Application Status of Sacrificial Biomaterials in 3D Bioprinting
Abstract
:1. Introduction
2. Sacrificial Biomaterials Based on Physical Principles
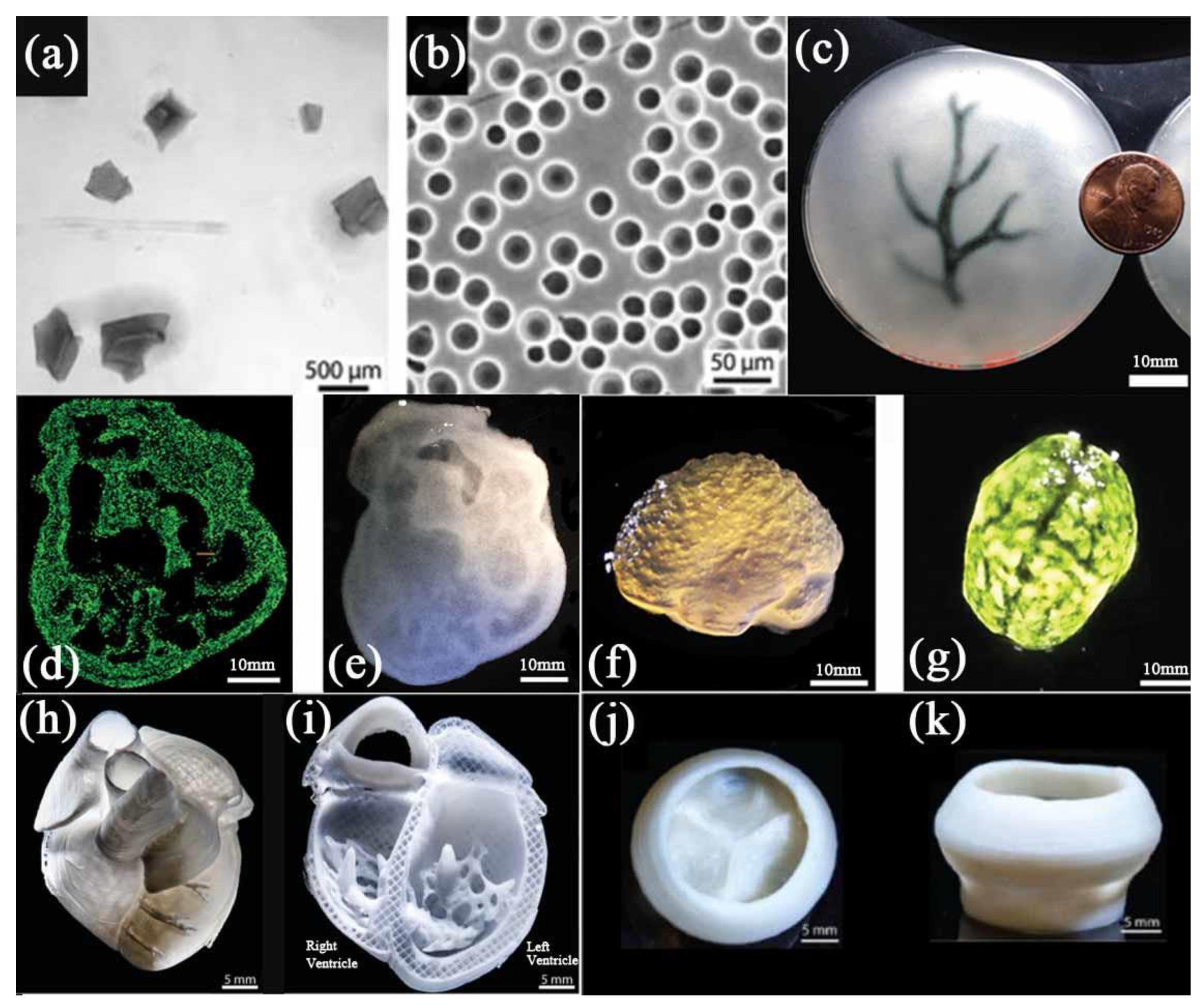
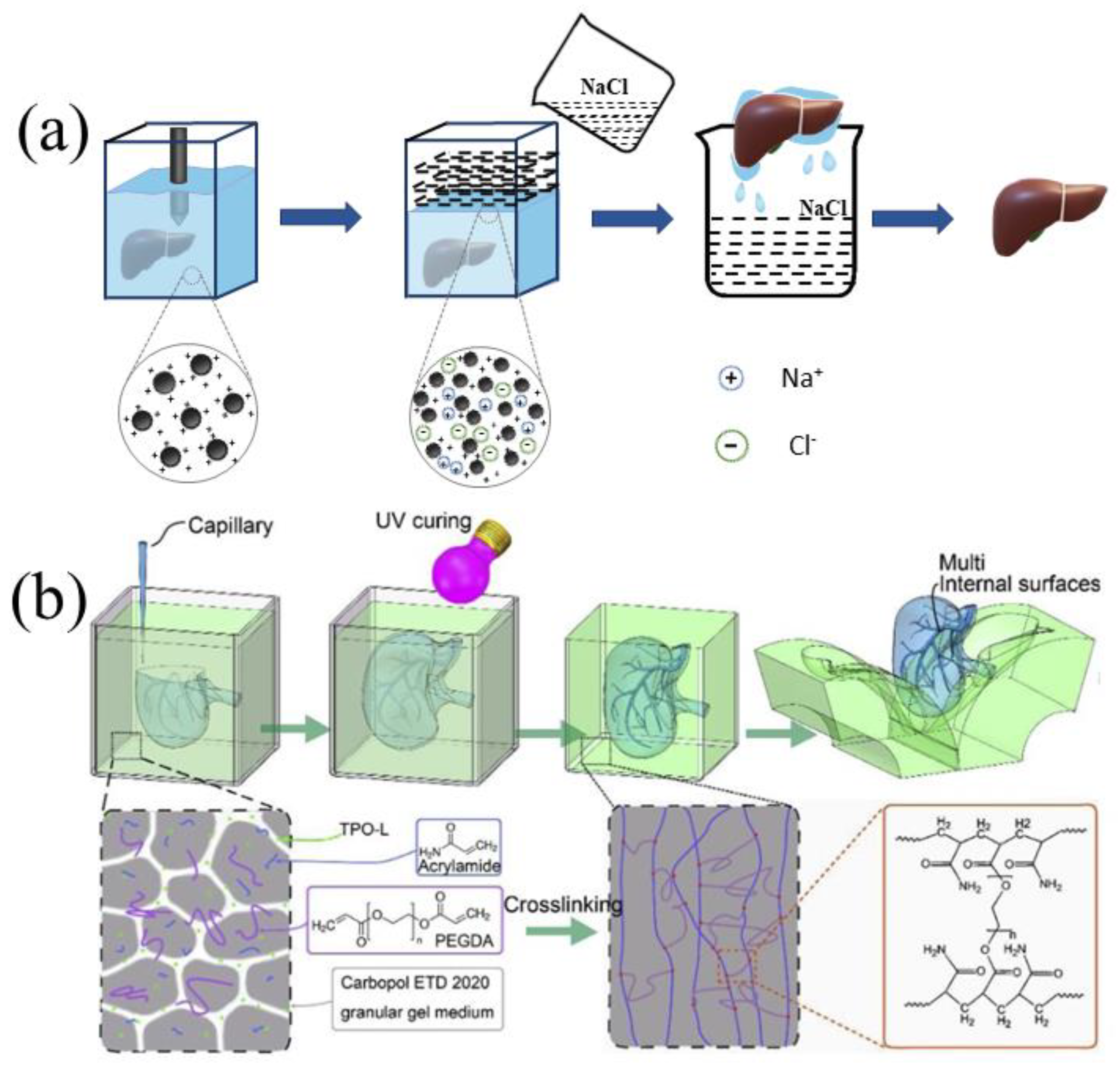
2.1. Polyvinyl Alcohol
2.2. Pluronic F127
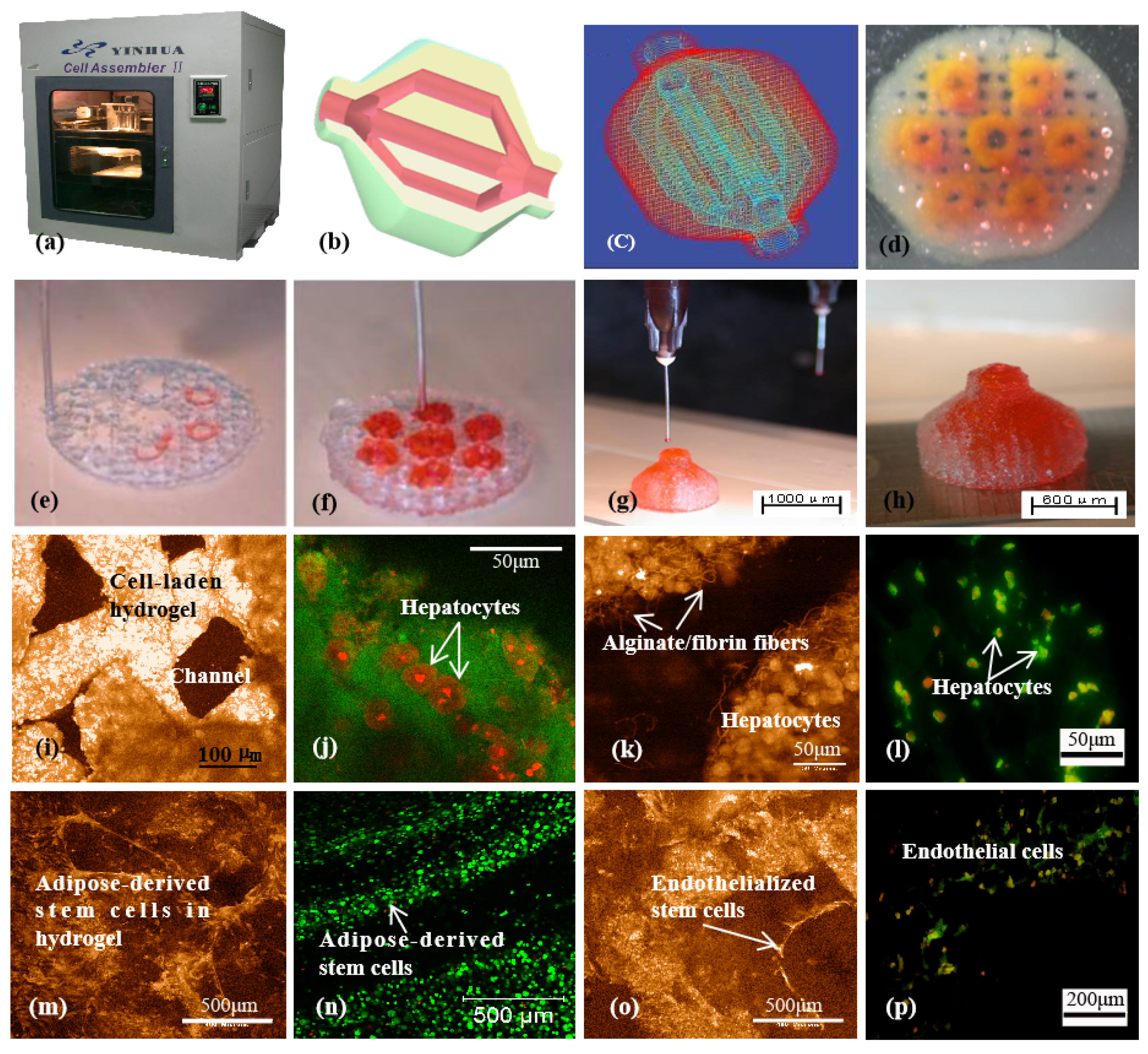
2.3. Gelatin Microgel
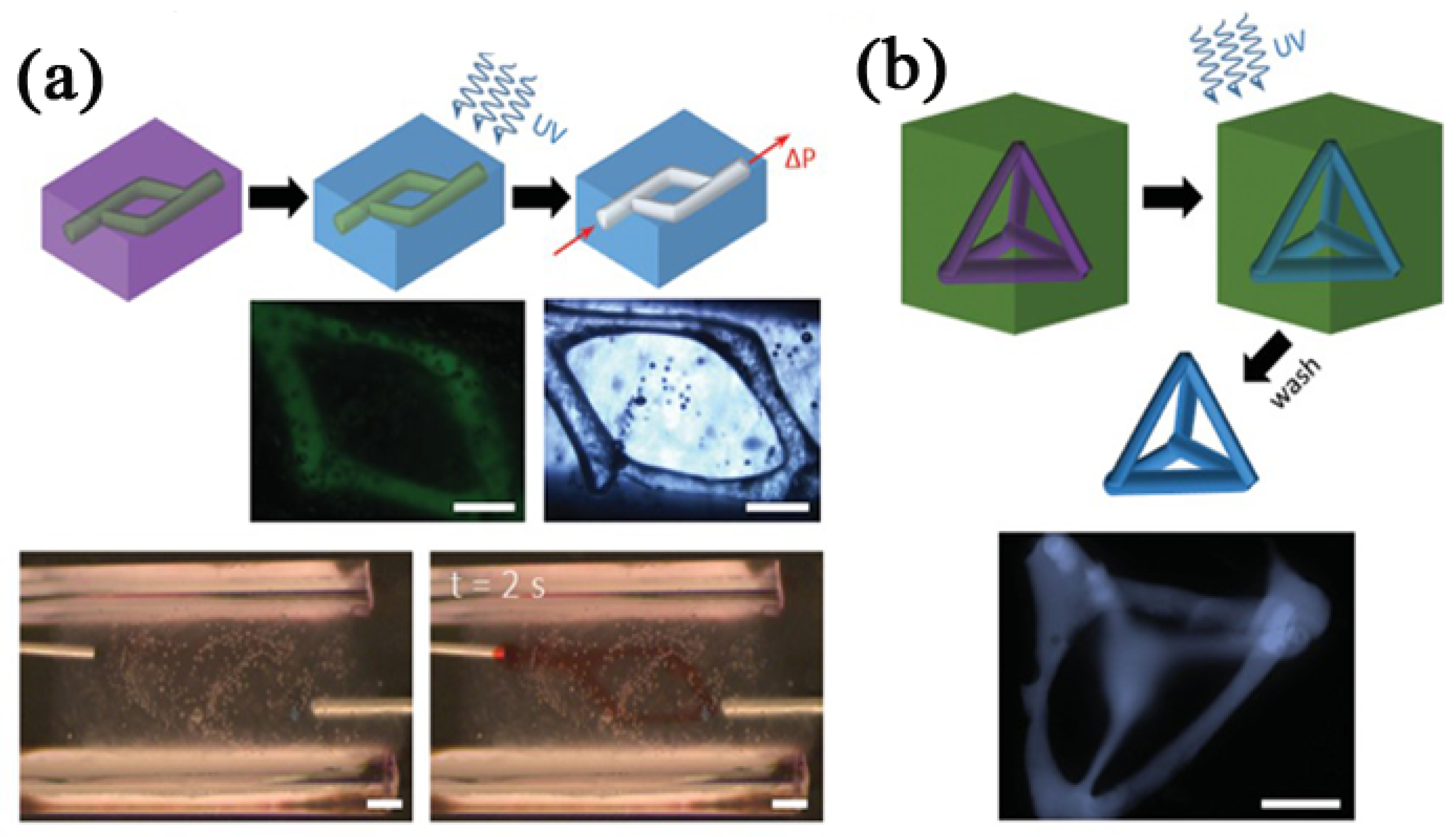
2.4. Carbopol
3. Sacrificial Biomaterials Based on Chemical Principles
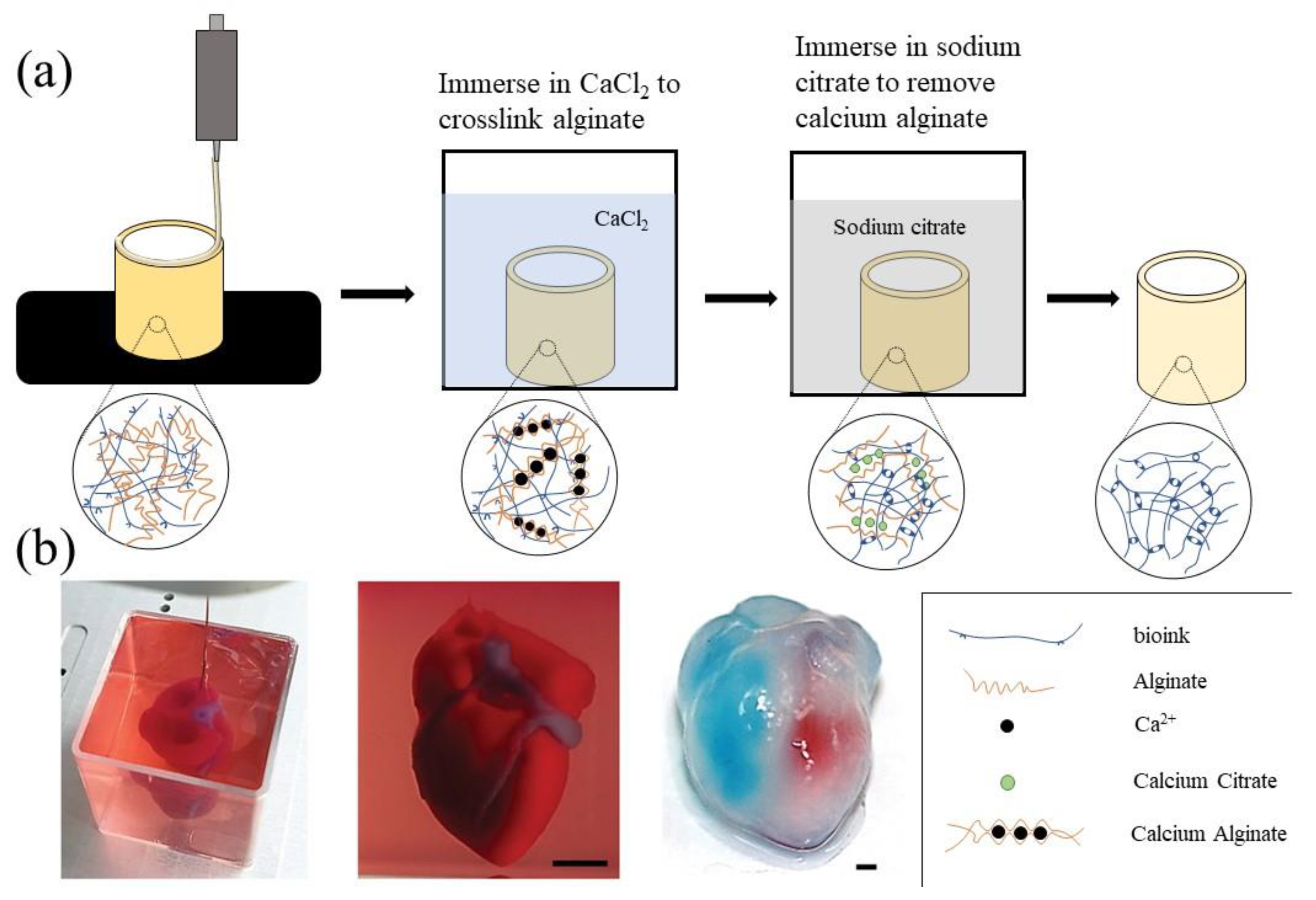
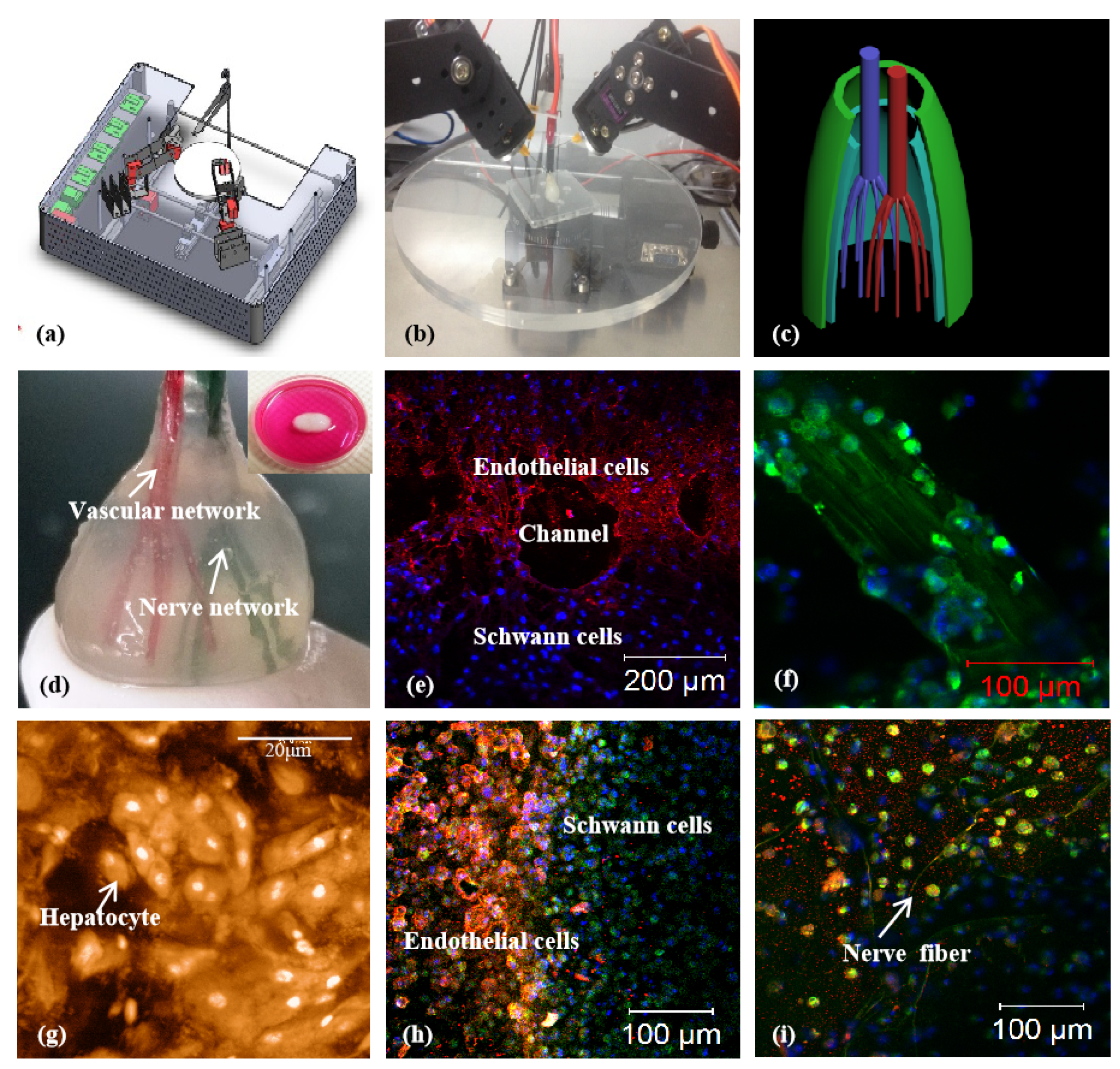
3.1. Alginate
3.2. Modified Hyaluronic Acid
| Biomaterials | Principle | Bioprinting Method | Advantage | Deficiency | Application | References |
|---|---|---|---|---|---|---|
| PVA | Physical | Fused deposition modeling | Biocompat ibility; water soluble | High printing temperature; not bioactive | Microtubule network | [56,65,66,67,68,69,70,71,72] |
| Pluronic F127 | Physical | Extrusion 3D printing | Bio-friendly; easy to remove; shear-thinning | Not bioactive | Microtubule network | [9,16,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95] |
| Gelatin | Physical | Extrusion 3D printing | bio-friendly; easy to remove; yield stress fluid behavior | Complex manufact uring process | Suspension medium/ increased porosity/sup port ing structure | [38,45,96,97,98,99,100,101,102,103,104,105,106,107,108] |
| Carbopol® | Physical | Extrusion 3D printing | bio-friendly; high transparency; lower dosage; easy to remove | - | Suspension medium | [43,109,110,111,112,113,114,115,116,117] |
| Alginate | Chemical | Inkjet 3D printing/ extrusion 3D printing | Bio-friendly; shear-thinning | Difficult to remove | Tubular tissue/supporting structure/ suspension medium | [47,118,119,120,121,122,123,124,125,126,127,128,129,130] |
| Modified hyalur- onic acid | Chemical | Extrusion 3D printing/stereo lithography 3D printing | Bio-friendly; shear-thinning; self- recovery | Difficult to synthesize; difficult to remove | Suspension medium | [119,120,131,132,133,134,135,136,137,138,139,140] |
4. Challenges and Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahrubudin, N.; Koshy, P.; Alipal, J.; Kadir, M.H.A.; Lee, T.C. Challenges of 3D printing technology for manufacturing biomedical products: A case study of Malaysian manufacturing firms. Heliyon 2020, 6, e03734. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I. The use of composite materials in 3D printing. J. Compos. Sci. 2020, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Blanco, I. A brief review of the applications of selected thermal analysis methods to 3D printing. Thermo 2022, 2, 6. [Google Scholar] [CrossRef]
- Lind, J.U.; Busbee, T.A.; Valentine, A.D.; Pasqualini, F.S.; Yuan, H.; Yadid, M.; Park, S.J.; Kotikian, A.; Nesmith, A.P.; Campbell, P.H.; et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater. 2017, 16, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.S.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.; Zanella, F.; et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef] [Green Version]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.H.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X.; et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping as a tool for manufacturing bioartificial livers. Trends Biotechnol. 2007, 25, 505–513. [Google Scholar] [CrossRef]
- Wang, X. Bioartificial Organ manufacturing technologies. Cell Transplant. 2019, 28, 5–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Liu, C. Fibrin hydrogels for endothelialized liver tissue engineering with a predesigned vascular network. Polymers 2018, 10, 1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.M.; Suen, S.K.Q.; Ng, W.L.; Ma, W.C.; Yeong, W.Y. Bioprinting of collagen: Considerations, potentials, and applications. Macromol. Biosci. 2021, 21, e2000280. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef] [PubMed]
- Natal, C.; Osés-Prieto, J.A.; Pelacho, B.; Iraburu, M.J.; López-Zabalza, M.J. Regulation of apoptosis by peptides of fibronectin in human monocytes. Apoptosis 2006, 11, 209–219. [Google Scholar] [CrossRef]
