren, A.E.; Tezcaner, A.; Evis, Z. Historical development of simulated body fluids used in biomedical applications: A review. Microchem. J. 2020, 155, 104713. [Google Scholar] [CrossRef]
- Olsen, B.R.; Reginato, A.M.; Wang, W. Bone Development. Annu. Rev. Cell Dev. Biol. 2000, 16, 191–220. [Google Scholar] [CrossRef]
- Schaffler, M.B.; Cheung, W.-Y.; Majeska, R.; Kennedy, O. Osteocytes: Master orchestrators of bone. Calcif. Tissue Int. 2014, 94, 5–24. [Google Scholar] [CrossRef] [Green Version]
- Florencio-Silva, R.; Sasso, G.R.d.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.C.; de Saint-Georges, L.; Bowman, B.M.; Jee, W.S. Bone lining cells: Structure and function. Scanning Microsc. 1989, 3, 953–960. [Google Scholar]
- Le, B.Q.; Nurcombe, V.; Cool, S.M.; Van Blitterswijk, C.A.; De Boer, J.; LaPointe, V.L.S. The Components of Bone and What They Can Teach Us about Regeneration. Materials 2018, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Viguet-Carrin, S.; Garnero, P.; Delmas, P.D. The role of collagen in bone strength. Osteoporos. Int. 2006, 17, 319–336. [Google Scholar] [CrossRef]
- Tzaphlidou, M. Bone architecture: Collagen structure and calcium/phosphorus maps. J. Biol. Phys. 2008, 34, 39–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossert, J.; de Crombrugghe, B. Chapter 12—Type I Collagen: Structure, Synthesis, and Regulation. In Principles of Bone Biology, 2nd ed.; Bilezikian, J.P., Raisz, L.G., Rodan, G.A., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 189–210. [Google Scholar] [CrossRef]
- Tanaka, T.; Moriya, K.; Tsunenaga, M.; Yanagawa, T.; Morita, H.; Minowa, T.; Tagawa, Y.-i.; Hanagata, N.; Inagaki, Y.; Ikoma, T. Visualized procollagen Iα1 demonstrates the intracellular processing of propeptides. Life Sci. Alliance 2022, 5, e202101060. [Google Scholar] [CrossRef] [PubMed]
- Ramshaw, J.A.M.; Shah, N.K.; Brodsky, B. Gly-X-Y Tripeptide Frequencies in Collagen: A Context for Host–Guest Triple-Helical Peptides. J. Struct. Biol. 1998, 122, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.-Z.; Li, Y.; Ge, J. Self-assembly of mineralized collagen composites. Mater. Sci. Eng. R Rep. 2007, 57, 1–27. [Google Scholar] [CrossRef]
- Giraud Guille, M.M.; Mosser, G.; Helary, C.; Eglin, D. Bone matrix like assemblies of collagen: From liquid crystals to gels and biomimetic materials. Micron 2005, 36, 602–608. [Google Scholar] [CrossRef]
- Church, R.L.; Pfeiffer, S.E.; Tanzer, M.L. Collagen biosynthesis: Synthesis and secretion of a high molecular weight collagen precursor (procollagen). Proc. Natl. Acad. Sci. USA 1971, 68, 2638–2642. [Google Scholar] [CrossRef] [Green Version]
- Miyahara, M.; Hayashi, K.; Berger, J.; Tanzawa, K.; Njieha, F.K.; Trelstad, R.L.; Prockop, D.J. Formation of collagen fibrils by enzymic cleavage of precursors of type I collagen in vitro. J. Biol. Chem. 1984, 259, 9891–9898. [Google Scholar] [CrossRef]
- Holmes, D.F.; Lu, Y.; Starborg, T.; Kadler, K.E. Chapter Three—Collagen Fibril Assembly and Function. In Current Topics in Developmental Biology; Litscher, E.S., Wassarman, P.M., Eds.; Academic Press: San Diego, CA, USA, 2018; Volume 130, pp. 107–142. [Google Scholar]
- Chung, H.J.; Steplewski, A.; Chung, K.Y.; Uitto, J.; Fertala, A. Collagen Fibril Formation: A new target TO limit fibrosis *. J. Biol. Chem. 2008, 283, 25879–25886. [Google Scholar] [CrossRef] [Green Version]
- Porter, S.; Clark, I.M.; Kevorkian, L.; Edwards, D.R. The ADAMTS metalloproteinases. Biochem J. 2005, 386, 15–27. [Google Scholar] [CrossRef]
