Polymeric Micelles Enhance Mucosal Contact Time and Deposition of Fluocinolone Acetonide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polymeric Micelles Loaded with FA
2.2.1. Formulation Development
2.2.2. Blank Polymeric Micelles
2.2.3. Dried Powder of FPMs and BPMs
2.3. Physicochemical Characteristics
2.3.1. Particle Size, Polydispersity Index, and Zeta Potential
2.3.2. Study of Polymeric Micelle Solutions Using Small Angle X-ray Scattering
2.3.3. Viscosity and Mucoadhesion
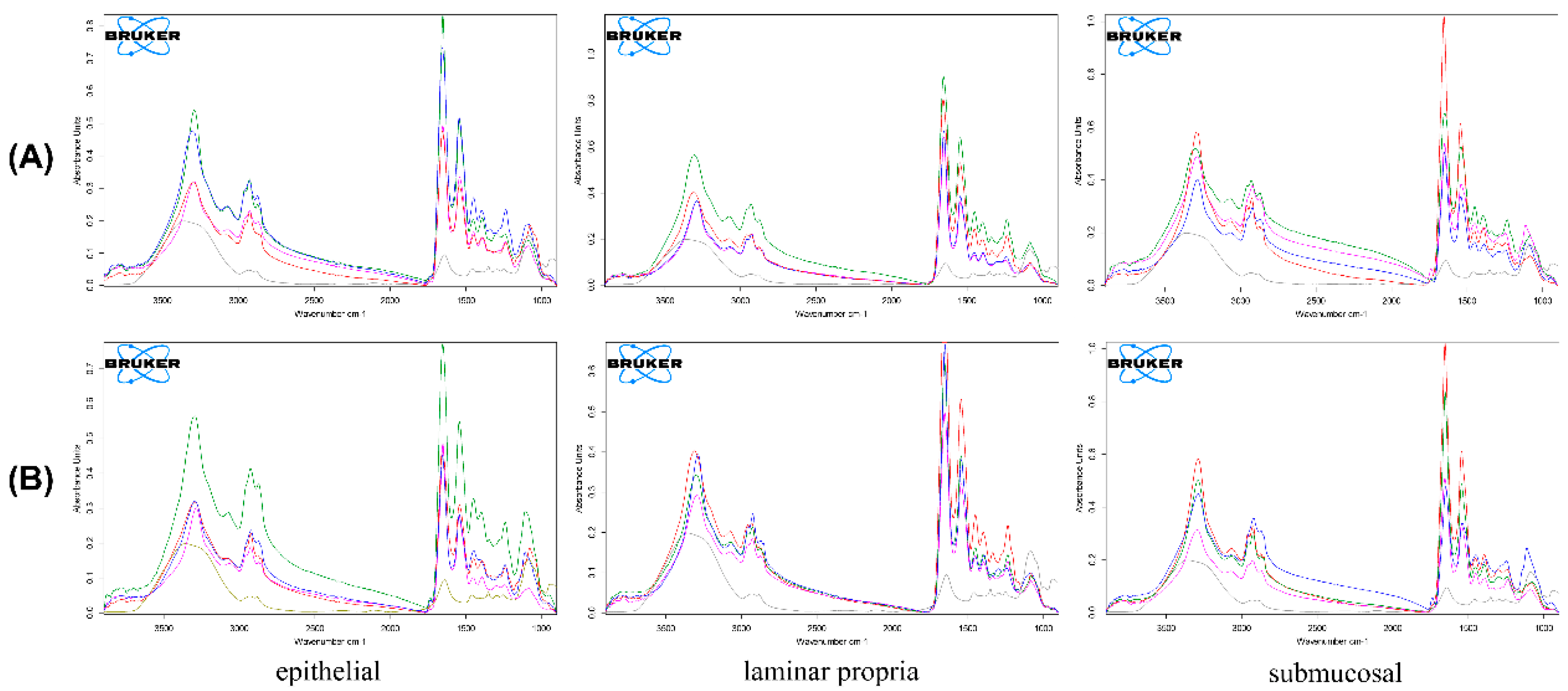
2.3.4. Fourier Transform Infrared Spectroscopy
2.3.5. Thermal Analysis Using Differential Scanning Calorimetry, Thermal Gravimetric Analysis, and Powder X-ray Diffraction Analysis
2.3.6. Microscopic Morphology
2.3.7. Prelimination Stability Testing
2.4. In Vitro Release and Permeation
2.5. Ex Vivo Drug Accumulation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effects of Independent Variables on Characterization of Polymeric Micelles
3.2. Effects of Independent Variables on Responses in Design Formulation
3.3. Percentage of FA Release and Permeation in Vitro Studies
3.4. Prelimination Stability Testing
3.5. Polymeric Micellar Characteristics and Physicochemical Properties
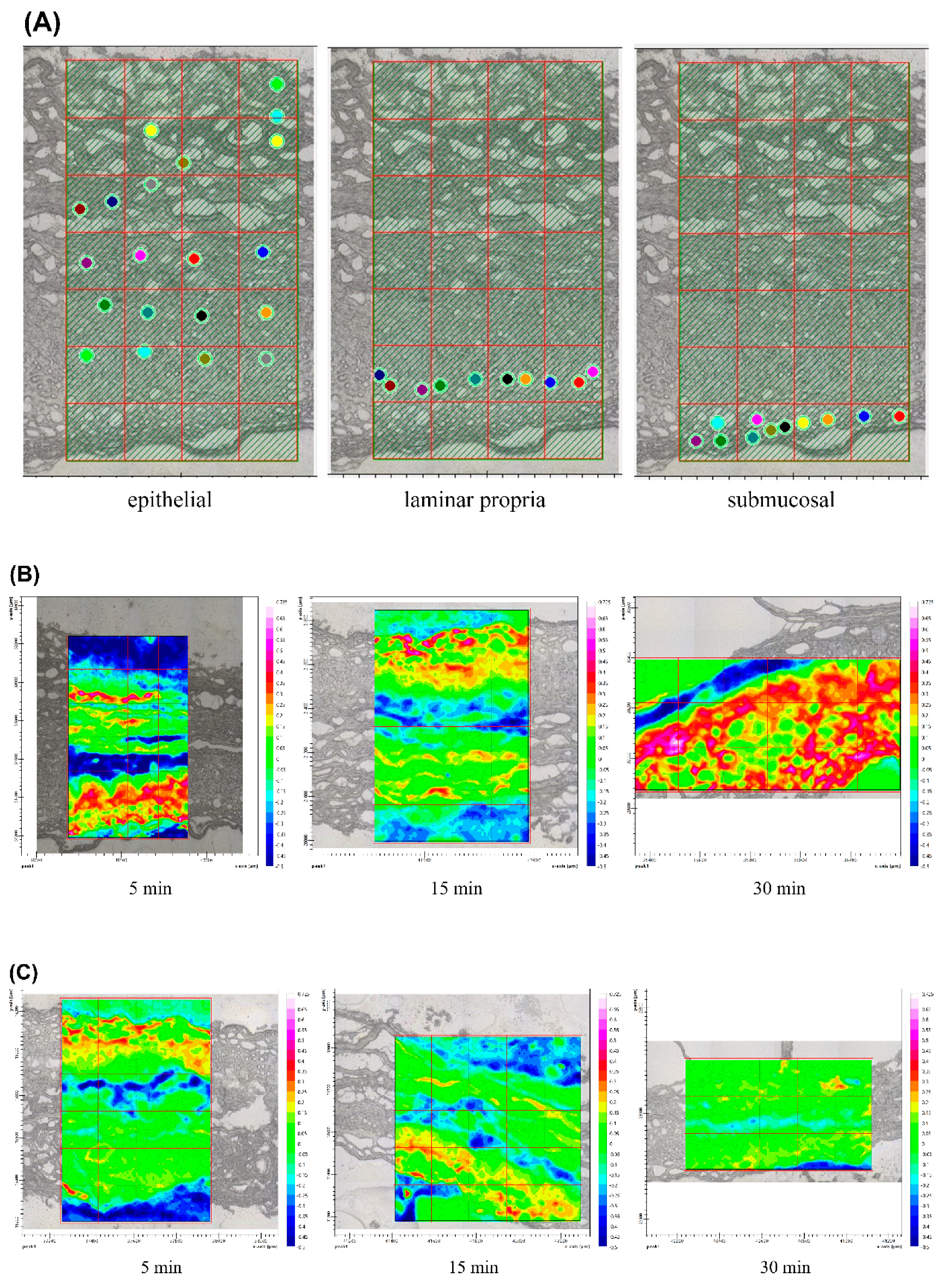
3.6. Ex Vivo Drug Accumulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lodi, G.; Manfredi, M.; Mercadante, V.; Murphy, R.; Carrozzo, M. Interventions for treating oral lichen planus: Corticosteroid therapies. Cochrane Database Syst. Rev. 2020, 2, CD001168. [Google Scholar] [CrossRef] [PubMed]
- Voûte, A.B.; Schulten, E.A.; Langendijk, P.N.; Kostense, P.J.; van der Waal, I. Fluocinonide in an adhesive base for treatment of oral lichen planus. A double-blind, placebo-controlled clinical study. Oral Surg. Oral Med. Oral Pathol. 1993, 75, 181–185. [Google Scholar] [CrossRef]
