The Use of Waste Hazelnut Shells as a Reinforcement in the Development of Green Biocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
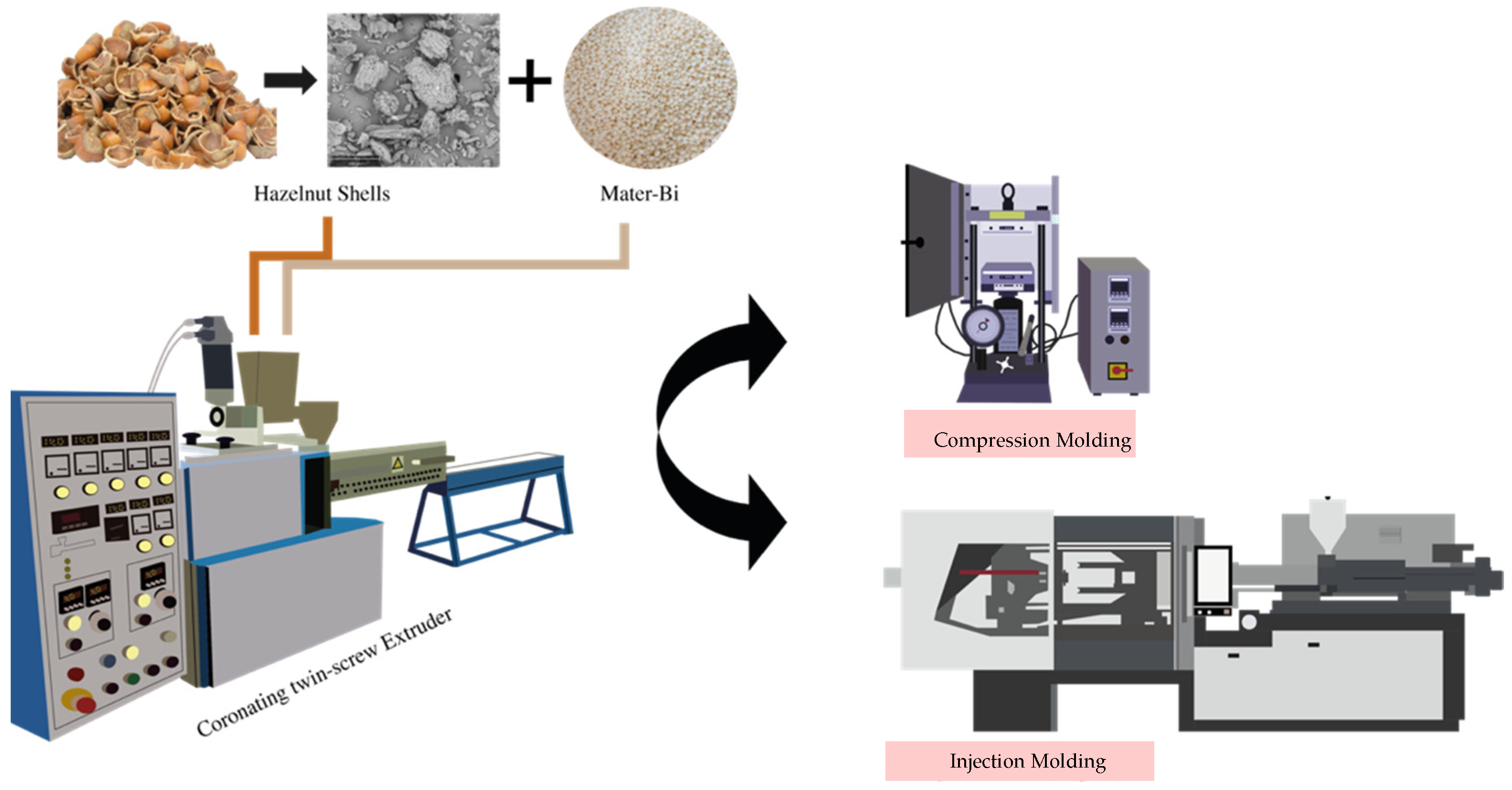