- Colige, A.; Vandenberghe, I.; Thiry, M.; Lambert, C.A.; Van Beeumen, J.; Li, S.-W.; Prockop, D.J.; Lapière, C.M.; Nusgens, B.V. Cloning and Characterization of ADAMTS-14, a Novel ADAMTS Displaying High Homology with ADAMTS-2 and ADAMTS-3. J. Biol. Chem. 2002, 277, 5756–5766. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, D.R.; Keles, S.; Greenspan, D.S. The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biol. 2007, 26, 508–523. [Google Scholar] [CrossRef] [Green Version]
- Feng, X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Curr. Chem. Biol. 2009, 3, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Burger, C.; Zhou, H.-W.; Wang, H.; Sics, I.; Hsiao, B.S.; Chu, B.; Graham, L.; Glimcher, M.J. Lateral Packing of Mineral Crystals in Bone Collagen Fibrils. Biophys. J. 2008, 95, 1985–1992. [Google Scholar] [CrossRef] [Green Version]
- Flynn, A. The role of dietary calcium in bone health. Proc. Nutr. Soc. 2003, 62, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Goretti Penido, M.; Alon, U.S. Phosphate homeostasis and its role in bone health. Pediatric Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef] [Green Version]
- Glimcher, M.J. Chapter 2—The Nature of the Mineral Phase in Bone: Biological and Clinical Implications. In Metabolic Bone Disease and Clinically Related Disorders, 3rd ed.; Avioli, L.V., Krane, S.M., Eds.; Academic Press: San Diego, CA, USA, 1998; pp. 23–52e. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Fratzl, P.; Gupta, H.S.; Paschalis, E.P.; Roschger, P. Structure and mechanical quality of the collagen–mineral nano-composite in bone. J. Mater. Chem. 2004, 14, 2115–2123. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.-G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Hanson, D.A.; Eyre, D.R. Molecular Site Specificity of Pyridinoline and Pyrrole Cross-links in Type I Collagen of Human Bone*. J. Biol. Chem. 1996, 271, 26508–26516. [Google Scholar] [CrossRef] [Green Version]
- Cundy, T.; Reid, I.R.; Grey, A. CHAPTER 31—Metabolic bone disease. In Clinical Biochemistry: Metabolic and Clinical Aspects, 3rd ed.; Marshall, W.J., Lapsley, M., Day, A.P., Ayling, R.M., Eds.; Churchill Livingstone: London, UK, 2014; pp. 604–635. [Google Scholar] [CrossRef]
- Risteli, J.; Eriksen, H.; Risteli, L.; Mansell, J.P.; Bailey, A.J. Pyrrolic cross-links are as abundant in human bone type I collagen as pyridinolines. J. Bone Miner. Res. 1994, 9, S186. [Google Scholar]
- Filipowska, J.; Tomaszewski, K.A.; Niedźwiedzki, Ł.; Walocha, J.A.; Niedźwiedzki, T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017, 20, 291–302. [Google Scholar] [CrossRef] [Green Version]
- Ott, S.M. Cortical or Trabecular Bone: What’s the Difference? Am. J. Nephrol. 2018, 47, 373–375. [Google Scholar] [CrossRef]
- Ascenzi, M.G.; Roe, A.K. The osteon: The micromechanical unit of compact bone. Front. Biosci. 2012, 17, 1551–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Cowin, S.C.; Weinbaum, S. Electrical signal transmission and gap junction regulation in a bone cell network: A cable model for an osteon. Ann. Biomed. Eng. 1997, 25, 357–374. [Google Scholar] [CrossRef]
- Galea, G.L.; Zein, M.R.; Allen, S.; Francis-West, P. Making and shaping endochondral and intramembranous bones. Dev. Dyn. 2021, 250, 414–449. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Behonick, D.J.; Werb, Z. Matrix remodeling during endochondral ossification. Trends Cell Biol. 2004, 14, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, B. Normal Bone Anatomy and Physiology. Clin. J. Am. Soc. Nephrol. 2008, 3, S131–S139. [Google Scholar] [CrossRef] [Green Version]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef]