- Jarnagin, W.R.; Rockey, D.C.; Koteliansky, V.E.; Wang, S.S.; Bissell, D.M. Expression of variant fibronectins in wound healing: Cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J. Cell Biol. 1994, 127, 2037–2048. [Google Scholar] [CrossRef]
- Patten, J.; Wang, K. Fibronectin in development and wound healing. Adv. Drug Deliv. Rev. 2021, 170, 353–368. [Google Scholar] [CrossRef]
- Barros, D.; Amaral, I.F.; Pêgo, A.P. Laminin-inspired cell-instructive microenvironments for neural stem cells. Biomacromolecules 2020, 21, 276–293. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D bioprinting. Polymers 2018, 11, 1278. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Wang, X.; Pan, Y.; Liu, H.; Cheng, J.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials 2005, 26, 5864–5871. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, Y.; Zhang, R. Gelatin-based hydrogels for controlled cell assembly. In Biomedical Applications of Hydrogels Handbook; Ottenbrite, R.M., Ed.; Springer: New York, NY, USA, 2010; pp. 269–284. [Google Scholar]
- Li, S.; Yan, Y.; Xiong, Z.; Weng, C.; Zhang, R.; Wang, X. Gradient hydrogel construct based on an improved cell assembling system. J. Bioact. Compat. Polym. 2009, 24, 84–99. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Pan, Y.; Xiong, Z.; Liu, H.; Cheng, J.; Liu, F.; Lin, F.; Wu, R.; Zhang, R.; et al. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng. 2006, 12, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; He, K.; Wang, X. Rapid prototyping of a hybrid hierarchical polyurethane-cell/hydrogel construct for regenerative medicine. Mater. Sci. Eng. C 2013, 33, 3220–3229. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Z.; Wang, X.; Yan, Y.; Liu, H.; Zhang, R. Direct fabrication of a hybrid cell/hydrogel construct by a double-nozzle assembling technology. J. Bioact. Compat. Polym. 2009, 24, 249–265. [Google Scholar]
- Wang, X.; Xu, H. Incorporation of DMSO and dextran-40 into a gelatin/alginate hydrogel for controlled assembled cell cryopreservation. Cryobiology 2010, 61, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tuomi, J.; Mäkitie, A.A.; Poloheimo, K.-S.; Partanen, J.; Yliperttula, M. The integrations of biomaterials and rapid prototyping techniques for intelligent manufacturing of complex organs. In Advances in Biomaterials Science and Applications in Biomedicine; Lazinica, R., Ed.; InTech: London, UK, 2013; pp. 437–463. [Google Scholar]
- Liu, F.; Liu, C.; Chen, Q.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Progress in organ 3D bioprinting. Int. J. Bioprinting 2018, 4, 128. [Google Scholar] [CrossRef] [Green Version]
- Muthusamy, S.; Kannan, S.; Lee, M.; Sanjairaj, V.; Lu, W.F.; Fuh, J.Y.H.; Sriram, G.; Cao, T. 3D bioprinting and microscale organization of vascularized tissue constructs using collagen-based bioink. Biotechnol. Bioeng. 2021, 118, 3150–3163. [Google Scholar] [CrossRef]
- Chen, Q.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. An interpenetrating alginate/gelatin network for three-dimensional (3D) cell cultures and organ bioprinting. Molecules 2020, 25, 756. [Google Scholar] [CrossRef] [Green Version]
- Truong, N.F.; Kurt, E.; Tahmizyan, N.; Lesher-Pérez, S.C.; Chen, M.; Darling, N.J.; Xi, W.; Segura, T. Microporous annealed particle hydrogel stiffness, void space size, and adhesion properties impact cell proliferation, cell spreading, and gene transfer. Acta Biomater. 2019, 94, 160–172. [Google Scholar] [CrossRef]
- Nih, L.R.; Sideris, E.; Carmichael, S.T.; Segura, T. Injection of microporous annealing particle (MAP) hydrogels in the stroke cavity reduces gliosis and inflammation and promotes NPC migration to the lesion. Adv. Mater. 2017, 29, adma.20160647. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Lippens, E.; Lee, K.; Mehta, M.; Koshy, S.T.; Darnell, M.C.; Desai, R.M.; Madl, C.M.; Xu, M.; Zhao, X.; et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat. Mater. 2015, 14, 1269–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.-H.; Yu, S.; Wang, T.; Behn, A.W.; Yang, F. Microribbon-like elastomers for fabricating macroporous and highly flexible scaffolds that support cell proliferation in 3D. Adv. Funct. Mater. 2013, 23, 346–358. [Google Scholar] [CrossRef]