2.2. Manufacturing of Biocomposites
2.2.1. Compression Molding (CM)
2.2.2. Injection Molding (IM)
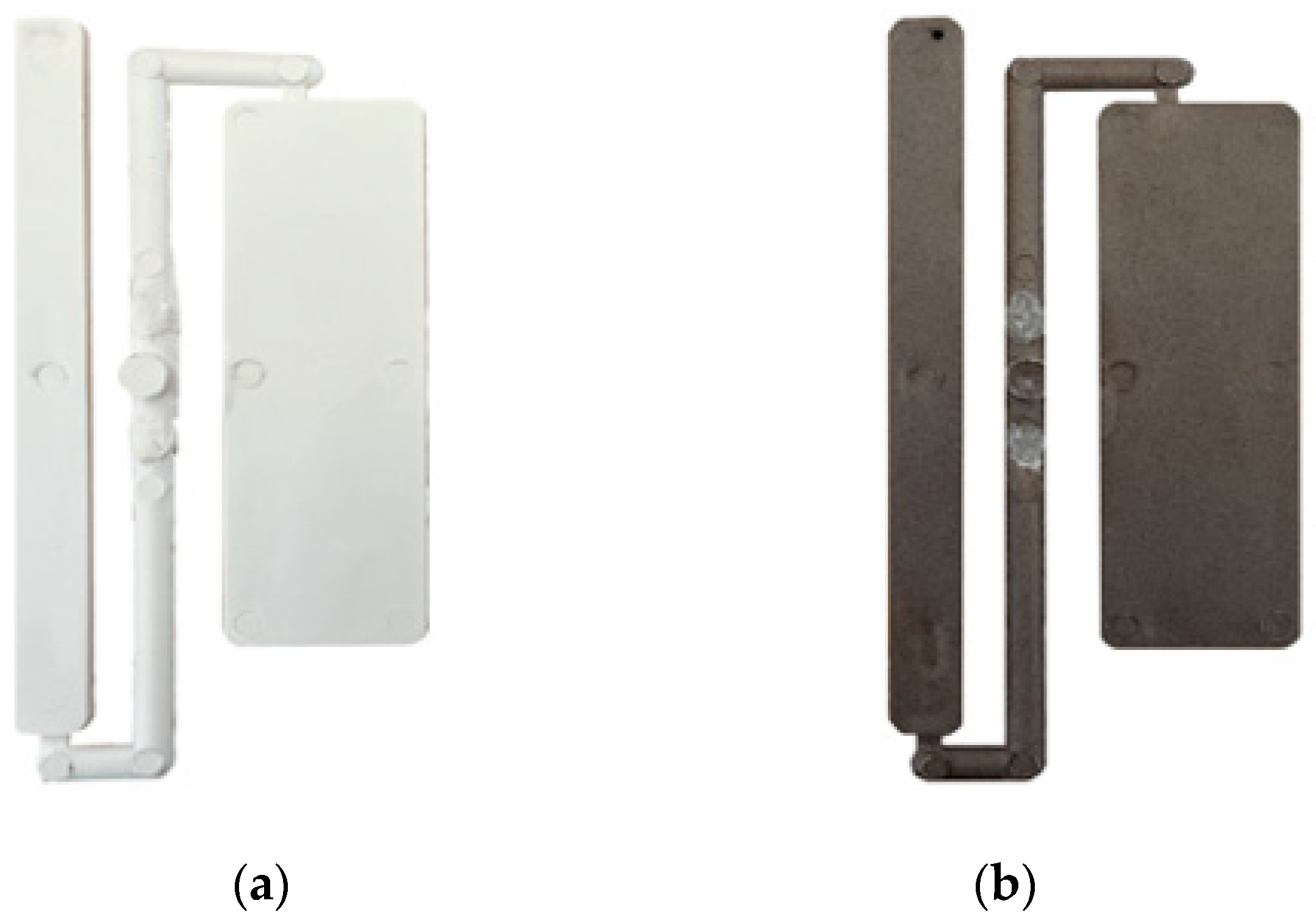
2.3. Characterization of Biocomposites