- Schell, H.; Duda, G.N.; Peters, A.; Tsitsilonis, S.; Johnson, K.A.; Schmidt-Bleek, K. The haematoma and its role in bone healing. J. Exp. Orthop. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Bigham-Sadegh, A.; Oryan, A. Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. Int. Wound J. 2015, 12, 238–247. [Google Scholar] [CrossRef]
- Khajavi, R.; Abbasipour, M.; Bahador, A. Electrospun biodegradable nanofibers scaffolds for bone tissue engineering. J. Appl. Polym. Sci. 2016, 133, 42883. [Google Scholar] [CrossRef]
- Kim, S.H.; Hur, W.; Kim, J.E.; Min, H.J.; Kim, S.; Min, H.S.; Kim, B.K.; Kim, S.H.; Choi, T.H.; Jung, Y. Self-assembling peptide nanofibers coupled with neuropeptide substance P for bone tissue engineering. Tissue Eng. Part A 2015, 21, 1237–1246. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Han, W.; Chen, H.; Tu, M.; Zeng, R.; Shi, Y.; Cha, Z.; Zhou, C. Preparation, structure and crystallinity of chitosan nano-fibers by a solid–liquid phase separation technique. Carbohydr. Polym. 2011, 83, 1541–1546. [Google Scholar] [CrossRef]
- Yin, L.; Yang, S.; He, M.; Chang, Y.; Wang, K.; Zhu, Y.; Liu, Y.; Chang, Y.; Yu, Z. Physicochemical and biological characteristics of BMP-2/IGF-1-loaded three-dimensional coaxial electrospun fibrous membranes for bone defect repair. J. Mater. Sci. Mater. Med. 2017, 28, 94. [Google Scholar] [CrossRef]
- Nagiah, N.; Murdock, C.J.; Bhattacharjee, M.; Nair, L.; Laurencin, C.T. Development of Tripolymeric Triaxial Electrospun Fibrous Matrices for Dual Drug Delivery Applications. Sci. Rep. 2020, 10, 609. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Melt electrospinning today: An opportune time for an emerging polymer process. Prog. Polym. Sci. 2016, 56, 116–166. [Google Scholar] [CrossRef]
- Lian, H.; Meng, Z. Melt electrospinning vs. solution electrospinning: A comparative study of drug-loaded poly (ε-caprolactone) fibres. Mater. Sci. Eng. C 2017, 74, 117–123. [Google Scholar] [CrossRef]
- Fee, T.; Surianarayanan, S.; Downs, C.; Zhou, Y.; Berry, J. Nanofiber Alignment Regulates NIH3T3 Cell Orientation and Cytoskeletal Gene Expression on Electrospun PCL+Gelatin Nanofibers. PLoS ONE 2016, 11, e0154806. [Google Scholar] [CrossRef] [Green Version]
- Doan, T.Q.; Leslie, L.S.; Kim, S.Y.; Bhargava, R.; White, S.R.; Sottos, N.R. Characterization of core-shell microstructure and self-healing performance of electrospun fiber coatings. Polymer 2016, 107, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, P.K.; Adhikari, J.; Saha, P. Facile fabrication of electrospun regenerated cellulose nanofiber scaffold for potential bone-tissue engineering application. Int. J. Biol. Macromol. 2019, 122, 644–652. [Google Scholar] [CrossRef]
- Karbowniczek, J.E.; Kaniuk, Ł.; Berniak, K.; Gruszczyński, A.; Stachewicz, U. Enhanced Cells Anchoring to Electrospun Hybrid Scaffolds with PHBV and HA Particles for Bone Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 632029. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Xiong, J.; Li, J.; Miao, X.; Lan, X.; Liu, X.; Wang, W.; Cai, N.; Tang, Y. Fabrication and in vitro evaluation of PCL/gelatin hierarchical scaffolds based on melt electrospinning writing and solution electrospinning for bone regeneration. Mater. Sci. Eng. C 2021, 128, 112287. [Google Scholar] [CrossRef]
- Xie, J.; Shen, H.; Yuan, G.; Lin, K.; Su, J. The effects of alignment and diameter of electrospun fibers on the cellular behaviors and osteogenesis of BMSCs. Mater. Sci. Eng. C 2021, 120, 111787. [Google Scholar] [CrossRef] [PubMed]
