Preparation and Properties of Municipal Solid Waste Incineration Alkali-Activated Lightweight Materials through Spontaneous Bubbles
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Materials
2.1.1. Municipal Solid Waste Incineration Bottom Ash Pretreatment
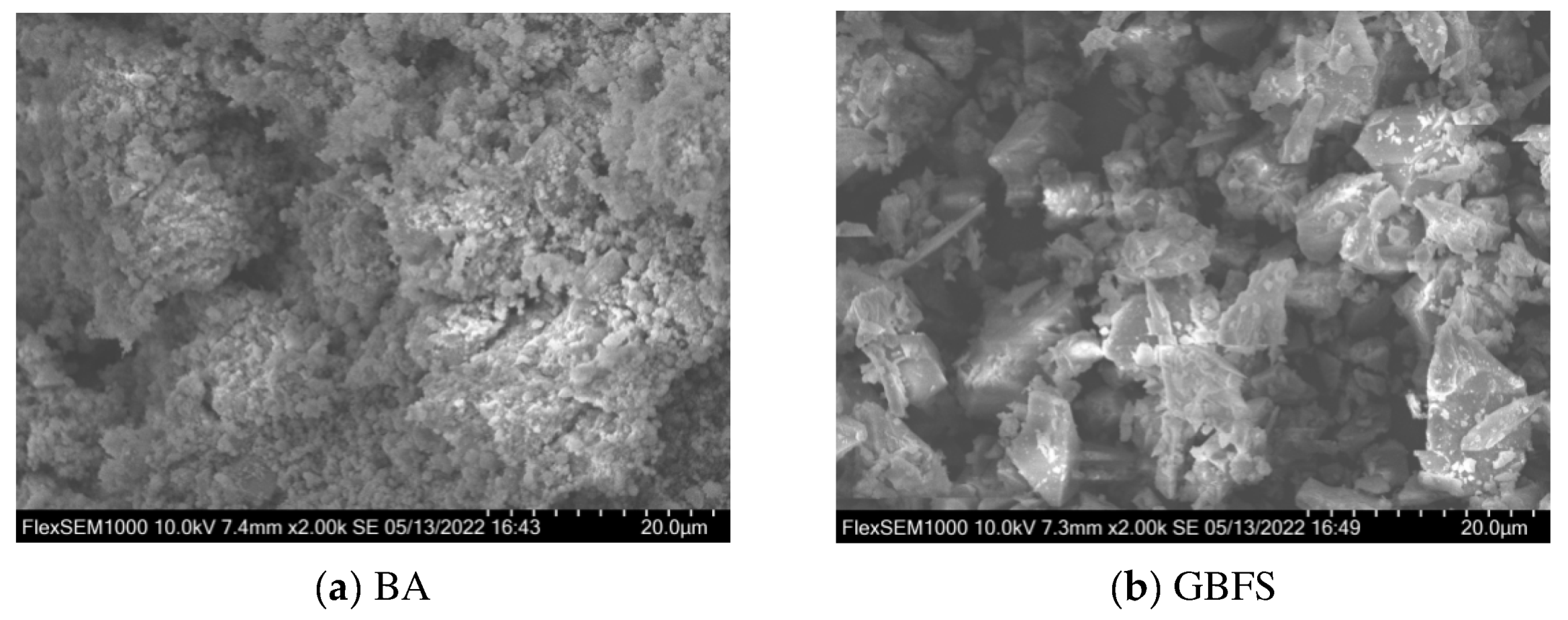
2.1.2. Blast Furnace Granulated Slag
2.1.3. Calcium Hydroxide and Sodium Hydroxide
2.1.4. Liquid Sodium Silicate
2.1.5. Other Ingredients
2.2. Preparation and Curing of Test Specimens
2.2.1. Experimental Mix Proportion