- Fatimi, A. Hydrogel-Based Bioinks for Three-Dimensional Bioprinting: Patent Analysis. In Proceedings of the 2nd International Online Conference on Polymer Science—Polymers and Nanotechnology for Industry 4.0, Online, 1–15 November 2021. [Google Scholar]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural Hydrogel-Based Bio-Inks for 3D Bioprinting in Tissue Engineering: A Review. Gels 2022, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Almeida, E.; Guvendiren, M. 3D bioprinting of complex channels within cell-laden hydrogels. Acta Biomater. 2019, 95, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Armstrong, J.P.K.; Chen, Q.; Lin, Y.; Stevens, M.M. Void-free 3D bioprinting for in situ endothelialization and microfluidic perfusion. Adv. Funct. Mater. 2019, 30, 8349. [Google Scholar] [CrossRef]
- Li, H.; Tan, Y.J.; Kiran, R.; Tor, S.B.; Zhou, K. Submerged and non-submerged 3D bioprinting approaches for the fabrication of complex structures with the hydrogel pair GelMA and alginate/methylcellulose. Addit. Manuf. 2021, 37, 101640. [Google Scholar] [CrossRef]
- Schoneberg, J.; De Lorenzi, F.; Theek, B.; Blaeser, A.; Rommel, D.; Kuehne, A.J.C.; Kiessling, F.; Fischer, H. Engineering biofunctional in vitro vessel models using a multilayer bioprinting technique. Sci. Rep. 2018, 8, 10430. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.Z.; Hussain, M.; Wang, M.N.; Li, Z.Y.; He, N.Y. Embedded 3D printing of multi-internal surfaces of hydrogels. Addit. Manuf. 2020, 32, 101097. [Google Scholar] [CrossRef]
- Bhattacharjee, T.; Zehnder, S.M.; Rowe, K.G.; Jain, S.; Nixon, R.M.; Sawyer, W.G.; Angelini, T.E. Writing in the granular gel medium. Sci. Adv. 2015, 1, e1500655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, O.; Lee, Y.B.; Jeong, H.; Lee, S.J.; Wells, D.; Alsberg, E. Individual cell-only bioink and photocurable supporting medium for 3D printing and generation of engineered tissues with complex geometries. Mater. Horiz. 2019, 6, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- McCormack, A.; Highley, C.B.; Leslie, N.R.; Melchels, F.P.W. 3D Printing in Suspension Baths: Keeping the Promises of Bioprinting Afloat. Trends Biotechnol. 2020, 38, 584–593. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Boustta, M.; Colombo, P.E.; Lenglet, S.; Poujol, S.; Vert, M. Versatile UCST-based thermoresponsive hydrogels for loco-regional sustained drug delivery. J. Control. Release 2014, 174, 1–6. [Google Scholar] [CrossRef]
- Ye, H.; Owh, C.; Jiang, S.; Ng, C.Z.Q.; Wirawan, D.; Loh, X.J. A thixotropic polyglycerol sebacate-based supramolecular hydrogel as an injectable drug delivery matrix. Polymers 2016, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Lu, Q.; Sun, L.; Cebe, P.; Wang, X.; Zhang, X.; Kaplan, D.L. Biomaterials from ultrasonication-induced silk fibroin-hyaluronic acid hydrogels. Biomacromolecules 2010, 11, 3178–3188. [Google Scholar] [CrossRef]
- Wang, X.; Kluge, J.A.; Leisk, G.G.; Kaplan, D.L. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials 2008, 29, 1054–1064. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, X.; Wang, S.; Zhao, J.; Xu, L.; Zhu, C.; Zeng, D.; Chen, J.; Zhang, Z.; Kaplan, D.L.; et al. The use of injectable sonication-induced silk hydrogel for VEGF(165) and BMP-2 delivery for elevation of the maxillary sinus floor. Biomaterials 2011, 32, 9415–9424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Mohanty, S.; Sanger, K.; Heiskanen, A.; Trifol, J.; Szabo, P.; Dufva, M.; Emneus, J.; Wolff, A. Fabrication of scalable tissue engineering scaffolds with dual-pore microarchitecture by combining 3D printing and particle leaching. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 180–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compaan, A.M.; Song, K.; Chai, W.; Huang, Y. Cross-linkable microgel composite matrix bath for embedded bioprinting of perfusable tissue constructs and sculpting of solid objects. ACS Appl. Mater. Interfaces 2020, 12, 7855–7868. [Google Scholar] [CrossRef]