- Said, Z.; Murdoch, C.; Hansen, J.; Madsen, L.S.; Colley, H.E. Corticosteroid delivery using oral mucosa equivalents for the treatment of inflammatory mucosal diseases. Eur. J. Oral Sci. 2021, 129, e12761. [Google Scholar] [CrossRef] [PubMed]
- Ungphaiboon, S.; Nittayananta, W.; Vuddhakul, V.; Maneenuan, D.; Kietthubthew, S.; Wongpoowarak, W.; Phadoongsombat, N. Formulation and efficacy of triamcinolone acetonide mouthwash for treating oral lichen planus. Am. J. Health Syst. Pharm. 2005, 1, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Kim, S.Y.; Ha, K.W.; Kang, M.J.; Huh, J.S.; Kim, Y.M.; Park, Y.M.; Kang, K.H.; Lee, S.; Chang, J.Y. Pharmaceutical evaluation of genistein-loaded pluronic micelles for oral delivery. Arch. Pharm. Res. 2007, 30, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Uchegbu, I.F.; Breznikar, J.; Zaffalon, A.; Odunze, U.; Schätzlein, A.G. Polymeric Micelles for the Enhanced Deposition of Hydrophobic Drugs into Ocular Tissues, without Plasma Exposure. Pharmaceutics 2021, 13, 744. [Google Scholar] [CrossRef] [PubMed]
- Eshel-Green, T.; Bianco-Peled, H. Mucoadhesive acrylated block copolymers micelles for the delivery of hydrophobic drugs. Colloids Surf. B Biointerfaces 2016, 139, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, H.M.; Lavasanifar, A. Polymeric micelles for drug delivery. Expert Opin. Drug Deliv. 2006, 3, 139–162. [Google Scholar] [CrossRef]
- Aboud, H.M.; Mahmoud, M.O.; Abdeltawab Mohammed, M.; Shafiq Awad, M.; Sabry, D. Preparation and appraisal of self-assembled valsartan-loaded amalgamated Pluronic F127/Tween 80 polymeric micelles: Boosted cardioprotection via regulation of Mhrt/Nrf2 and Trx1 pathways in cisplatin-induced cardiotoxicity. J. Drug Target. 2020, 28, 282–299. [Google Scholar] [CrossRef]