2.2.2. Specimen Preparation and Curing
2.3. Experimental Method
2.3.1. Compressive Strength Test
2.3.2. Determination of Dry and Wet Density and Water Absorption
2.3.3. Scanning Electron Microscopy
3. Results and Discussion
3.1. Analysis of Foaming and Expansion Mechanism of Bottom Ash in the Case of Alkali
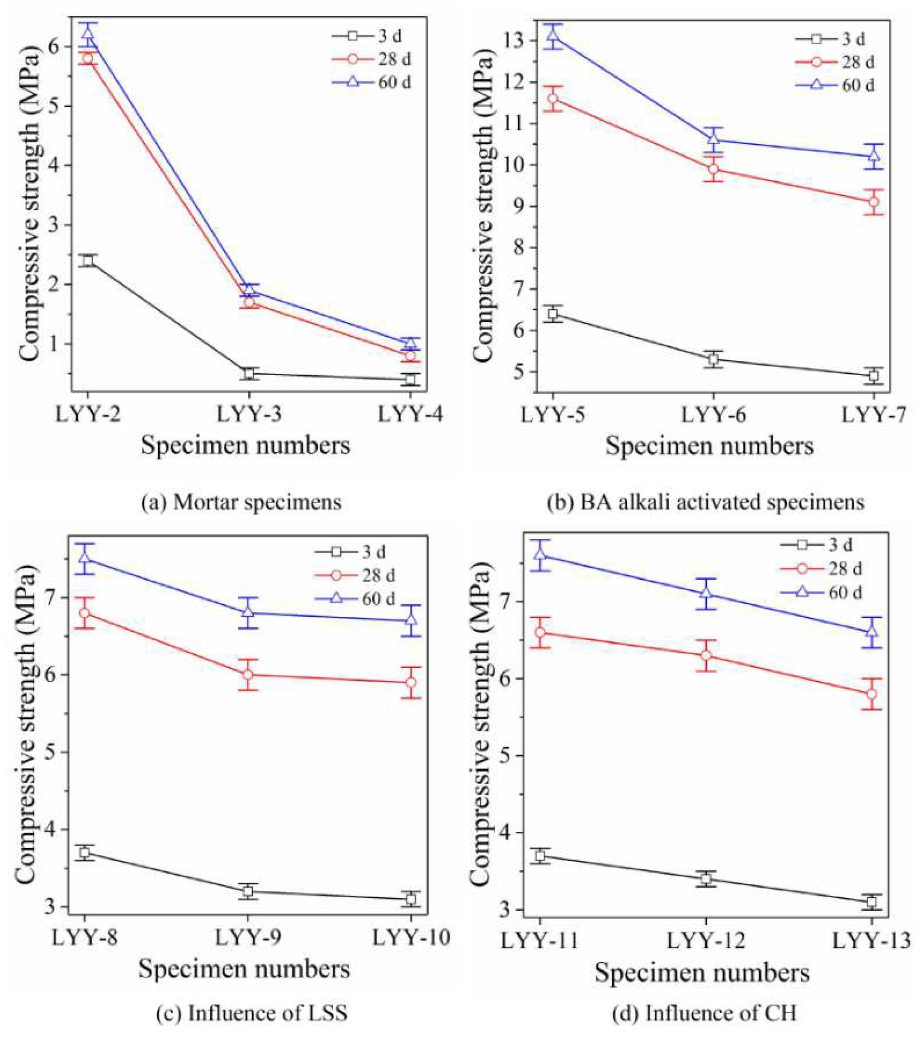
3.2. Analysis of Compressive Strength
3.2.1. Analysis of Compressive Strength of Cement Specimens
3.2.2. Analysis of Compressive Strength of BA Alkali-Activated Specimens