- Sun, K.; Wang, Z.; Xin, J.; Wang, Z.; Xie, P.; Fan, G.; Murugadoss, V.; Fan, R.; Fan, J.; Guo, Z. Hydrosoluble graphene/polyvinyl alcohol membranous composites with negative permittivity behavior. Macromol. Mater. Eng. 2020, 305, 709. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef]
- Litvinchuk, S.; Lu, Z.; Rigler, P.; Hirt, T.D.; Meier, W. Calcein release from polymeric vesicles in blood plasma and PVA hydrogel. Pharm. Res. 2009, 26, 1711–1717. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Y.; Lan, Y.; Zuo, Q.; Li, C.; Zhang, Y.; Guo, R.; Xue, W. Acceleration of skin regeneration in full-thickness burns by incorporation of bFGF-loaded alginate microspheres into a CMCS-PVA hydrogel. J. Tissue Eng. Regen. Med. 2017, 11, 1562–1573. [Google Scholar] [CrossRef]
- Dashtdar, H.; Murali, M.R.; Abbas, A.A.; Suhaeb, A.M.; Selvaratnam, L.; Tay, L.X.; Kamarul, T. PVA-chitosan composite hydrogel versus alginate beads as a potential mesenchymal stem cell carrier for the treatment of focal cartilage defects. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1368–1377. [Google Scholar] [CrossRef]
- Blumenrother, E.; Melchert, O.; Wollweber, M.; Roth, B. Detection, numerical simulation and approximate inversion of optoacoustic signals generated in multi-layered PVA hydrogel based tissue phantoms. Photoacoustics 2016, 4, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Zou, Q.; Tian, X.; Luo, S.; Yuan, D.; Xu, S.; Yang, L.; Ma, M.; Ye, C. Agarose composite hydrogel and PVA sacrificial materials for bioprinting large-scale, personalized face-like with nutrient networks. Carbohydr. Polym. 2021, 269, 118222. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, E.M.; Nakamura, S.; Lee, K.W.; Clampffer, J.; Ijima, H.; Wang, Y. Micropatterning electrospun scaffolds to create intrinsic vascular networks. Macromol. Biosci. 2014, 14, 1514–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Q.; Grottkau, B.E.; He, Z.; Shu, L.; Yang, L.; Ma, M.; Ye, C. Biofabrication of valentine-shaped heart with a composite hydrogel and sacrificial material. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110205. [Google Scholar] [CrossRef]
- Shimizu, A.; Goh, W.H.; Itai, S.; Karyappa, R.; Hashimoto, M.; Onoe, H. ECM-based microfluidic gradient generator for tunable surface environment by interstitial flow. Biomicrofluidics 2020, 14, 044106. [Google Scholar] [CrossRef]
- Shimizu, A.; Goh, W.H.; Itai, S.; Hashimoto, M.; Miura, S.; Onoe, H. ECM-based microchannel for culturing in vitro vascular tissues with simultaneous perfusion and stretch. Lab Chip 2020, 20, 1917–1927. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.Y.; Liu, L.J.; Hu, Q.X. A versatile method for fabricating tissue engineering scaffolds with a three-dimensional channel for prevasculature networks. ACS Appl. Mater. Interfaces 2016, 8, 25096–25103. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Belaid, H.; Radhakrishnan, S.; Teyssier, C.; Balme, S.; Miele, P.; Cornu, D.; Subbaraya, N.K.; Cavaillès, V.; Bechelany, M. Sacrificial mold-assisted 3D printing of stable biocompatible gelatin scaffolds. Bioprinting 2021, 22, e00140. [Google Scholar] [CrossRef]
- Yapar, E.A.; Ýnal, Ö. Poly(ethylene oxide)–poly(propylene oxide)-based copolymers for transdermal drug delivery: An overview. Trop. J. Pharm. Res. 2012, 11, 855–866. [Google Scholar] [CrossRef] [Green Version]
- Quintans-Júnior, L.J.; Brito, R.G.; Quintans, J.S.S.; Santos, P.L.; Camargo, Z.T.; Barreto, P.A.; Arrigoni-Blank, M.F.; Lucca-Júnior, W.; Scotti, L.; Scotti, M.T.; et al. Nanoemulsion thermoreversible Pluronic F127-based hydrogel containing Hyptis pectinata (Lamiaceae) leaf essential oil produced a lasting anti-hyperalgesic effect in chronic noninflammatory widespread pain in mice. Mol. Neurobiol. 2018, 55, 1665–1675. [Google Scholar] [CrossRef]
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci. Rep. 2016, 6, 34845. [Google Scholar] [CrossRef] [Green Version]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, S.; Huang, J.; Fu, H.; Ning, X.; He, Y.; Zhang, Z. HBC-nanofiber hydrogel scaffolds with 3D printed internal microchannels for enhanced cartilage differentiation. J. Mater. Chem. B 2020, 8, 6115–6127. [Google Scholar] [CrossRef]