- Jose, M.V.; Thomas, V.; Johnson, K.T.; Dean, D.R.; Nyairo, E. Aligned PLGA/HA nanofibrous nanocomposite scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, Y.; Cui, D.; Liu, Y.; Zou, Q.; Xu, S.; Luo, S.; Ye, C. Coaxial bioelectrospinning of P34HB/PVA microfibers biomimetic scaffolds with simultaneity cell-laden for improving bone regeneration. Mater. Des. 2022, 213, 110349. [Google Scholar] [CrossRef]
- Panzavolta, S.; Gualandi, C.; Fiorani, A.; Bracci, B.; Focarete, M.L.; Bigi, A. Fast Coprecipitation of Calcium Phosphate Nanoparticles inside Gelatin Nanofibers by Tricoaxial Electrospinning. J. Nanomater. 2016, 2016, 4235235. [Google Scholar] [CrossRef] [Green Version]
- Udomluck, N.; Koh, W.-G.; Lim, D.-J.; Park, H. Recent Developments in Nanofiber Fabrication and Modification for Bone Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 99. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Venugopal, J.; Ramakrishna, S. Electrospun nanostructured scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 2884–2893. [Google Scholar] [CrossRef]
- Hu, Y.; Grainger, D.W.; Winn, S.R.; Hollinger, J.O. Fabrication of poly(α-hydroxy acid) foam scaffolds using multiple solvent systems. J. Biomed. Mater. Res. 2002, 59, 563–572. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Sahoo, S.; Ang, L.T.; Goh, J.C.-H.; Toh, S.-L. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J. Biomed. Mater. Res. Part A 2010, 93A, 1539–1550. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, X.; He, H.-W.; Yu, M.; Ning, X.; Long, Y.-Z. Solvent-free electrospinning: Opportunities and challenges. Polym. Chem. 2017, 8, 333–352. [Google Scholar] [CrossRef]
- Kim, B.S.; Park, K.E.; Kim, M.H.; You, H.K.; Lee, J.; Park, W.H. Effect of nanofiber content on bone regeneration of silk fibroin/poly(ε-caprolactone) nano/microfibrous composite scaffolds. Int. J. Nanomed. 2015, 10, 485–502. [Google Scholar] [CrossRef]
- Saidy, N.T.; Shabab, T.; Bas, O.; Rojas-González, D.M.; Menne, M.; Henry, T.; Hutmacher, D.W.; Mela, P.; De-Juan-Pardo, E.M. Melt Electrowriting of Complex 3D Anatomically Relevant Scaffolds. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- McColl, E.; Groll, J.; Jungst, T.; Dalton, P.D. Design and fabrication of melt electrowritten tubes using intuitive software. Mater. Des. 2018, 155, 46–58. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Abbasi, N.; Gwiazda, M.; Hamlet, S.; Ivanovski, S. Novel polycaprolactone/hydroxyapatite nanocomposite fibrous scaffolds by direct melt-electrospinning writing. Eur. Polym. J. 2018, 105, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Liu, L.; Xie, J.; Shen, L.; Tao, J.; Zhu, J. Facile Strategy to Generate Aligned Polymer Nanofibers: Effects on Cell Adhesion. ACS Appl. Mater. Interfaces 2018, 10, 1566–1574. [Google Scholar] [CrossRef]
- Vogel, V.; Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265–275. [Google Scholar] [CrossRef]
- Wang, B.; Cai, Q.; Zhang, S.; Yang, X.; Deng, X. The effect of poly (L-lactic acid) nanofiber orientation on osteogenic responses of human osteoblast-like MG63 cells. J. Mech. Behav. Biomed. Mater. 2011, 4, 600–609. [Google Scholar] [CrossRef]
- Reible, B.; Schmidmaier, G.; Prokscha, M.; Moghaddam, A.; Westhauser, F. Continuous stimulation with differentiation factors is necessary to enhance osteogenic differentiation of human mesenchymal stem cells in-vitro. Growth Factors 2017, 35, 179–188. [Google Scholar] [CrossRef]
- Gregory, C.A.; Grady Gunn, W.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of Collagen Nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef]