3.2.3. Effect of Liquid Sodium Silicate on Compressive Strength
3.2.4. Effect of Calcium Hydroxide on Compressive Strength
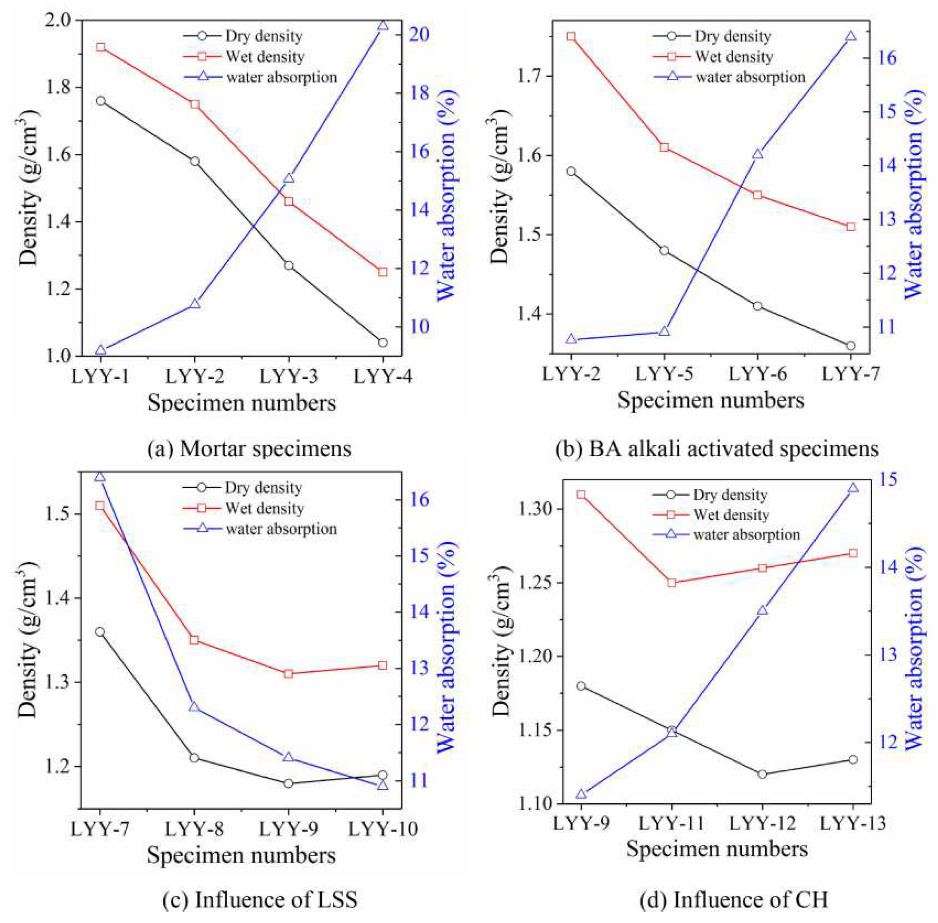
3.3. Analysis of Dry and Wet Density and Water Absorption
3.3.1. Cement Paste Specimens
3.3.2. Bottom Ash Alkali-Activated Paste Specimen
3.3.3. Influence of Liquid Sodium Silicate
3.3.4. Effect of Calcium Hydroxide
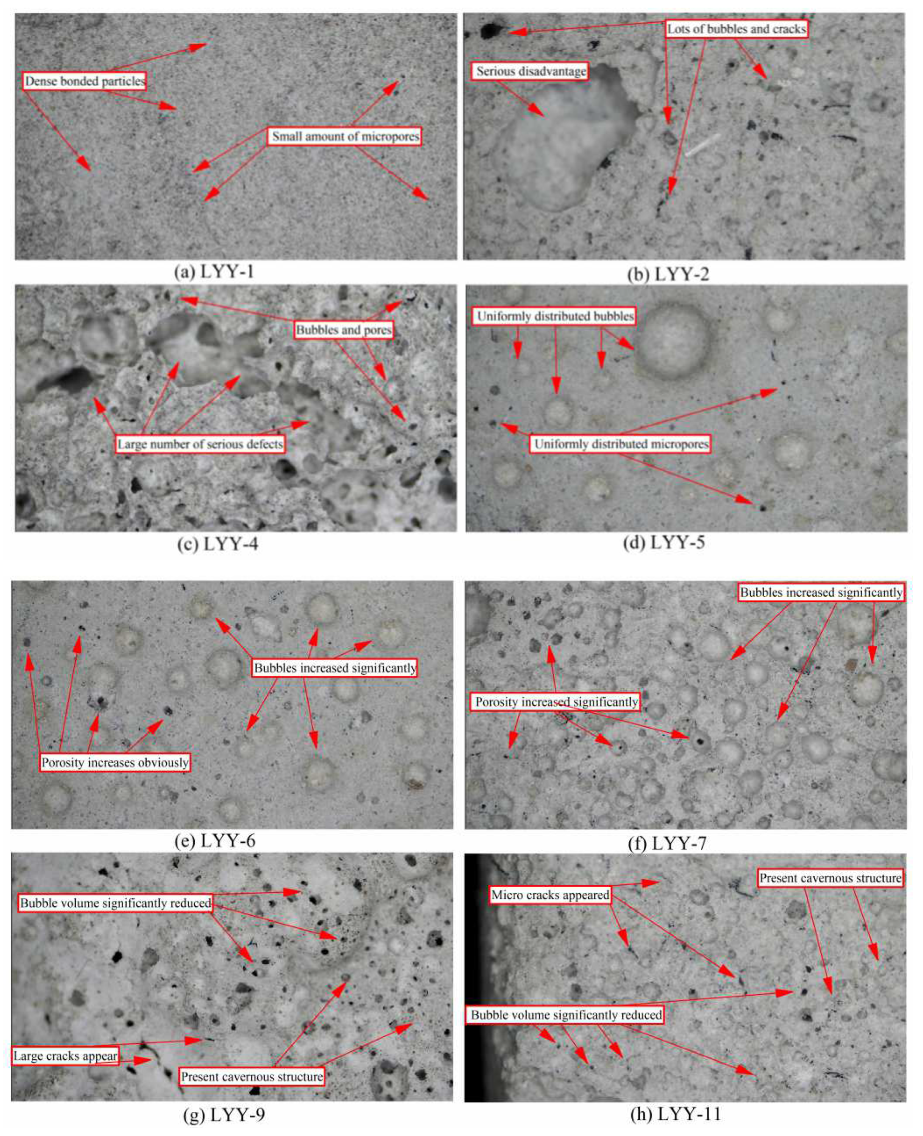
3.4. Optical Microscopy Analysis
3.4.1. Cement Lightweight Specimen
3.4.2. Bottom Ash Alkali-Activated Light Specimen
3.4.3. Influence of LSS and CH