- Sultan, A.A.; El-Gizawy, S.A.; Osman, M.A.; El Maghraby, G.M. Self-dispersing mixed micelles forming systems for enhanced dissolution and intestinal permeability of hydrochlorothiazide. Colloids Surf. B 2017, 149, 206–216. [Google Scholar] [CrossRef]
- Graça, A.; Gonçalves, L.; Raposo, S.; Ribeiro, H.; Marto, J. Useful in vitro techniques to evaluate the mucoadhesive properties of hyaluronic acid-based ocular delivery systems. Pharmaceutics 2018, 10, 110. [Google Scholar] [CrossRef] [Green Version]
- da Silva, J.; Ferreira, S.; Reis, A.; Cook, M.; Bruschi, M.L. 2018. Assessing mucoadhesion in polymer gels: The effect of method type and instrument variables. Polymers 2018, 10, 254. [Google Scholar] [CrossRef] [Green Version]
- Supachawaroj, N.; Damrongrungruang, T.; Limsitthichaikoon, S. Formulation development and evaluation of lidocaine hydrochloride loaded in chitosan-pectin-hyaluronic acid polyelectrolyte complex for dry socket treatment. Saudi Pharm. J. 2021, 29, 1070–1081. [Google Scholar] [CrossRef]
- Diaz-Del Consuelo, I.; Jacques, Y.; Pizzolato, G.P.; Guy, R.H.; Falson, F. Comparison of the lipid composition of porcine buccal and esophageal permeability barriers. Arch. Oral Biol. 2005, 50, 981–987. [Google Scholar] [CrossRef]
- Diaz-Del Consuelo, I.; Pizzolato, G.-P.; Falson, F.; Guy, R.H.; Jacques, Y. Evaluation of pig esophageal mucosa as a permea-bility barrier model for buccal tissue. J. Pharm. Sci. 2005, 94, 2777–2788. [Google Scholar] [CrossRef]
- Limsitthichaikoon, S.; Khampaenjiraroch, B.; Damrongrungruang, T.; Limphirat, W.; Thapphasaraphong, S.; Priprem, A. Topical oral wound healing potential of anthocyanin complex: Animal and clinical studies. Ther. Deliv. 2018, 9, 359–374. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Fakhari, A.; Corcoran, M.; Schwarz, A. Thermogelling properties of purified poloxamer 407. Heliyon 2017, 3, e00390. [Google Scholar] [CrossRef]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef] [Green Version]