- Wu, Y.; Carnell, L.A.; Clark, R.L. Control of electrospun mat width through the use of parallel auxiliary electrodes. Polymer 2007, 48, 5653–5661. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning of Polymeric and Ceramic Nanofibers as Uniaxially Aligned Arrays. Nano Lett. 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Katta, P.; Alessandro, M.; Ramsier, R.D.; Chase, G.G. Continuous Electrospinning of Aligned Polymer Nanofibers onto a Wire Drum Collector. Nano Lett. 2004, 4, 2215–2218. [Google Scholar] [CrossRef]
- Afifi, A.M.; Nakajima, H.; Yamane, H.; Kimura, Y.; Nakano, S. Fabrication of Aligned Poly(L-lactide) Fibers by Electrospinning and Drawing. Macromol. Mater. Eng. 2009, 294, 658–665. [Google Scholar] [CrossRef]
- Ajao, J.A.; Abiona, A.A.; Chigome, S.; Fasasi, A.Y.; Osinkolu, G.A.; Maaza, M. Electric-magnetic field-induced aligned electrospun poly (ethylene oxide) (PEO) nanofibers. J. Mater. Sci. 2010, 45, 2324–2329. [Google Scholar] [CrossRef]
- Kancheva, M.; Toncheva, A.; Manolova, N.; Rashkov, I. Advanced centrifugal electrospinning setup. Mater. Lett. 2014, 136, 150–152. [Google Scholar] [CrossRef]
- Ma, J.; He, X.; Jabbari, E. Osteogenic differentiation of marrow stromal cells on random and aligned electrospun poly(L-lactide) nanofibers. Ann. Biomed Eng. 2011, 39, 14–25. [Google Scholar] [CrossRef]
- Delaine-Smith, R.M.; Hann, A.J.; Green, N.H.; Reilly, G.C. Electrospun Fiber Alignment Guides Osteogenesis and Matrix Organization Differentially in Two Different Osteogenic Cell Types. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef]
- Moghe, A.K.; Gupta, B.S. Co-axial Electrospinning for Nanofiber Structures: Preparation and Applications. Polym. Rev. 2008, 48, 353–377. [Google Scholar] [CrossRef]
- Han, D.; Steckl, A.J. Triaxial Electrospun Nanofiber Membranes for Controlled Dual Release of Functional Molecules. ACS Appl. Mater. Interfaces 2013, 5, 8241–8245. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, J.; Yu, G.; Cardenas, R.; Wei, S.; Wujcik, E.K.; Guo, Z. Coaxial electrospun fibers: Applications in drug delivery and tissue engineering. WIREs Nanomed. Nanobiotechnology 2016, 8, 654–677. [Google Scholar] [CrossRef] [PubMed]
- Kareem, M.M.; Hodgkinson, T.; Sanchez, M.S.; Dalby, M.J.; Tanner, K.E. Hybrid core–shell scaffolds for bone tissue engineering. Biomed. Mater. 2019, 14, 025008. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; He, J.; Sang, F.; Ding, B.; Chen, L.; Cui, S.; Li, K.; Han, Q.; Tan, W. Coaxial electrospun aligned tussah silk fibroin nanostructured fiber scaffolds embedded with hydroxyapatite-tussah silk fibroin nanoparticles for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 342–351. [Google Scholar] [CrossRef]
- Asli, M.M.; Pourdeyhimi, B.; Loboa, E.G. Release Profiles of Tricalcium Phosphate Nanoparticles from Poly(L-lactic acid) Electrospun Scaffolds with Single Component, Core–Sheath, or Porous Fiber Morphologies: Effects on hASC Viability and Osteogenic Differentiation. Macromol. Biosci. 2012, 12, 893–900. [Google Scholar] [CrossRef]
- Nair, A.K.; Gautieri, A.; Chang, S.-W.; Buehler, M.J. Molecular mechanics of mineralized collagen fibrils in bone. Nat. Commun. 2013, 4, 1724. [Google Scholar] [CrossRef] [Green Version]
- Kokubo, T. Bioactive glass ceramics: Properties and applications. Biomaterials 1991, 12, 155–163. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Lee, J.T.Y.; Leng, Y.; Chow, K.L.; Ren, F.; Ge, X.; Wang, K.; Lu, X. Cell culture medium as an alternative to conventional simulated body fluid. Acta Biomater. 2011, 7, 2615–2622. [Google Scholar] [CrossRef]
- Oyane, A.; Onuma, K.; Ito, A.; Kim, H.M.; Kokubo, T.; Nakamura, T. Formation and growth of clusters in conventional and new kinds of simulated body fluids. J. Biomed. Mater. Research. Part A 2003, 64, 339–348. [Google Scholar] [CrossRef]