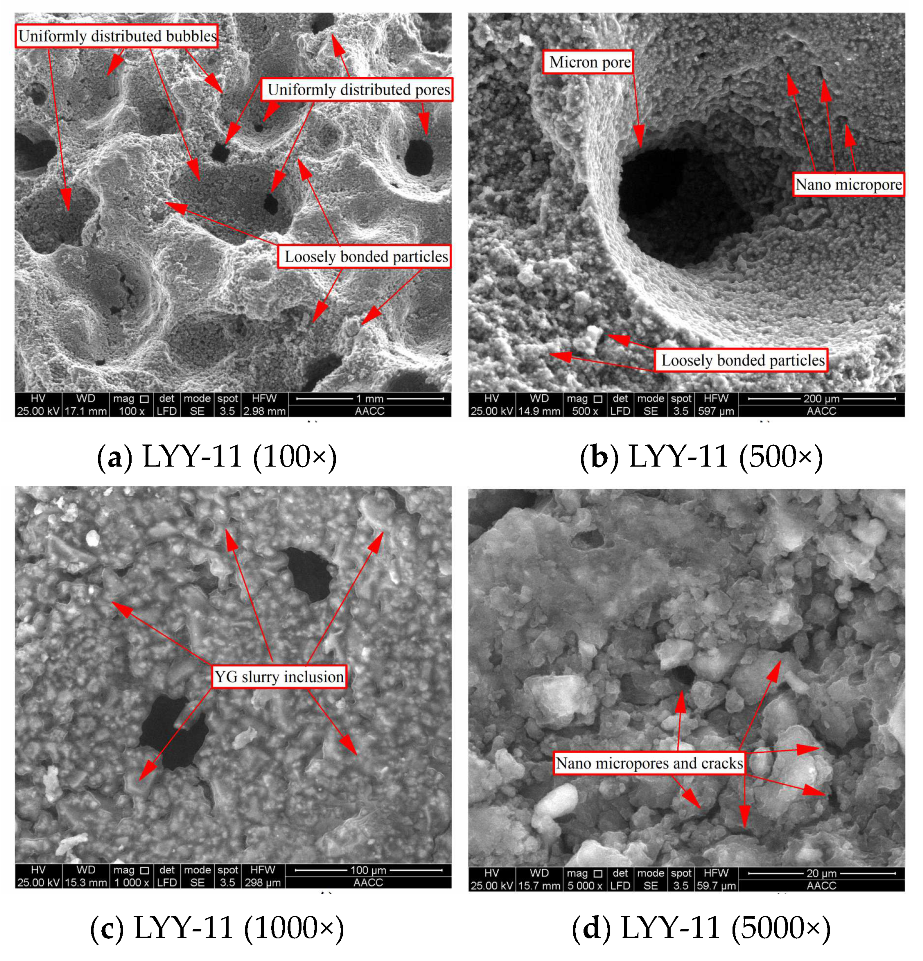
3.5. Scanning Electron Microscopy Analysis
4. Conclusions
- (1)
- Owing to the uneven distribution of bubbles, lightweight cement materials prepared using a foaming agent produced penetrating pores and cracks. These adversely impacted the compressive strength, thermal insulation, and the durability of the specimens. With the increasing content of the foaming agent, the defects became increasingly severe, ultimately resulting in the sudden decline of all aspects of the properties.