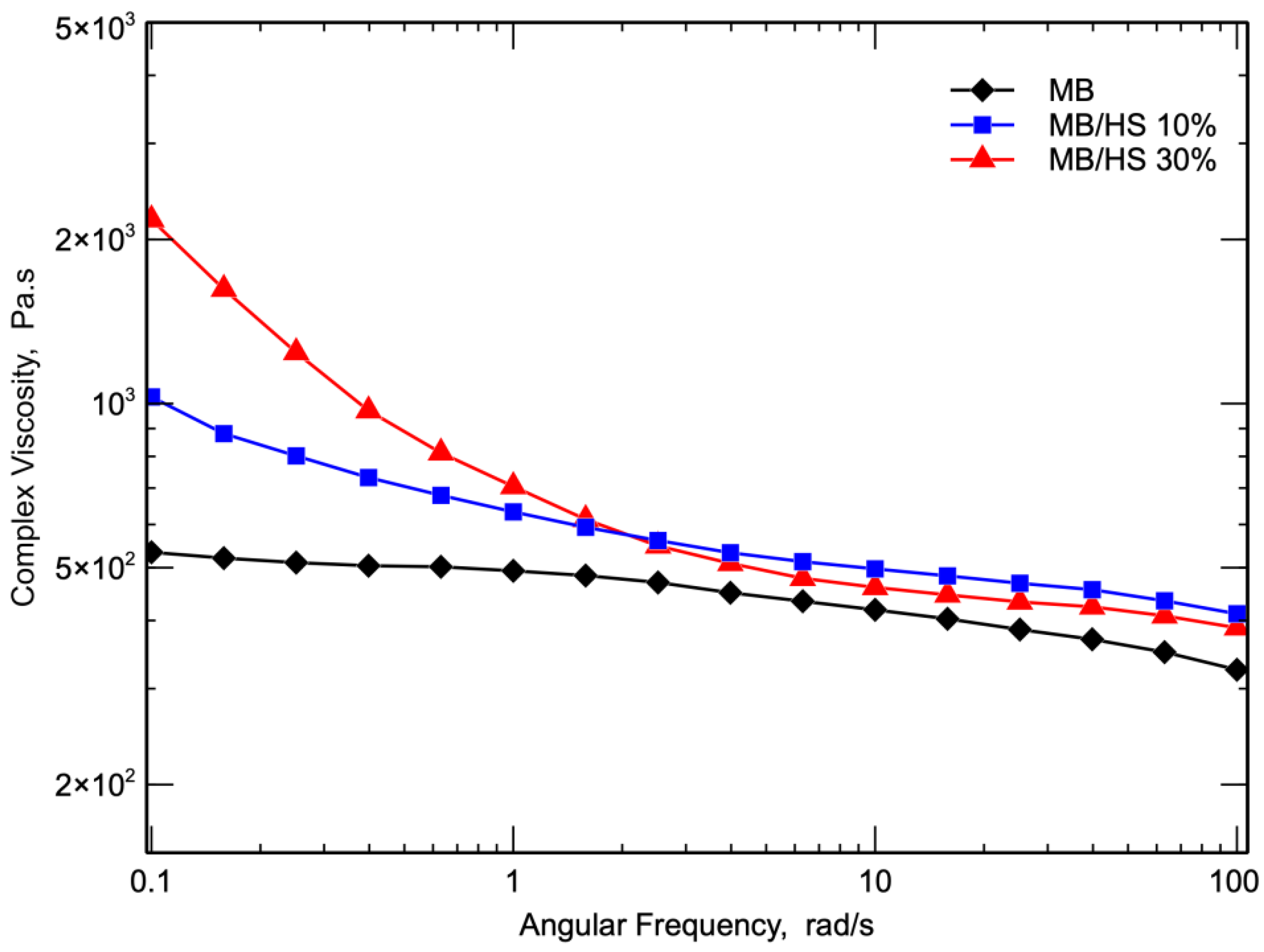
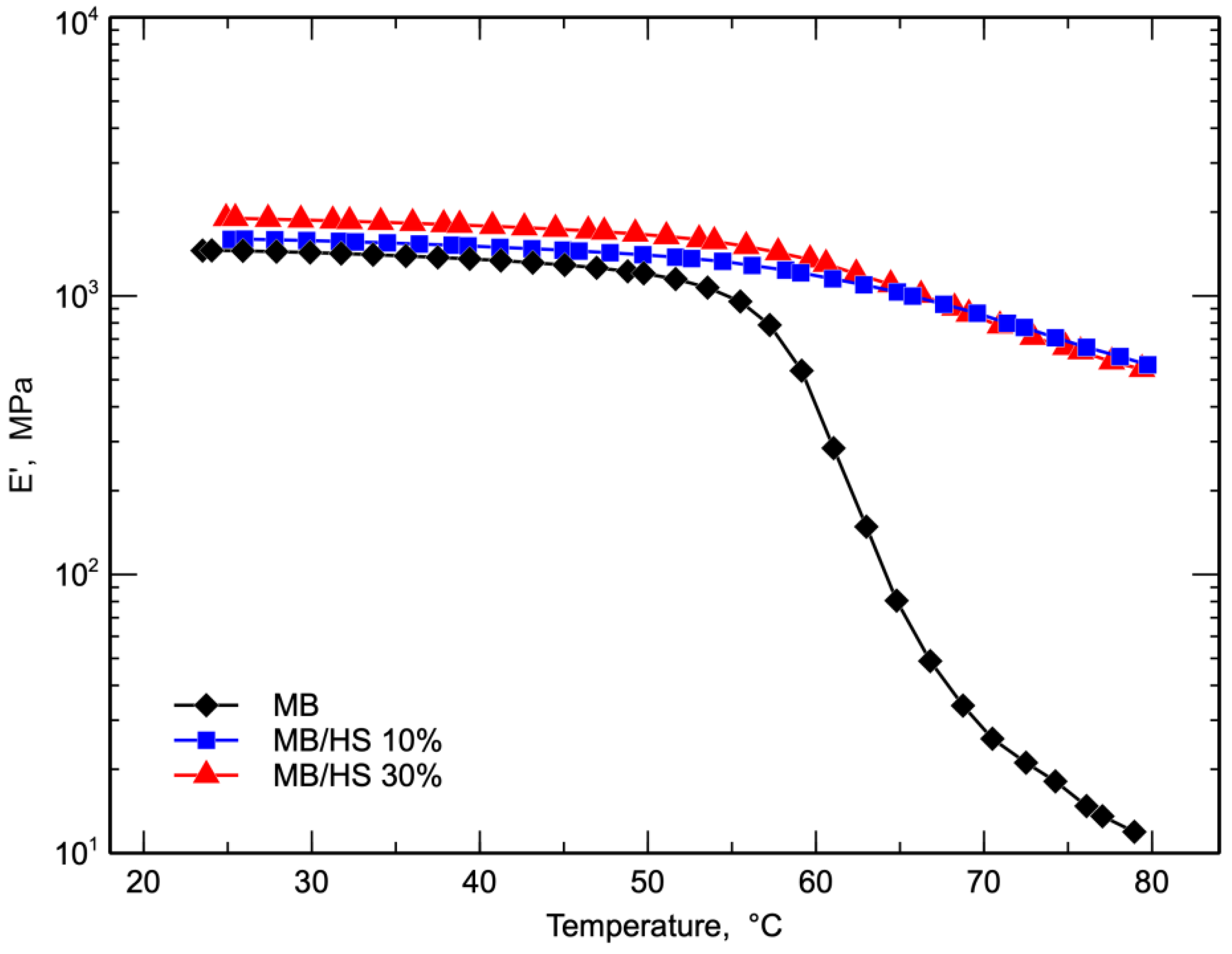
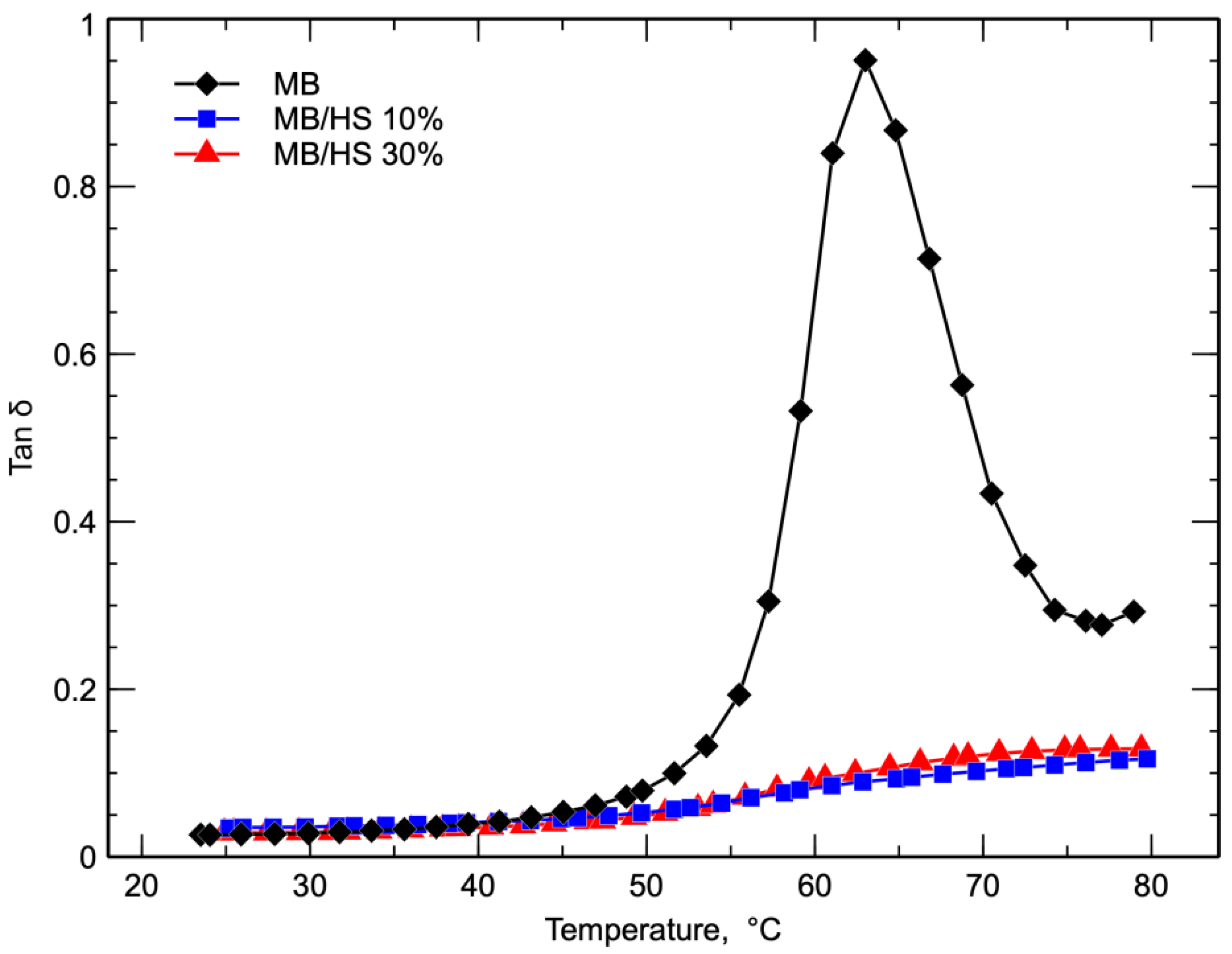
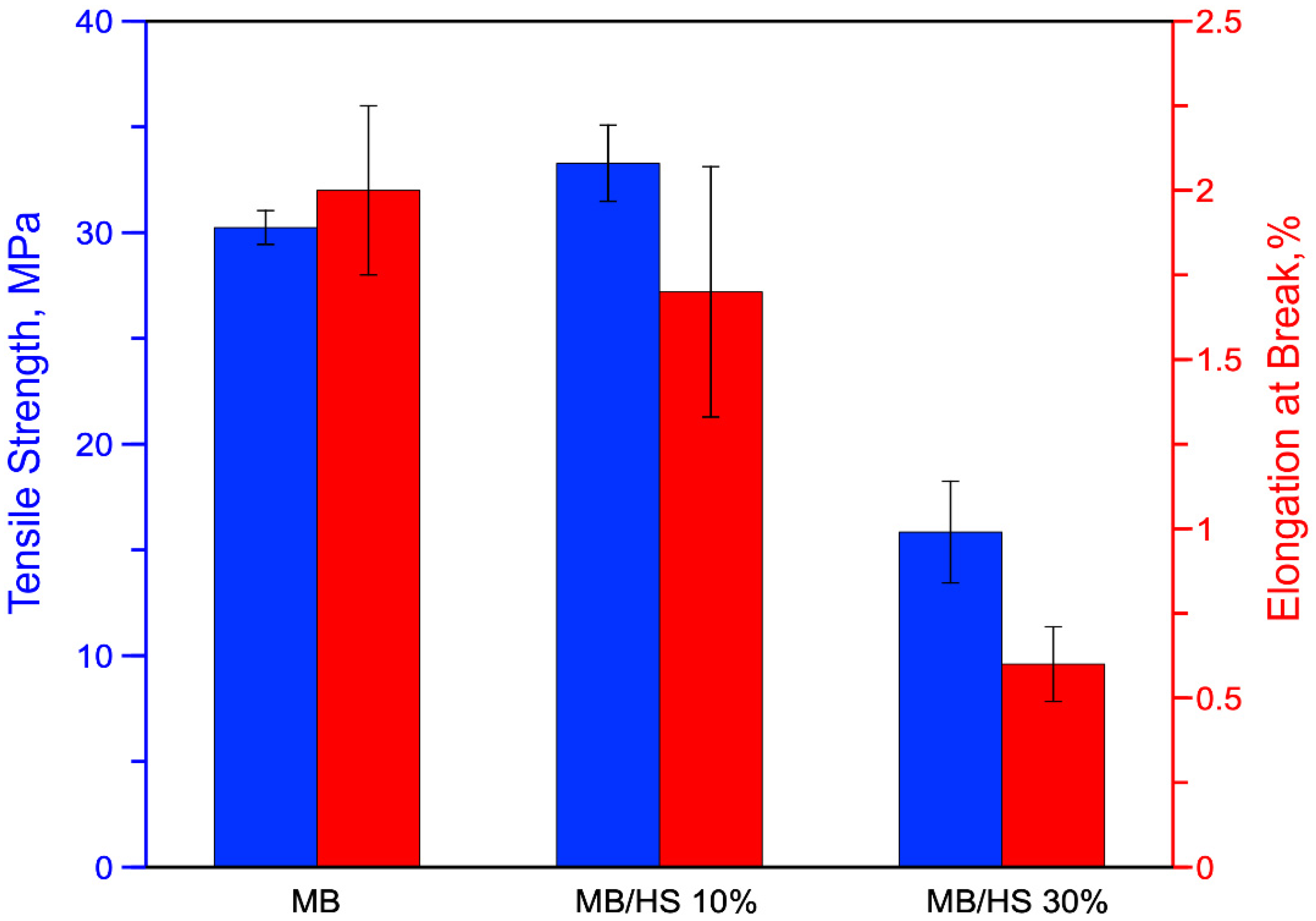
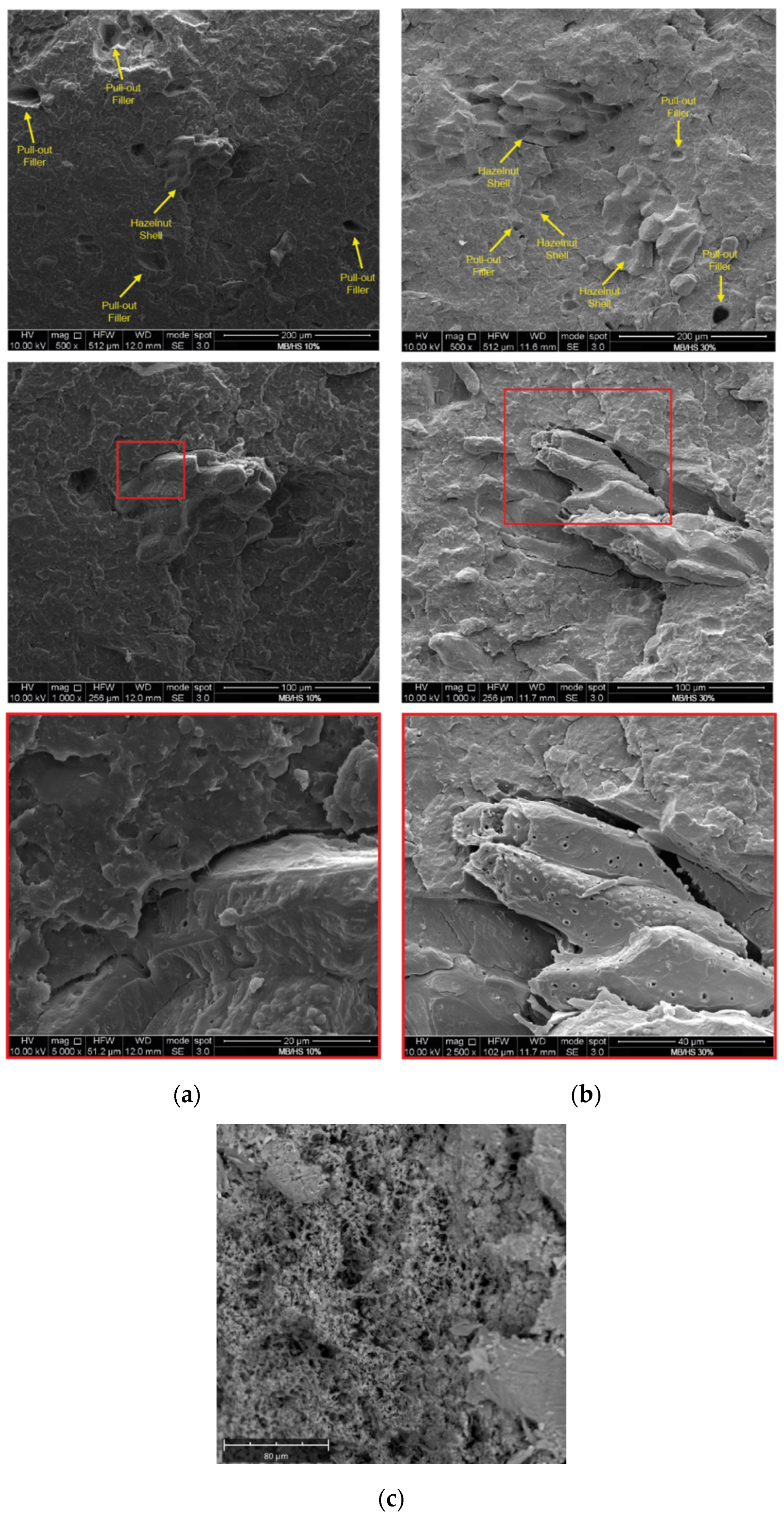
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.C.; Moore, C.J.; Saal, F.S.V.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- The New Plastics Economy: Rethinking the Future of Plastics. Available online: https://ellenmacarthurfoundation.org/the-new-plastics-economy-rethinking-the-future-of-plastics (accessed on 28 December 2021).