- Takadama, H.; Hashimoto, M.; Mizuno, M.; Kunio, I.; Kokubo, T. Newly Improved Simulated Body Fluid. Key Eng. Mater. 2004, 254–256, 115–118. [Google Scholar] [CrossRef]
- Zvicer, J.; Medic, A.; Veljovic, D.; Jevtic, S.; Novak, S.; Obradovic, B. Biomimetic characterization reveals enhancement of hydroxyapatite formation by fluid flow in gellan gum and bioactive glass composite scaffolds. Polym. Test. 2019, 76, 464–472. [Google Scholar] [CrossRef]
- Yu, H.-S.; Jang, J.-H.; Kim, T.-I.; Lee, H.-H.; Kim, H.-W. Apatite-mineralized polycaprolactone nanofibrous web as a bone tissue regeneration substrate. J. Biomed. Mater. Res. Part A 2009, 88A, 747–754. [Google Scholar] [CrossRef]
- Wang, D.; Christensen, K.; Chawla, K.; Xiao, G.; Krebsbach, P.H.; Franceschi, R.T. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J. Bone Miner. Res. 1999, 14, 893–903. [Google Scholar] [CrossRef]
- Belgheisi, G.; Nazarpak, M.H.; Hashjin, M.S. Bone tissue engineering electrospun scaffolds based on layered double hydroxides with the ability to release vitamin D3: Fabrication, characterization and in vitro study. Appl. Clay Sci. 2020, 185, 105434. [Google Scholar] [CrossRef]
- Andric, T.; Wright, L.D.; Freeman, J.W. Rapid Mineralization of Electrospun Scaffolds for Bone Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2011, 22, 1535–1550. [Google Scholar] [CrossRef]
- Fu, Q.W.; Zi, Y.P.; Xu, W.; Zhou, R.; Cai, Z.Y.; Zheng, W.J.; Chen, F.; Qian, Q.R. Electrospinning of calcium phosphate-poly (d,l-lactic acid) nanofibers for sustained release of water-soluble drug and fast mineralization. Int. J. Nanomed. 2016, 11, 5087–5097. [Google Scholar] [CrossRef] [Green Version]
- Boda, S.K.; Almoshari, Y.; Wang, H.; Wang, X.; Reinhardt, R.A.; Duan, B.; Wang, D.; Xie, J. Mineralized nanofiber segments coupled with calcium-binding BMP-2 peptides for alveolar bone regeneration. Acta Biomater. 2019, 85, 282–293. [Google Scholar] [CrossRef]
- Wang, D.; Jang, J.; Kim, K.; Kim, J.; Park, C.B. “Tree to Bone”: Lignin/Polycaprolactone Nanofibers for Hydroxyapatite Biomineralization. Biomacromolecules 2019, 20, 2684–2693. [Google Scholar] [CrossRef]
- Ataie, M.; Shabani, I.; Seyedjafari, E. Surface mineralized hybrid nanofibrous scaffolds based on poly(l-lactide) and alginate enhances osteogenic differentiation of stem cells. J. Biomed. Mater. Res. Part A 2019, 107, 586–596. [Google Scholar] [CrossRef]
- Nie, D.; Luo, Y.; Li, G.; Jin, J.; Yang, S.; Li, S.; Zhang, Y.; Dai, J.; Liu, R.; Zhang, W. The Construction of Multi-Incorporated Polylactic Composite Nanofibrous Scaffold for the Potential Applications in Bone Tissue Regeneration. Nanomaterials 2021, 11, 2402. [Google Scholar] [CrossRef]
- Rajzer, I.; Menaszek, E.; Kwiatkowski, R.; Chrzanowski, W. Bioactive nanocomposite PLDL/nano-hydroxyapatite electrospun membranes for bone tissue engineering. J. Mater. Sci. Mater. Med. 2014, 25, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Kanjwal, M.A.; Sheikh, F.A.; Nirmala, R.; Macossay, J.; Kim, H.Y. Fabrication of poly(caprolactone) nanofibers containing hydroxyapatite nanoparticles and their mineralization in a simulated body fluid. Fibers Polym. 2011, 12, 50–56. [Google Scholar] [CrossRef]
- Guo, Y.; Lan, J.; Zhang, C.; Cao, M.; Cai, Q.; Yang, X. Mineralization on polylactide/gelatin composite nanofibers using simulated body fluid containing amino acid. Appl. Surf. Sci. 2015, 349, 538–548. [Google Scholar] [CrossRef]