- (2)
- BA exhibits the characteristics of lightweight and alkali-foaming expansion, and the generated bubbles were evenly distributed, thus avoiding the formation of defects and alleviating the impact of the foaming expansion on all aspects of the properties. With an increasing BA content, the foaming effect was increased significantly, and the dry and wet densities of the specimen continued to decrease. However, water absorption increased significantly, but the declining range of compressive strength was significantly lower than that of cement foaming specimens.
- (3)
- LSS could provide active silicon for the polymerization reaction, promote the reaction progress, and accelerate the formation of polymerization products. This accelerated the condensation of specimens, improved the bubble sealing efficiency, reduced the opening diameter of bubbles, further reduced the dry and wet densities and water absorption of specimens, and alleviated the decline of compressive strength. With an increasing LSS content, the dry and wet densities of the specimen first decreased and then basically remained unchanged; however, the water absorption gradually decreased further.
- (4)
- CH provides active calcium for polymerization, promotes the formation of hydrated calcium silicate gels, further accelerates the condensation of specimens, enhances the efficiency of foam fixation, and further reduces the dry and wet densities. However, CH readily absorbs water, which leads to an apparent increase in water absorption. With an increasing LSS content, the dry and wet densities increased slowly; however, the water absorption exhibited a clearly increasing trend.
Author Contributions
Funding
Conflicts of Interest
References
- Shi, C.; Fernández-Jiménez, A.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Ghomi, E.R.; Khosravi, F.; Ardahaei, A.S.; Dai, Y.; Neisiany, R.; Foroughi, F.; Wu, M.; Das, O.; Ramakrishna, S. The Life Cycle Assessment for Polylactic Acid (PLA) to Make It a Low-Carbon Material. Polymers 2021, 13, 1854. [Google Scholar] [CrossRef]
- Lo, K.W.; Lin, Y.W.; Cheng, T.W.; Lin, K.L.; Lin, W.T. Recycling of Silicon Carbide Sludge on the Preparation and Characterization of Lightweight Foamed Geopolymer Materials. Polymers 2021, 13, 4029. [Google Scholar] [CrossRef] [PubMed]
- Kurda, R.; Silva, R.; De Brito, J. Incorporation of Alkali-Activated Municipal Solid Waste Incinerator Bottom Ash in Mortar and Concrete: A Critical Review. Materials 2020, 13, 3428. [Google Scholar] [CrossRef] [PubMed]
- Vateva, I.; Laner, D. Grain-Size Specific Characterisation and Resource Potentials of Municipal Solid Waste Incineration (MSWI) Bottom Ash: A German Case Study. Resources 2020, 9, 66. [Google Scholar] [CrossRef]
- Hujova, M.; Monich, P.R.; Sedlacek, J.; Hnatko, M.; Kraxner, J.; Galusek, D.; Bernardo, E. Glass-Ceramic Foams from Alkali-Activated Vitrified Bottom Ash and Waste Glasses. Appl. Sci. 2020, 10, 5714. [Google Scholar] [CrossRef]
- Taherlou, A.; Asadollahfardi, G.; Salehi, A.M.; Katebi, A. Sustainable use of municipal solid waste incinerator bottom ash and the treated industrial wastewater in self-compacting concrete. Constr. Build. Mater. 2021, 297, 123814. [Google Scholar] [CrossRef]
- Ferone, C.; Colangelo, F.; Messina, F.; Santoro, L.; Cioffi, R. Recycling of Pre-Washed Municipal Solid Waste Incinerator Fly Ash in the Manufacturing of Low Temperature Setting Geopolymer Materials. Materials 2013, 6, 3420–3437. [Google Scholar] [CrossRef] [Green Version]