- Briassoulis, D.; Pikasi, A.; Hiskakis, M. Recirculation potential of post-consumer/industrial bio-based plastics through mechanical recycling—Techno-economic sustainability criteria and indicators. Polym. Degrad. Stab. 2021, 183, 109217. [Google Scholar] [CrossRef]
- Winterstetter, A.; Grodent, M.; Kini, V.; Ragaert, K.; Vrancken, K.C. A review of technological solutions to prevent or reduce marine plastic litter in developing countries. Sustainability 2021, 13, 4894. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament: The Council, The European Economic and Social Committee and the Committee of the Regions—A European Strategy for Plastics in a Circular Economy. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2018%3A28%3AFIN (accessed on 28 December 2021).
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Huang, W.; Wen, X.; Tang, W. Are biodegradable plastics a promising solution to solve the global plastic pollution? Environ. Pollut. 2020, 263, 114469. [Google Scholar] [CrossRef]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of Biodegradable Polymers in Food Packaging Industry: A Comprehensive Review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Thakur, M.; Majid, I.; Hussain, S.; Nanda, V. Poly(ε-caprolactone): A potential polymer for biodegradable food packaging applications. Packag. Technol. Sci. 2021, 34, 449–461. [Google Scholar] [CrossRef]
- Rydz, J.; Musiol, M.; Zawidlak-Wegrzyńska, B.; Sikorska, W. Present and Future of Biodegradable Polymers for Food Packaging Applications. In Biopolymers for Food Design; Academic Press: Bucharest, Romania, 2018. [Google Scholar]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour. Technol. 2021, 325, 124739. [Google Scholar] [CrossRef]
- Lengalova, A.; Vesel, A.; Feng, Y.; Sencadas, V. Biodegradable Polymers for Medical Applications. Int. J. Polym. Sci. 2016, 2016, 6047284. [Google Scholar] [CrossRef]
- Feig, V.R.; Tran, H.; Bao, Z. Biodegradable Polymeric Materials in Degradable Electronic Devices. ACS Cent. Sci. 2018, 4, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Jin, F.L.; Hu, R.R.; Park, S.J. Improvement of thermal behaviors of biodegradable poly(lactic acid) polymer: A review. Compos. Part B Eng. 2019, 164, 287–296. [Google Scholar] [CrossRef]
- Villegas, C.; Arrieta, M.P.; Rojas, A.; Torres, A.; Faba, S.; Toledo, M.J.; Gutierrez, M.A.; Zavalla, E.; Romero, J.; Galotto, M.J.; et al. PLA/organoclay bionanocomposites impregnated with thymol and cinnamaldehyde by supercritical impregnation for active and sustainable food packaging. Compos. Part B Eng. 2019, 176, 107336. [Google Scholar] [CrossRef]
- Wang, R.; Sun, X.; Chen, L.; Liang, W. Morphological and mechanical properties of biodegradable poly(glycolic acid)/poly(butylene adipate-co-terephthalate) blends with in situ compatibilization. RSC Adv. 2021, 11, 1241–1249. [Google Scholar] [CrossRef]
- de La Rosa-Ramírez, H.; Aldas, M.; Ferri, J.M.; López-Martínez, J.; Samper, M.D. Modification of poly (lactic acid) through the incorporation of gum rosin and gum rosin derivative: Mechanical performance and hydrophobicity. J. Appl. Polym. Sci. 2020, 137, 44. [Google Scholar] [CrossRef]
- Fiore, V.; Botta, L.; Scaffaro, R.; Valenza, A.; Pirrotta, A. PLA based biocomposites reinforced with Arundo donax fillers. Compos. Sci. Technol. 2014, 105, 110–117. [Google Scholar] [CrossRef]