- Alexandridis, P.; Olsson, U.; Lindman, B. A Record Nine Different Phases (Four Cubic, Two Hexagonal, and One Lamellar Lyotropic Liquid Crystalline and Two Micellar Solutions) in a Ternary Isothermal System of an Amphiphilic Block Copolymer and Selective Solvents (Water and Oil). Langmuir 1998, 14, 2627–2638. [Google Scholar] [CrossRef]
- Yuan, X.; Krueger, S.; Sztucki, M.; Jones, R.L.; Curtis, J.E.; Shalaev, E. Phase Behavior of Poloxamer 188 Aqueous Solutions at Subzero Temperatures: A Neutron and X-ray Scattering Study. J. Phys. Chem. B 2021, 125, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhi, M.; Formela, K.; et al. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, M.; Yang, X.; Sun, W.; Lu, C.; Hui, Q.; Shi, C.; Li, X.; Wang, X. Modified poloxamer 407 and hyaluronic acid thermosensitive hydrogel-encapsulated keratinocyte growth factor 2 improves knee osteoarthritis in rats. Mater. Des. 2021, 210, 110086. [Google Scholar] [CrossRef]
- Artzner, F.; Geiger, S.; Olivier, A.; Allais, C.; Finet, S.; Agnely, F. Interactions between Poloxamers in Aqueous Solutions: Micellization and Gelation Studied by Differential Scanning Calorimetry, Small Angle X-ray Scattering, and Rheology. Langmuir 2007, 23, 5085–5092. [Google Scholar] [CrossRef]
- Ivanova, R.; Lindman, B.; Alexandridis, P. Effect of Pharmaceutically Acceptable Glycols on the Stability of the Liquid Crystalline Gels Formed by Poloxamer 407 in Water. J. Colloid Interf. Sci. 2002, 252, 226–235. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int. J. Nanomed. 2018, 13, 2921–2942. [Google Scholar] [CrossRef] [Green Version]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, R.; Li, L.; Li, B.; Zhang, X.; Su, J. Mechanical, rheological and release behaviors of a poloxamer 407/poloxamer 188/carbopol 940 thermosensitive composite hydrogel. Molecules 2003, 18, 12415–12425. [Google Scholar] [CrossRef]