- Czop, M.; Łazniewska-Piekarczyk, B. Use of Slag from the Combustion of Solid Municipal Waste as a Partial Replacement of Cement in Mortar and Concrete. Materials 2020, 13, 1593. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.; Wang, W.; Hua, D.; Wu, S.; Yao, Y. Preparation and Properties of a Sulphoaluminate Magnesium-Potassium Phosphate Green Cementitious Composite Material from Industrial Solid Wastes. Materials 2021, 14, 7340. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, K.; Baskar, K. Recycling of E-plastic waste as a construction material in developing countries. J. Mater. Cycles Waste Manag. 2015, 17, 718–724. [Google Scholar] [CrossRef]
- Agrawal, Y.; Gupta, T.; Siddique, S.; Sharma, R.K. Potential of dolomite industrial waste as construction material:a review. Innov. Infrastruct. Solut. 2021, 6, 205. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Zhang, l.; Hou, Z.; Zhang, L.; Wu, S. Influence of calcium content on structure and strength of MSWI bottom ash-based geopolymer. Mag. Concr. Res. 2019, 71, 362–372. [Google Scholar] [CrossRef]
- Huang, G.; Yang, K.; Chen, L.; Lu, Z.; Sun, Y.; Zhang, X.; Feng, Y.; Ji, Y.; Xu, Z. Use of pretreatment to prevent expansion and foaming in highperformance MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2020, 245, 118471. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Zhang, L.; Li, J.; Hou, Z. Advances in Understanding and Analyzing the Anti-diffusion Phenomenon in Complete Carbonization Zone of MSWI Bottom Ash-based Alkali-activated Concrete. Constr. Build. Mater. 2018, 186, 1072–1081. [Google Scholar] [CrossRef]
- GB/T18046-2017; Ground Granulated Blast Furnace Slag Used for Cement and Concrete. Foryou Tech Co., Ltd.: Beijing, China, 2017.
- GB/T17671-2021; Method of Testing Cements—Determination of Strength—ISO. Foryou Tech Co., Ltd.: Beijing, China, 2021.
- Tang, P.; Florea, M.V.A.; Spiesz, P.; Brouwers, H.J.H. Characteristics and application potential of municipal solid waste incineration (MSWI) bottom ashes from two waste-to-energy plants. Constr. Build. Mater. 2015, 83, 77–94. [Google Scholar] [CrossRef]
- Wongsa, A.; Boonserm, K.; Waisurasingha, C.; Sata, V.; Chindaprasirt, P. Use of municipal solid waste incinerator (MSWI) bottom ash in high calcium fly ash geopolymer matrix. J. Clean. Prod. 2017, 148, 49–59. [Google Scholar] [CrossRef]
- Chou, J.D.; Wey, M.Y.; Liang, H.H.; Chang, S.H. Biotoxicity evaluation of fly ash and bottom ash from different municipal solid waste incinerators. J. Hazard. Mater. 2009, 168, 197–202. [Google Scholar] [CrossRef]
- Angulo-Ramírez, D.E.; de Gutiérrez, R.M.; Puertas, F. Alkali-activated Portland blast-furnace slag cement: Mechanical properties and hydration. Constr. Build. Mater. 2017, 140, 119–128. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Dong, Z. Improving strength of calcinated coal gangue geopolymer mortars via increasing calcium content. Constr. Build. Mater. 2018, 166, 760–768. [Google Scholar] [CrossRef]
- Huang, G.; Li, Y.; Zhang, Y.; Zhu, J.; Li, D.; Wang, B. Effect of Sodium Hydroxide, Liquid Sodium Silicate, Calcium Hydroxide, and Slag on the Mechanical Properties and Mineral Crystal Structure Evolution of Polymer Materials. Crystals 2021, 11, 1586. [Google Scholar] [CrossRef]
- Jin, L.; Huang, G.; Li, Y.; Zhang, X.; Ji, Y.; Xu, Z. Positive Influence of Liquid Sodium Silicate on the Setting Time, Polymerization, and Strength Development Mechanism of MSWI Bottom Ash Alkali-Activated Mortars. Materials 2021, 14, 1927. [Google Scholar] [CrossRef] [PubMed]