- Botta, L.; Teresi, R.; Titone, V.; Salvaggio, G.; la Mantia, F.P.; Lopresti, F. Use of biochar as filler for biocomposite blown films: Structure-processing-properties relationships. Polymers 2021, 13, 3953. [Google Scholar] [CrossRef]
- Botta, L.; Titone, V.; Mistretta, M.C.; La Mantia, F.P.; Modica, A.; Bruno, M.; Sottile, F.; Lopresti, F. PBAT based composites reinforced with microcrystalline cellulose obtained from softwood almond shells. Polymers 2021, 13, 16. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Han, L.; Wang, B.; Xu, H.; Zhong, Y.; Zhang, L.; Mao, Z.; Sui, X. Biodegradable regenerated cellulose-dispersed composites with improved properties via a pickering emulsion process. Carbohydr. Polym. 2018, 179, 86–92. [Google Scholar] [CrossRef]
- Priyodip, P.; Balaji, S.; Kini, M.V. Physio-chemico-thermo-mechanical properties of selected biodegradable polymers. Green Mater. 2013, 1, 191–200. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; Galgano, A. Biomass screening for the production of furfural via thermal decomposition. Ind. Eng. Chem. Res. 2010, 49, 2658–2671. [Google Scholar] [CrossRef]
- Demirbas, A. Furfural production from fruit shells by acid-catalyzed hydrolysis. Energy Sources Part A 2006, 28, 157–165. [Google Scholar] [CrossRef]
- García, A.I.; García, A.M.; Bou, S.F. Study of the influence of the almond shell variety onthe mechanical properties of starch-based polymer biocomposites. Polymers 2020, 12, 2049. [Google Scholar] [CrossRef] [PubMed]
- La Mantia, F.P.; TzankovaDintcheva, N.; Morreale, M.; Vaca-Garcia, C. Green composites of organic materials and recycled post-consumer polyethylene. Polym. Int. 2004, 53, 1888–1891. [Google Scholar] [CrossRef] [Green Version]
- Balart, J.F.; García-Sanoguera, D.; Balart, R.; Boronat, T.; Sánchez-Nacher, L. Manufacturing and properties of biobased thermoplastic composites from poly(lactid acid) and hazelnut shell wastes. Polym. Compos. 2018, 39, 848–857. [Google Scholar] [CrossRef]
- Balart, J.F.; Fombuena, V.; Fenollar, O.; Boronat, T.; Sánchez-Nacher, L. Processing and characterization of high environmental efficiency composites based on PLA and hazelnut shell flour (HSF) with biobased plasticizers derived from epoxidized linseed oil (ELO). Compos. Part B Eng. 2016, 86, 168–177. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Bonacchi, D.; Coltelli, M.B.; Lazzeri, A. Analysis, development, and scaling-up of poly(Lactic)acid (PLA) biocomposites with hazelnuts shell powder (HSP). Polymers 2021, 13, 4080. [Google Scholar] [CrossRef]
- Pradhan, P.; Satapathy, A. Physico-mechanical characterization and thermal property evaluation of polyester composites filled with walnut shell powder. Polym. Polym. Compos. 2022, 30, 09673911221077808. [Google Scholar] [CrossRef]
- Kufel, A.; Kuciel, S. Hybrid composites based on polypropylene with basalt/hazelnut shell fillers: The influence of temperature, thermal aging, and water absorption on mechanical properties. Polymers 2020, 12, 18. [Google Scholar] [CrossRef] [Green Version]
- Bastioli, C. Properties and applications of mater-Bi starch-based materials. Polym. Degrad. Stab. 1998, 59, 263–272. [Google Scholar] [CrossRef]