- Vecchi, C.F.; Cesar, G.B.; Souza, P.R.; Caetano, W.; Bruschi, M.L. Mucoadhesive polymeric films comprising polyvinyl alcohol, polyvinylpyrrolidone, and poloxamer 407 for pharmaceutical applications. Pharm. Dev. Technol. 2021, 26, 138–149. [Google Scholar] [CrossRef]
- Sasaki, S.; Machida, G.; Nakanishi, R.; Kinoshita, M.; Akiba, I. Elucidation of Spatial Distribution of Hydrophobic Aromatic Compounds Encapsulated in Polymer Micelles by Anomalous Small-Angle X-ray Scattering. Polymers 2018, 10, 180. [Google Scholar] [CrossRef] [Green Version]
- Tundisi, L.L.; Yang, R.; Borelli, L.P.P.; Alves, T.; Mehta, M.; Chaud, M.V.; Mazzola, P.G.; Kohane, D.S. Enhancement of the Mechanical and Drug-Releasing Properties of Poloxamer 407 Hydrogels with Casein. Pharm. Res. 2021, 38, 515–522. [Google Scholar] [CrossRef]
- Mhlanga, N.; Ray, S.S. Kinetic models for the release of the anticancer drug doxorubicin from biodegradable polylactide/metal oxide-based hybrids. Int. J. Biol. Macromol. 2015, 72, 1301–1307. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Tsai, H.C.; Chang, C.H.; Chiu, Y.C.; Lin, S.Y.; Lin, C.P.; Hsiue, G.H. In vitro evaluation of hexagonal polymeric micelles in macrophage phagocytosis. Macromol Rapid Commun. 2011, 32, 1442–1446. [Google Scholar] [CrossRef]
- Dong, Y.-D.; Boyd, B.J. Applications of X-ray scattering in pharmaceutical science. Int. J. Pharm. 2011, 417, 101–111. [Google Scholar] [CrossRef]
- Brizuela, M.; Winters, R. Histology, Oral Mucosa. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572115/ (accessed on 9 June 2021).