- Molley, T.G.; Jalandhra, G.K.; Nemec, S.R.; Tiffany, A.S.; Patkunarajah, A.; Poole, K.; Harley, B.A.C.; Hung, T.T.; Kilian, K.A. Heterotypic tumor models through freeform printing into photostabilized granular microgels. Biomater. Sci. 2021, 9, 4496–4509. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Derby, B.; Wong, J. Fabrication of microvascular constructs using high resolution electrohydrodynamic inkjet printing. Biofabrication 2020, 13, 035006. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Dey, M.; Ayan, B.; Zhang, Z.; Ozbolat, V.; Kim, M.H.; Khristov, V.; Ozbolat, I.T. Fabrication of PDMS microfluidic devices using nanoclay-reinforced Pluronic F-127 as a sacrificial ink. Biomed. Mater. 2021, 16, 045005. [Google Scholar] [CrossRef]
- Pattappa, G.; Li, Z.; Peroglio, M.; Wismer, N.; Alini, M.; Grad, S. Diversity of intervertebral disc cells: Phenotype and function. J. Anat. 2012, 221, 480–496. [Google Scholar] [CrossRef]
- Kondiah, P.J.; Kondiah, P.P.D.; Choonara, Y.E.; Marimuthu, T.; Pillay, V. A 3D bioprinted pseudo-bone drug delivery scaffold for bone tissue engineering. Pharmaceutics 2020, 12, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, J.P.; Burke, M.; Carter, B.M.; Davis, S.A.; Perriman, A.W. 3D bioprinting using a templated porous bioink. Adv. Healthc. Mater. 2016, 5, 1724–1730. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, F.; Liu, W.; Cui, L.; Shang, Q.; Xia, W.; Wang, J.; Cui, Y.; Yang, G.; Liu, D.; et al. Repairing large porcine full-thickness defects of articular cartilage using autologous chondrocyte-engineered cartilage. Tissue Eng. 2002, 8, 709–721. [Google Scholar] [CrossRef]
- Ma, X.; Sun, Y.; Cheng, X.; Gao, Y.; Hu, B.; Wen, G.; Qian, Y.; Gu, W.; Mao, Y.; Liu, W. Repair of osteochondral defects by mosaicplasty and allogeneic BMSCs transplantation. Int. J. Clin. Exp. Med. 2015, 8, 6053–6059. [Google Scholar]
- Vayas, R.; Reyes, R.; Arnau, M.R.; Évora, C.; Delgado, A. Injectable scaffold for bone marrow stem cells and bone morphogenetic protein-2 to repair cartilage. Cartilage 2021, 12, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Li, Y.; Gui, C.; Ma, Y.; Ge, Y.; Dai, H.; Zhang, K.; Du, J.; Guo, Y.; Jiang, Y.; et al. Fibronectin enhances cartilage repair by activating progenitor cells through integrin α5β1 receptor. Tissue Eng. Part A 2018, 24, 1112–1124. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Liu, S.; Jiao, W.; Wang, X. Mesenchymal stem cell-derived extracellular vesicles prevent the development of osteoarthritis via the circHIPK3/miR-124-3p/MYH9 axis. J. Nanobiotechnol. 2021, 19, 194. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Liu, S.; Jiao, W.; Wang, X. Chitosan oligosaccharides packaged into rat adipose mesenchymal stem cells-derived extracellular vesicles facilitating cartilage injury repair and alleviating osteoarthritis. J. Nanobiotechnol. 2021, 19, 343. [Google Scholar] [CrossRef]
- Wu, W.; DeConinck, A.; Lewis, J.A. Omnidirectional printing of 3D microvascular networks. Adv. Mater. 2011, 23, H178–H183. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Jiang, W.; Peng, Y.; Luo, J.; Xie, S.; Zhong, S.; Pu, H.; Liu, N.; Yue, T. A novel biodegradable multilayered bioengineered vascular construct with a curved structure and multi-branches. Micromachines 2019, 10, 275. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Hu, Y.; Liu, C.; Yao, H.; Liu, B.; Mi, S. A novel strategy for creating tissue-engineered biomimetic blood vessels using 3D bioprinting technology. Materials 2018, 11, 1581. [Google Scholar] [CrossRef] [Green Version]
- Lewis, P.L.; Yan, M.; Su, J.; Shah, R.N. Directing the growth and alignment of biliary epithelium within extracellular matrix hydrogels. Acta Biomater. 2019, 85, 84–93. [Google Scholar] [CrossRef]