- Titone, V.; Correnti, A.; la Mantia, F.P. Effect of moisture content on the processing and mechanical properties of a biodegradable polyester. Polymers 2021, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Mistretta, M.C.; Botta, L.; Morreale, M.; Rifici, S.; Ceraulo, M.; la Mantia, F.P. Injection molding and mechanical properties of bio-based polymer nanocomposites. Materials 2018, 11, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ASTM-D638-14; Standard Test Method for Tensile Properties of Plastics. ASTM: Philadelphia, PA, USA, 2014.
- ASTM D 256-02; Standard Test Methods for Determining the Izod Pendulum Impact Resistance ASTM. ASTM: Philadelphia, PA, USA, 2002.
- Perinović, S.; Andriić, B.; Erceg, M. Thermal properties of poly(l-lactide)/olive stone flour composites. Thermochim. Acta 2010, 510, 97–102. [Google Scholar] [CrossRef]
- Salazar-Cruz, B.A.; Chávez-Cinco, M.Y.; Morales-Cepeda, A.B.; Ramos-Galván, C.E.; Rivera-Armenta, J.L. Evaluation of Thermal Properties of Composites Prepared from Pistachio Shell Particles Treated Chemically and Polypropylene. Molecules 2022, 27, 426. [Google Scholar] [CrossRef]
- Herschel, W.H.; Bulkley, R. Konsistenzmessungen von Gummi-Benzollösungen. Kolloid-Z. 1926, 39, 291–300. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Botta, L.; Mistretta, M.C.; Fiore A di Titone, V. Recycling of a biodegradablepolymer blend. Polymers 2020, 12, 2297. [Google Scholar] [CrossRef]
- Bek, M.; Gonzalez-Gutierrez, J.; Kukla, C.; Črešnar, K.P.; Maroh, B.; Perše, L.S. Rheological behaviour of highly filled materials for injection moulding and additive manufacturing: Effect of particle material and loading. Appl. Sci. 2020, 10, 7993. [Google Scholar] [CrossRef]
- Kader, M.A.; Bhowmick, A.K. Effect of Filler on the Mechanical, Dynamic Mechanical, and Aging Properties of Binary and Ternary Blends of Acrylic Rubber, Fluorocarbon Rubber, and Polyacrylate. J. Appl. Polym. Sci. 2003, 90, 278–286. [Google Scholar] [CrossRef]




| Polymer | Density, g/cm3 | MFR, g/10 min (2.16 kg at 230 °C) | Melting Point, °C |
|---|---|---|---|
| Mater-Bi® El51N0 | 1.23 | 19 | 167 |
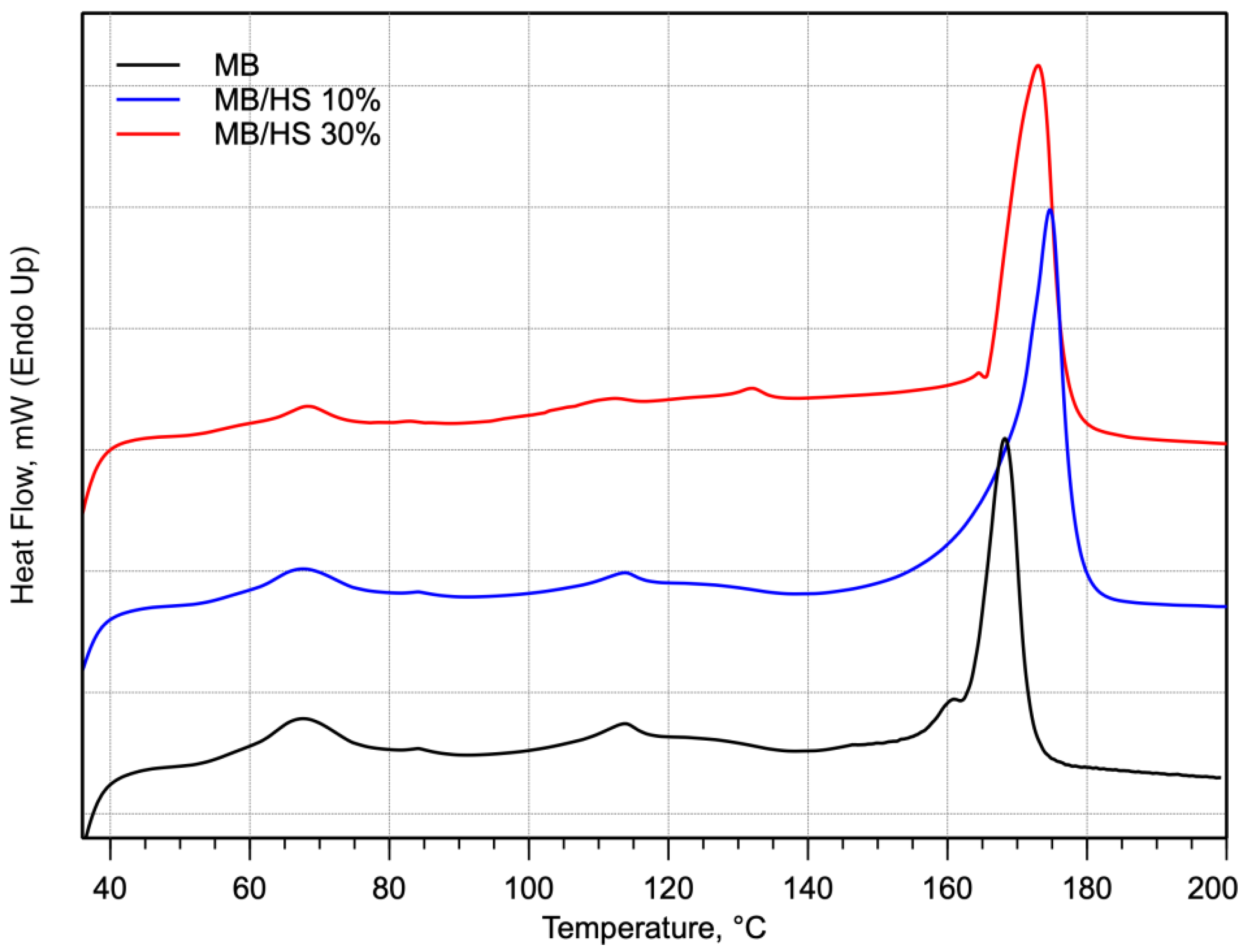
| Sample Name | Tm, °C | ΔHm(J/g) |
|---|---|---|
| MB | 168.5 | 27.1 |
| MB/HS 10% | 175.2 | 31.5 |
| MB/HS 30% | 173.1 | 39.8 |
| Sample Name | Elastic Modulus, MPa | Tensile Strength, MPa | Elongation at Break, % |
|---|---|---|---|
| MB (CM) | |||
| MB/HS 10% (CM) | |||
| MB (IM) | |||
| MB/HS 10% (IM) |
| Property | MB | MB/HS 10% |
|---|---|---|
| Impact strength, KJ/m2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceraulo, M.; La Mantia, F.P.; Mistretta, M.C.; Titone, V. The Use of Waste Hazelnut Shells as a Reinforcement in the Development of Green Biocomposites. Polymers 2022, 14, 2151. https://doi.org/10.3390/polym14112151
Ceraulo M, La Mantia FP, Mistretta MC, Titone V. The Use of Waste Hazelnut Shells as a Reinforcement in the Development of Green Biocomposites. Polymers. 2022; 14(11):2151. https://doi.org/10.3390/polym14112151
Chicago/Turabian StyleCeraulo, Manuela, Francesco Paolo La Mantia, Maria Chiara Mistretta, and Vincenzo Titone. 2022. "The Use of Waste Hazelnut Shells as a Reinforcement in the Development of Green Biocomposites" Polymers 14, no. 11: 2151. https://doi.org/10.3390/polym14112151
APA StyleCeraulo, M., La Mantia, F. P., Mistretta, M. C., & Titone, V. (2022). The Use of Waste Hazelnut Shells as a Reinforcement in the Development of Green Biocomposites. Polymers, 14(11), 2151. https://doi.org/10.3390/polym14112151