- Jie, Y.; Cai, Z.; Li, S.; Xie, Z.; Ma, M.; Huang, X. Hydroxyapatite nucleation and growth on collagen electrospun fibers controlled with different mineralization conditions and phosvitin. Macromol. Res. 2017, 25, 905–912. [Google Scholar] [CrossRef]
- Yilmaz, B.; Ağagündüz, D. Bioactivities of hen’s egg yolk phosvitin and its functional phosphopeptides in food industry and health. J. Food Sci. 2020, 85, 2969–2976. [Google Scholar] [CrossRef]
- Lao, L.; Zhu, Y.; Zhang, Y.; Gao, Z.; Zhou, F.; Chen, L.; Ouyang, H.; Gao, C. Mineralization of Collagen-Coated Electrospun Poly(lactide-co-glycolide) Nanofibrous Mesh to Enhance Growth and Differentiation of Osteoblasts and Bone Marrow Mesenchymal Stem Cells. Adv. Eng. Mater. 2012, 14, B123–B137. [Google Scholar] [CrossRef]
- Chahal, S.; Fathima, S.J.H.; Yusoff, M.B.M. Biomimetic growth of bone-like apatite via simulated body fluid on hydroxyethyl cellulose/polyvinyl alcohol electrospun nanofibers. Bio-Med. Mater. Eng. 2014, 24, 799–806. [Google Scholar] [CrossRef] [Green Version]
- Ercan, B.; Webster, T.J. Greater osteoblast proliferation on anodized nanotubular titanium upon electrical stimulation. Int. J. Nanomed. 2008, 3, 477–485. [Google Scholar] [CrossRef] [Green Version]
- Ferrier, J.; Ross, S.M.; Kanehisa, J.; Aubin, J.E. Osteoclasts and osteoblasts migrate in opposite directions in response to a constant electrical field. J. Cell. Physiol. 1986, 129, 283–288. [Google Scholar] [CrossRef]
- Samadian, H.; Mobasheri, H.; Hasanpour, S.; Ai, J.; Azamie, M.; Faridi-Majidi, R. Electro-conductive carbon nanofibers as the promising interfacial biomaterials for bone tissue engineering. J. Mol. Liq. 2020, 298, 112021. [Google Scholar] [CrossRef]
- Samadian, H.; Mobasheri, H.; Azami, M.; Faridi-Majidi, R. Osteoconductive and electroactive carbon nanofibers/hydroxyapatite nanocomposite tailored for bone tissue engineering: In vitro and in vivo studies. Sci. Rep. 2020, 10, 14853. [Google Scholar] [CrossRef] [PubMed]


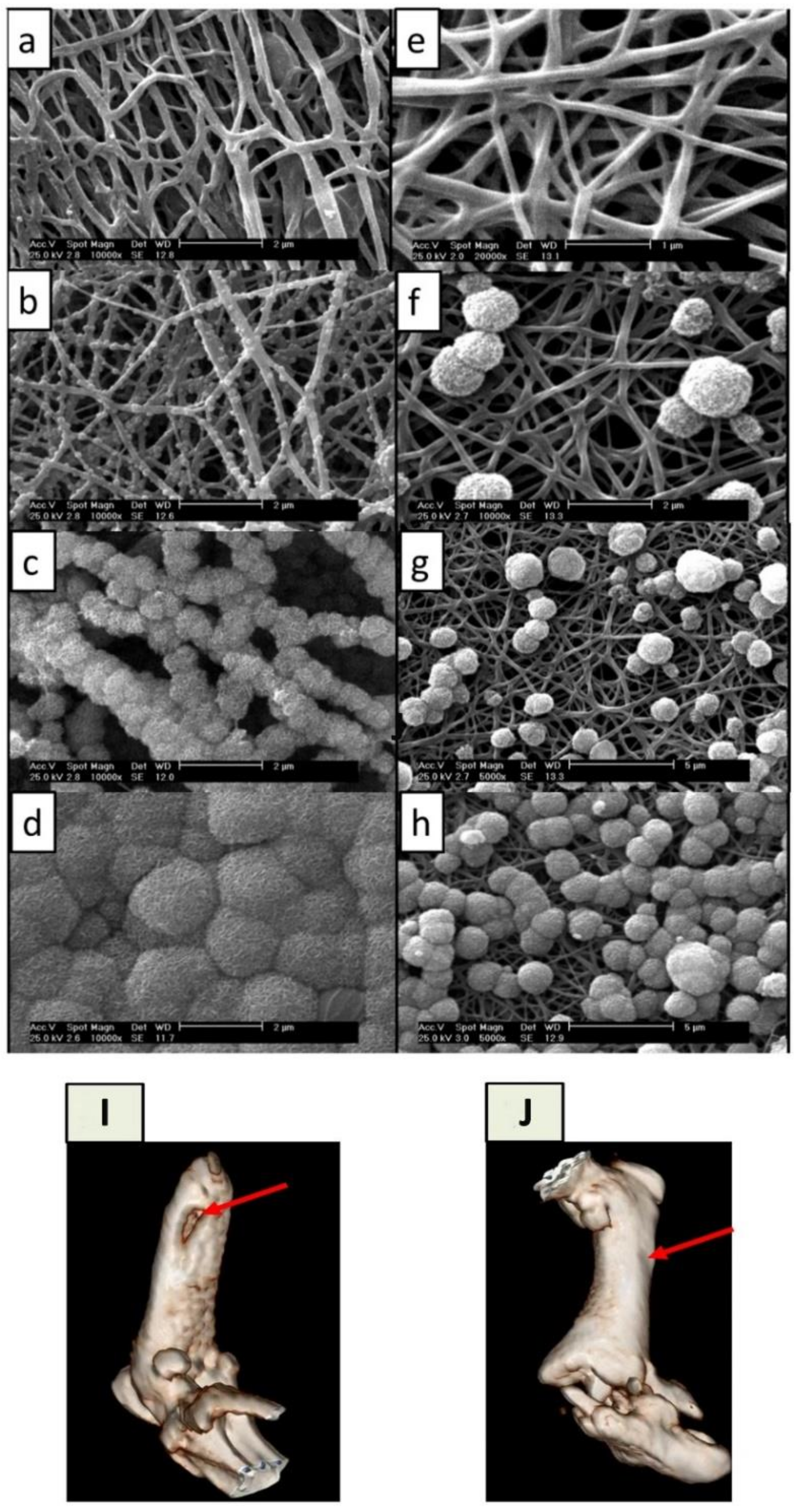
| Name | Fold-Change 2 | Descriptions | Purpose | Implications |
|---|---|---|---|---|
| modified SBF [37] | 1-fold | ions were incrementally supplemented. (5, 10, 15, 20, and 27 mM) | Addition of ions affected the composition and structure of formed calcium phosphates. | Under conditions lower than 20 mM, only B-type carbonated apatite precipitated, while 27 mM resulted in the formation of A-type carbonated apatite as well. |
| Selenate added 1.5× SBF [38] | 1.5-fold | 0.15 mM SeO42− ion was added, and ion concentration was increased to 1.5×. Subtractions: None | Incorporating Se into the bone-like apatite structure to obtain a coating with potential anti-cancer and anti-bacterial properties on the surface of Ti6Al4V. | Adding 0.15 mM selenate ion did not yield secondary calcium phosphate phases other than HA. Se was shown to inhibit the proliferation of osteosarcoma cells without affecting the proliferation of normal bone cells in vitro. The coating was also shown to inhibit the growth of Staphylococcus epidermidis. |
| Modified SBF [39] | 2-fold | Concentrations of CaCl2 and KH2PO4 were doubled. Subtractions: None | Deposition of CaP 4 onto electrospun chitosan and polyvinyl alcohol (PVA) fibers | Spherical CaP crystallites (average diameter of 350 nm) with nano-sized β-TCP 5 crystalline plates with low crystallinity formed on the fibers starting from the first day. |
| Modified SBF [40] | 2-fold | Concentrations of CaCl2 and KH2PO4 were doubled. Subtractions: None | Deposition of CaP on chitosan substrates, which were prepared by spin coating of chitosan on Ti | Mg ion-incorporated bone-like apatite was synthesized by incubating the chitosan-coated Ti in m-SBF. |