- Li, S.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. Chitosans for tissue repair and organ three-dimensional (3D) bioprinting. Micromachines 2019, 10, 765. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Du, S.; Chai, L.; Xu, Y.; Liu, L.; Zhou, X.; Wang, J.; Zhang, W.; Liu, C.-H.; Wang, X. Anti-cancer drug screening based on an adipose-derived stem cell/hepatocyte 3D printing technique. J. Stem Cell Res. Ther. 2015, 5, 273. [Google Scholar]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-based hydrogels for organ 3D bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eltaher, H.M.; Abukunna, F.E.; Ruiz-Cantu, L.; Stone, Z.; Yang, J.; Dixon, J.E. Human-scale tissues with patterned vascular networks by additive manufacturing of sacrificial sugar-protein composites. Acta Biomater. 2020, 113, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Khalighi, S.; Saadatmand, M. Bioprinting a thick and cell-laden partially oxidized alginate-gelatin scaffold with embedded micro-channels as future soft tissue platform. Int. J. Biol. Macromol. 2021, 193, 2153–2164. [Google Scholar] [CrossRef] [PubMed]
- Dranseikiene, D.; Schrufer, S.; Schubert, D.W.; Reakasame, S.; Boccaccini, A.R. Cell-laden alginate dialdehyde-gelatin hydrogels formed in 3D printed sacrificial gel. J. Mater. Sci. Mater. Med. 2020, 31, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Y.; Liu, H.; Cheng, W.; Liu, Y.; Kim, S.; Yuan, X.; Kusi-Appiah, A.; Lenhert, S.; Ma, T.; Ren, Y.; et al. Conjugating micropatches to living cells through membrane intercalation. ACS Appl. Mater. Interfaces 2020, 12, 29110–29121. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Advanced polymers for organ three-dimensional (3D) bioprinting. Micromachines 2019, 10, 814. [Google Scholar] [CrossRef] [Green Version]
- Spencer, A.R.; Shirzaei Sani, E.; Soucy, J.R.; Corbet, C.C.; Primbetova, A.; Koppes, R.A.; Annabi, N. Bioprinting of a cell-laden conductive hydrogel composite. ACS Appl. Mater. Interfaces 2019, 11, 30518–30533. [Google Scholar] [CrossRef]
- Wang, W.; Tan, B.; Chen, J.; Bao, R.; Zhang, X.; Liang, S.; Shang, Y.; Liang, W.; Cui, Y.; Fan, G.; et al. An injectable conductive hydrogel encapsulating plasmid DNA-eNOs and ADSCs for treating myocardial infarction. Biomaterials 2018, 160, 69–81. [Google Scholar] [CrossRef]
- Choi, Y.J.; Jun, Y.J.; Kim, D.Y.; Yi, H.G.; Chae, S.H.; Kang, J.; Lee, J.; Gao, G.; Kong, J.S.; Jang, J.; et al. A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss. Biomaterials 2019, 206, 160–169. [Google Scholar] [CrossRef]
- Jeon, O.; Bin Lee, Y.; Hinton, T.J.; Feinberg, A.W.; Alsberg, E. Cryopreserved cell-laden alginate microgel bioink for 3D bioprinting of living tissues. Mater. Today Chem. 2019, 12, 61–70. [Google Scholar] [CrossRef]
- Guex, A.G.; Puetzer, J.L.; Armgarth, A.; Littmann, E.; Stavrinidou, E.; Giannelis, E.P.; Malliaras, G.G.; Stevens, M.M. Highly porous scaffolds of PEDOT:PSS for bone tissue engineering. Acta Biomater. 2017, 62, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D. A pore way to heal and regenerate: 21st century thinking on biocompatibility. Regen. Biomater. 2016, 3, 107–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seymour, A.J.; Shin, S.; Heilshorn, S.C. 3D printing of microgel scaffolds with tunable void fraction to promote cell infiltration. Adv. Healthc. Mater. 2021, 10, e2100644. [Google Scholar] [CrossRef]
- Shao, L.; Gao, Q.; Xie, C.; Fu, J.; Xiang, M.; Liu, Z.; Xiang, L.; He, Y. Sacrificial microgel-laden bioink-enabled 3D bioprinting of mesoscale pore networks. Bio-Des. Manuf. 2020, 3, 30–39. [Google Scholar] [CrossRef]
- Konka, J.; Buxadera-Palomero, J.; Espanol, M.; Ginebra, M.P. 3D printing of hierarchical porous biomimetic hydroxyapatite scaffolds: Adding concavities to the convex filaments. Acta Biomater. 2021, 134, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, M.; Balhoff, M.; Hayman, N.W. Chemical and microstructural controls on viscoplasticity in Carbopol hydrogel. Polymer 2018, 139, 44–51. [Google Scholar] [CrossRef]
- Weber, E.; Moyers-González, M.; Burghelea, T.I. Thermorheological properties of a Carbopol gel under shear. J. Non-Newton. Fluid Mech. 2012, 183–184, 14–24. [Google Scholar] [CrossRef]
- Jin, Y.; Compaan, A.; Bhattacharjee, T.; Huang, Y. Granular gel support-enabled extrusion of three-dimensional alginate and cellular structures. Biofabrication 2016, 8, 025016. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Sani, E.S.; Spencer, A.R.; Guan, Y.; Weiss, A.S.; Annabi, N. Human-recombinant-elastin-based bioinks for 3D bioprinting of vascularized soft tissues. Adv. Mater. 2020, 32, e2003915. [Google Scholar] [CrossRef]