- Ben Haha, M.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si + Ca) and metakaolin (Si + Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Huang, G.; Yang, K.; Sun, Y.; Lu, Z.; Zhang, X.; Zuo, L.; Feng, Y.; Qian, R.; Qi, Y.; Ji, Y.; et al. Influence of NaOH content on the alkali conversion mechanism in MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2020, 248, 118582. [Google Scholar] [CrossRef]
- Huang, G.; Yuan, L.; Ji, Y.; Liu, B.; Xu, Z. Cooperative action and compatibility between Portland cement and MSWI bottom ash alkali-activated double gel system materials. Constr. Build. Mater. 2019, 209, 445–453. [Google Scholar] [CrossRef]
- Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S. The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar] [CrossRef]
- Bernal, S.A.; De Gutierrez, R.M.; John, L.P.; Rose, V. Effect of silicate modulus and metakaolin incorporation on the carbonation of alkali silicate-activated slags. Cem. Concr. Res. 2010, 40, 898–907. [Google Scholar] [CrossRef]
- Bernal, S.A.; de Gutiérrez, R.M.; Pedraza, A.L.; Provis, J.L.; Rodriguez, E.D.; Delvasto, S. Effect of binder content on the performance of alkali-activated slag concretes. Cem. Concr. Res. 2011, 41, 1–8. [Google Scholar] [CrossRef]
- Maraghechi, H.; Rajabipour, F.; Pantano, C.G.; Burgos, W.D. Effect of calciumon dissolution and precipitation reactions of amorphous silica at high alkalinity. Cem. Concr. Res. 2016, 87, 1–13. [Google Scholar] [CrossRef] [Green Version]

| Raw Materials | SiO2 | CaO | Al2O3 | Fe2O3 | MgO | K2O | Na2O | Others | Loss |
|---|---|---|---|---|---|---|---|---|---|
| BA | 53.8 | 14.4 | 14.2 | 6.2 | 3.3 | 2.5 | 2.2 | 0.6 | 1.6 |
| GBFS | 31.4 | 34.7 | 18.7 | 0.6 | 9.3 | - | - | 1.8 | 0.7 |
| Specimen Numbers | Cement | BA | GBFS | CH | LSS | NH | Foaming Agent | Water | Liquid–Solid Ratio |
|---|---|---|---|---|---|---|---|---|---|
| LYY-1 | 1000 | 0 | 0 | 0 | 0 | 0 | 0 | 500 | 0.5 |
| LYY-2 | 1000 | 0 | 0 | 0 | 0 | 0 | 10 | 500 | 0.5 |
| LYY-3 | 1000 | 0 | 0 | 0 | 0 | 0 | 20 | 500 | 0.5 |
| LYY-4 | 1000 | 0 | 0 | 0 | 0 | 0 | 30 | 500 | 0.5 |
| LYY-5 | 0 | 400 | 600 | 0 | 0 | 40 | 0 | 500 | 0.5 |
| LYY-6 | 0 | 500 | 500 | 0 | 0 | 40 | 0 | 500 | 0.5 |
| LYY-7 | 0 | 600 | 400 | 0 | 0 | 40 | 0 | 500 | 0.5 |
| LYY-8 | 0 | 600 | 400 | 0 | 100 | 40 | 0 | 435 | 0.5 |
| LYY-9 | 0 | 600 | 400 | 0 | 200 | 40 | 0 | 370 | 0.5 |
| LYY-10 | 0 | 600 | 400 | 0 | 300 | 40 | 0 | 305 | 0.5 |
| LYY-11 | 0 | 600 | 380 | 20 | 200 | 40 | 0 | 370 | 0.5 |
| LYY-12 | 0 | 600 | 350 | 50 | 200 | 40 | 0 | 370 | 0.5 |
| LYY-13 | 0 | 600 | 300 | 100 | 200 | 40 | 0 | 370 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, H.; Huang, G.; Cui, Y.; Feng, J.; Zhang, Y.; Li, D.; Zhu, J. Preparation and Properties of Municipal Solid Waste Incineration Alkali-Activated Lightweight Materials through Spontaneous Bubbles. Polymers 2022, 14, 2222. https://doi.org/10.3390/polym14112222
Li Y, Zhang H, Huang G, Cui Y, Feng J, Zhang Y, Li D, Zhu J. Preparation and Properties of Municipal Solid Waste Incineration Alkali-Activated Lightweight Materials through Spontaneous Bubbles. Polymers. 2022; 14(11):2222. https://doi.org/10.3390/polym14112222
Chicago/Turabian StyleLi, Yongyu, Hongxue Zhang, Guodong Huang, Yi Cui, Jiacheng Feng, Yuting Zhang, Dawei Li, and Jielei Zhu. 2022. "Preparation and Properties of Municipal Solid Waste Incineration Alkali-Activated Lightweight Materials through Spontaneous Bubbles" Polymers 14, no. 11: 2222. https://doi.org/10.3390/polym14112222