| 10× SBF [41] | 10-fold | Ion concentration was increased to 10×. Subtractions: and SO42− ions were omitted. No buffering agent was used. | The formation of HA 3 onto gelatin-siloxane microspheres was fabricated via a single emulsion method in modified 10× SBF solution using microwave energy (600 W). | The homogeneity and speed of mineralization increased in 10× SBF solution with the microwave-assisted method, compared to the conventional coating systems. Biomimetic monodispersed HA exhibited nanoscale morphology and good cytocompatibility with human osteosarcoma cell lines (MG-63). |
| Boron added SBF (B-SBF) [42] | 10-fold | 5–17 mg boric acid (H3BO3) was added, and the ion concentration was increased to 10×. Subtractions: and SO42− ions were omitted. No buffering agent was used. | Producing biomimetic boron-doped HA with the support of microwave for coating tissue scaffolds | Freeze-dried chitosan tissue scaffolds were coated with boron-doped HA via the microwave-assisted biomimetic process. No buffers were used in the preparation of 10× SBF. The addition of boron did not alter the crystallinity of HA. |
| ES Modes | Advantages | Limitations | Recent Examples |
|---|---|---|---|
| Monoaxial | Simple installation Easy to operate | Random patterns Lack of tensile strength | Regenerated cellulose non-woven electrospun scaffolds [92] HA-embedded poly(3-hydroxybutyric acid-co-3-hydrovaleric acid) (PHBV) random nanofibers [93] |
| Melt | Three-dimensional structure Larger pore size Diverse diameter range Eco-friendly method | Expensive setup Mostly amorphous fibers and thermal degradation | Multilayered PCL/ gelatin scaffolds (through both monoaxial and melt modes) [94] |
| Aligned | Aligned structure Guided oriented arrangement and elongation of cells Decreased size in diameter Good mechanical properties | Complex setup Clogging or jet instability | Aligned poly (L-lactic acids) (PLLA) nanofibers [95] Aligned nano-HA-incorporated poly(D,L-lactide-co-glycolide) (PLGA) electrospun scaffolds [96] |
| Multi-Axial | Core-shell structure Versatility and flexibility for functional scaffolds | Complex setup Difficult material selection and fabrication | Coaxial poly (3-hydroxybutyrate-co-4-hydroxybutyrate)/poly (vinyl alcohol) (P34HB/PVA) nanofibers [97] Triaxially in-situ calcium phosphate fabrication in gelatin electrospun nanofibers [98] |
| Ions (mM) | Blood Plasma | ||||||
|---|---|---|---|---|---|---|---|
| Total | Dissociated | c-SBF | r-SBF | i-SBF | m-SBF | n-SBF | |
| Na+ | 142.0 | 142.0 | 142.0 | 142.0 | 142.0 | 142.0 | 142.0 |
| K+ | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Mg2+ | 1.5 | 1.0 | 1.5 | 1.5 | 1.0 | 1.5 | 1.5 |
| Ca2+ | 2.5 | 1.3 | 2.5 | 2.5 | 1.6 | 2.5 | 2.5 |
| Cl− | 103.0 | 103.0 | 147.8 | 103.0 | 103.0 | 103.0 | 103.0 |
| HCO3− | 27.0 | 27.0 | 4.2 | 27.0 | 27.0 | 10.0 | 4.2 |
| HPO42− | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| SO42− | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, D.-J. Bone Mineralization in Electrospun-Based Bone Tissue Engineering. Polymers 2022, 14, 2123. https://doi.org/10.3390/polym14102123
Lim D-J. Bone Mineralization in Electrospun-Based Bone Tissue Engineering. Polymers. 2022; 14(10):2123. https://doi.org/10.3390/polym14102123
Chicago/Turabian StyleLim, Dong-Jin. 2022. "Bone Mineralization in Electrospun-Based Bone Tissue Engineering" Polymers 14, no. 10: 2123. https://doi.org/10.3390/polym14102123
APA StyleLim, D.-J. (2022). Bone Mineralization in Electrospun-Based Bone Tissue Engineering. Polymers, 14(10), 2123. https://doi.org/10.3390/polym14102123