- Annabi, N.; Zhang, Y.N.; Assmann, A.; Sani, E.S.; Cheng, G.; Lassaletta, A.D.; Vegh, A.; Dehghani, B.; Ruiz-Esparza, G.U.; Wang, X.; et al. Engineering a highly elastic human protein-based sealant for surgical applications. Sci. Transl. Med. 2017, 9, aai7466. [Google Scholar] [CrossRef] [Green Version]
- Soucy, J.R.; Shirzaei Sani, E.; Portillo Lara, R.; Diaz, D.; Dias, F.; Weiss, A.S.; Koppes, A.N.; Koppes, R.A.; Annabi, N. Photocrosslinkable gelatin/tropoelastin hydrogel adhesives for peripheral nerve repair. Tissue Eng. Part A 2018, 24, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Mehta, R.; Cao, C.; Theus, A.; Tomov, M.; Zhu, N.; Weeks, E.R.; Bauser-Heaton, H.; Serpooshan, V. Embedded 3D bioprinting of gelatin methacryloyl-based constructs with highly tunable structural fidelity. ACS Appl. Mater. Interfaces 2020, 12, 44563–44577. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, V.; Dey, M.; Ayan, B.; Ozbolat, I.T. Extrusion-based printing of sacrificial Carbopol ink for fabrication of microfluidic devices. Biofabrication 2019, 11, 034101. [Google Scholar] [CrossRef]
- Noria, S.; Xu, F.; McCue, S.; Jones, M.; Gotlieb, A.I.; Langille, B.L. Assembly and reorientation of stress fibers drives morphological changes to endothelial cells exposed to shear stress. Am. J. Pathol. 2004, 164, 1211–1223. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Xia, Y.; Qiu, Y.; Chen, X.; Shi, S. Preparation and property of starch nanoparticles reinforced aldehyde-hydrazide covalently crosslinked PNIPAM hydrogels. J. Appl. Polym. Sci. 2018, 135, 45761. [Google Scholar] [CrossRef]
- Wang, M.; Yang, X.; Liang, L. Fabrication of a microfluidic system using micromolded alginate gel as a sacrificial material for tissues engineering. J. Chem. 2020, 2020, 3148652. [Google Scholar] [CrossRef]
- Highley, C.B.; Rodell, C.B.; Burdick, J.A. Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv. Mater. 2015, 27, 5075–5079. [Google Scholar] [CrossRef]
- Song, K.H.; Highley, C.B.; Rouff, A.; Burdick, J.A. Complex 3D-printed microchannels within cell-degradable hydrogels. Adv. Funct. Mater. 2018, 28, 1801331. [Google Scholar] [CrossRef]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Mahdi, M.H.; Diryak, R.; Kontogiorgos, V.; Morris, G.A.; Smith, A.M. In situ rheological measurements of the external gelation of alginate. Food Hydrocoll. 2016, 55, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Compaan, A.M.; Christensen, K.; Huang, Y. Inkjet bioprinting of 3D silk fibroin cellular constructs using sacrificial alginate. ACS Biomater. Sci. Eng. 2017, 3, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, W.; Li, W.; Xie, M.; Deng, C.; Sun, X.; Wang, C.; Liu, Y.; Shi, G.; Xu, Y.; et al. Fabrication of Thermoresponsive hydrogel scaffolds with engineered microscale vasculatures. Adv. Funct. Mater. 2021, 31, 2102685. [Google Scholar] [CrossRef]
- Saeki, K.; Hiramatsu, H.; Hori, A.; Hirai, Y.; Yamada, M.; Utoh, R.; Seki, M. Sacrificial alginate-assisted microfluidic engineering of cell-supportive protein microfibers for hydrogel-based cell encapsulation. ACS Omega 2020, 5, 21641–21650. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, J.V.; McCarthy, A.; Wang, H.; Luo, Z.; Li, H.; Wang, Z.; Cheng, F.; Zhang, Y.S.; Xie, J. Freeze-casting with 3D-printed templates creates anisotropic microchannels and patterned macrochannels within biomimetic nanofiber aerogels for rapid cellular infiltration. Adv. Healthc. Mater. 2021, 10, e2100238. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.S.; Shi, Q.; Chen, S.; Bin Juhari, M.A.; Song, J. Recyclable and biocompatible microgel-based supporting system for positive 3D freeform printing of silicone rubber. Biomed. Eng. Lett. 2020, 10, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.S.; Juhari, M.A.B.; Shi, Q.; Chen, S.; Campolo, D.; Song, J. Development of a new additive manufacturing platform for direct freeform 3D printing of intrinsically curved flexible membranes. Addit. Manuf. 2020, 36, 101563. [Google Scholar] [CrossRef]
- Prestwich, G.D. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J. Control. Release 2011, 155, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Toole, B.P. Hyaluronan in morphogenesis. Semin. Cell Dev. Biol. 2001, 12, 79–87. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef]
- Shi, L.; Han, Y.; Hilborn, J.; Ossipov, D. “Smart” drug loaded nanoparticle delivery from a self-healing hydrogel enabled by dynamic magnesium-biopolymer chemistry. Chem. Commun. 2016, 52, 11151–11154. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Carstensen, H.; Hölzl, K.; Lunzer, M.; Li, H.; Hilborn, J.; Ovsianikov, A.; Ossipov, D.A. Dynamic coordination chemistry enables free directional printing of biopolymer hydrogel. Chem. Mater. 2017, 29, 5816–5823. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Heitmiller, K.; Ring, C.; Saedi, N. Rheologic properties of soft tissue fillers and implications for clinical use. J. Cosmet. Dermatol. 2021, 20, 28–34. [Google Scholar] [CrossRef]
- Al-Khateeb, R.; Olszewska-Czyz, I. Biological molecules in dental applications: Hyaluronic acid as a companion biomaterial for diverse dental applications. Heliyon 2020, 6, e03722. [Google Scholar] [CrossRef]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced bioinks for 3D printing: A materials science perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef]
- Thomas, A.; Orellano, I.; Lam, T.; Noichl, B.; Geiger, M.A.; Amler, A.K.; Kreuder, A.E.; Palmer, C.; Duda, G.; Lauster, R.; et al. Vascular bioprinting with enzymatically degradable bioinks via multi-material projection-based stereolithography. Acta Biomater. 2020, 117, 121–132. [Google Scholar] [CrossRef]
- Wang, X. Overview on biocompatibilities of implantable biomaterials. In Advances in Biomaterials Science and Biomedical Applications; Pignatello, R., Ed.; InTech: Zagreb, Croatia, 2013; pp. 111–155. [Google Scholar]
- Zhao, J.; He, N. A mini-review of embedded 3D printing: Supporting media and strategies. J. Mater. Chem. B 2020, 8, 10474–10486. [Google Scholar] [CrossRef]
- Miller, J.S. The billion cell construct: Will three-dimensional printing get us there? PLoS Biol. 2014, 12, e1001882. [Google Scholar] [CrossRef]
- Miri, A.K.; Mirzaee, I.; Hassan, S.; Mesbah Oskui, S.; Nieto, D.; Khademhosseini, A.; Zhang, Y.S. Effective bioprinting resolution in tissue model fabrication. Lab Chip 2019, 19, 2019–2037. [Google Scholar] [CrossRef]
- Wang, X.; Rijff, B.L.; Khang, G. A building block approach into 3D printing a multi-channel organ regenerative scaffold. J. Tissue Eng. Regen. Med. 2015, 11, 1403. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, C.; Zhao, X.; Wang, X. A 3D bioprinting liver tumor model for drug screening. World J. Pharm. Pharm. Sci. 2016, 5, 196–213. [Google Scholar]
- Wang, X.; He, K.; Zhang, W. Optimizing the fabrication processes for manufacturing a hybrid hierarchical polyurethane-cell/hydrogel construct. J. Bioact. Compat. Polym. 2013, 28, 303–319. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X. Preparation of an adipose-derived stem cell/fibrin-poly(dl-lactic-co-glycolic acid) construct based on a rapid prototyping technique. J. Bioact. Compat. Polym. 2013, 28, 191–203. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C. 3D bioprinting of adipose-derived stem cells for organ manufacturing. In Enabling Cutting Edge Technology for Regenerative Medicine; Spirnger: New York, NY, USA, 2018; Chapter 1; pp. 3–14. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Wang, T.; Li, S.; Wang, X. Application Status of Sacrificial Biomaterials in 3D Bioprinting. Polymers 2022, 14, 2182. https://doi.org/10.3390/polym14112182
Liu S, Wang T, Li S, Wang X. Application Status of Sacrificial Biomaterials in 3D Bioprinting. Polymers. 2022; 14(11):2182. https://doi.org/10.3390/polym14112182
Chicago/Turabian StyleLiu, Siyu, Tianlin Wang, Shenglong Li, and Xiaohong Wang. 2022. "Application Status of Sacrificial Biomaterials in 3D Bioprinting" Polymers 14, no. 11: 2182. https://doi.org/10.3390/polym14112182
APA StyleLiu, S., Wang, T., Li, S., & Wang, X. (2022). Application Status of Sacrificial Biomaterials in 3D Bioprinting. Polymers, 14(11), 2182. https://doi.org/10.3390/polym14112182