- Thongprasom, K. A Review of the Effectiveness and Side-Effects of Fluocinolone Acetonide 0.1% in the Treatment of Oral Mucosal Diseases. Acta Stomatol. Croat. 2017, 51, 240–247. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, J.S.M.; Volpato, M.C.; Muniz, B.V.; Xavier, G.G.A.; Martinelli, C.C.M.; Lopez, R.F.V.; Groppo, F.C.; Franz-Montan, M. Resistivity Technique for the Evaluation of the Integrity of Buccal and Esophageal Epithelium Mucosa for In Vitro Permeation Studies: Swine Buccal and Esophageal Mucosa Barrier Models. Pharmaceutics 2021, 30, 643. [Google Scholar] [CrossRef]
- Zielińska, K.A.; Van Moortel, L.; Opdenakker, G.; De Bosscher, K.; Van den Steen, P.E. Endothelial Response to Glucocorticoids in Inflammatory Diseases. Front. Immunol. 2016, 7, 592. [Google Scholar] [CrossRef]
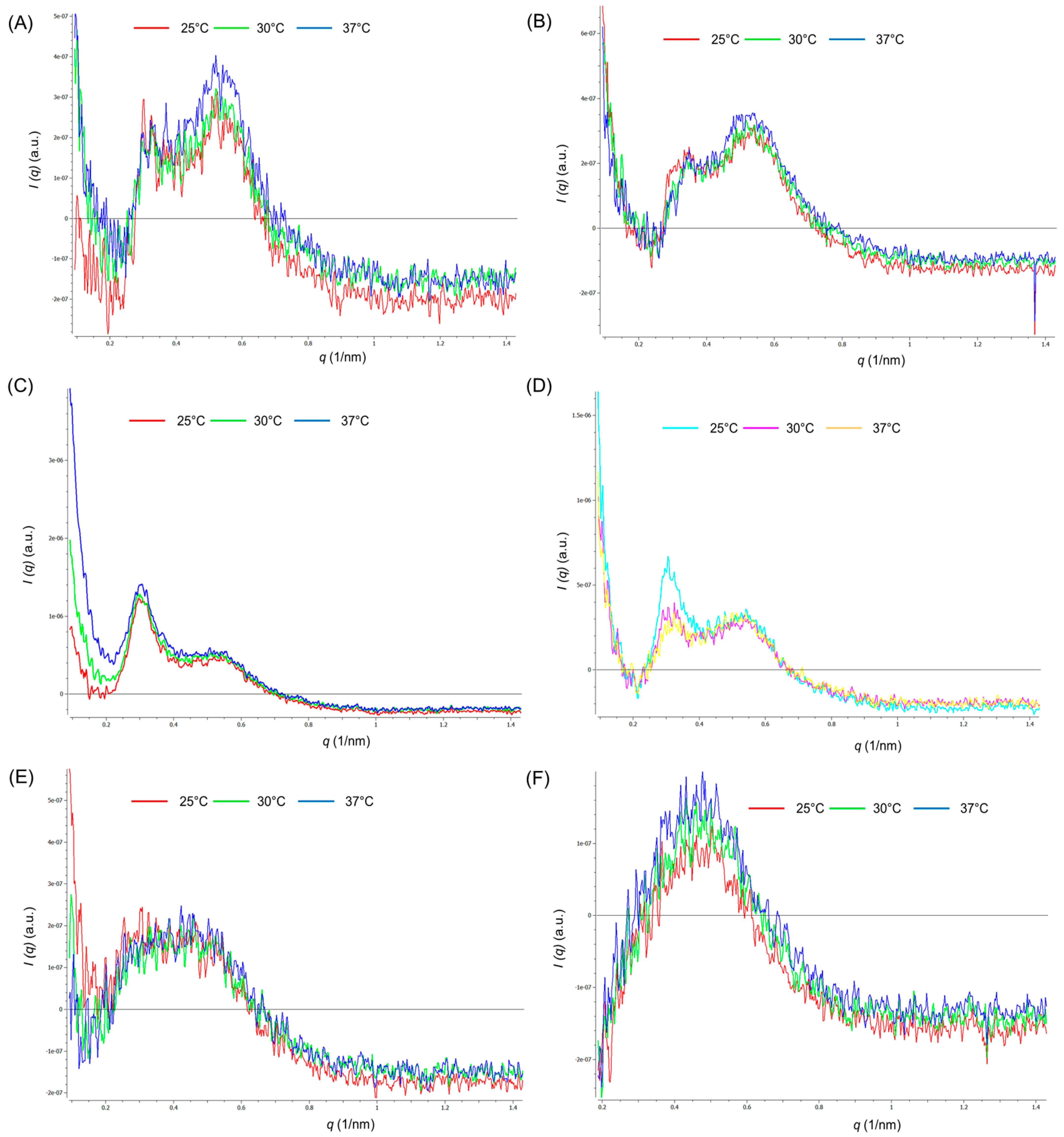
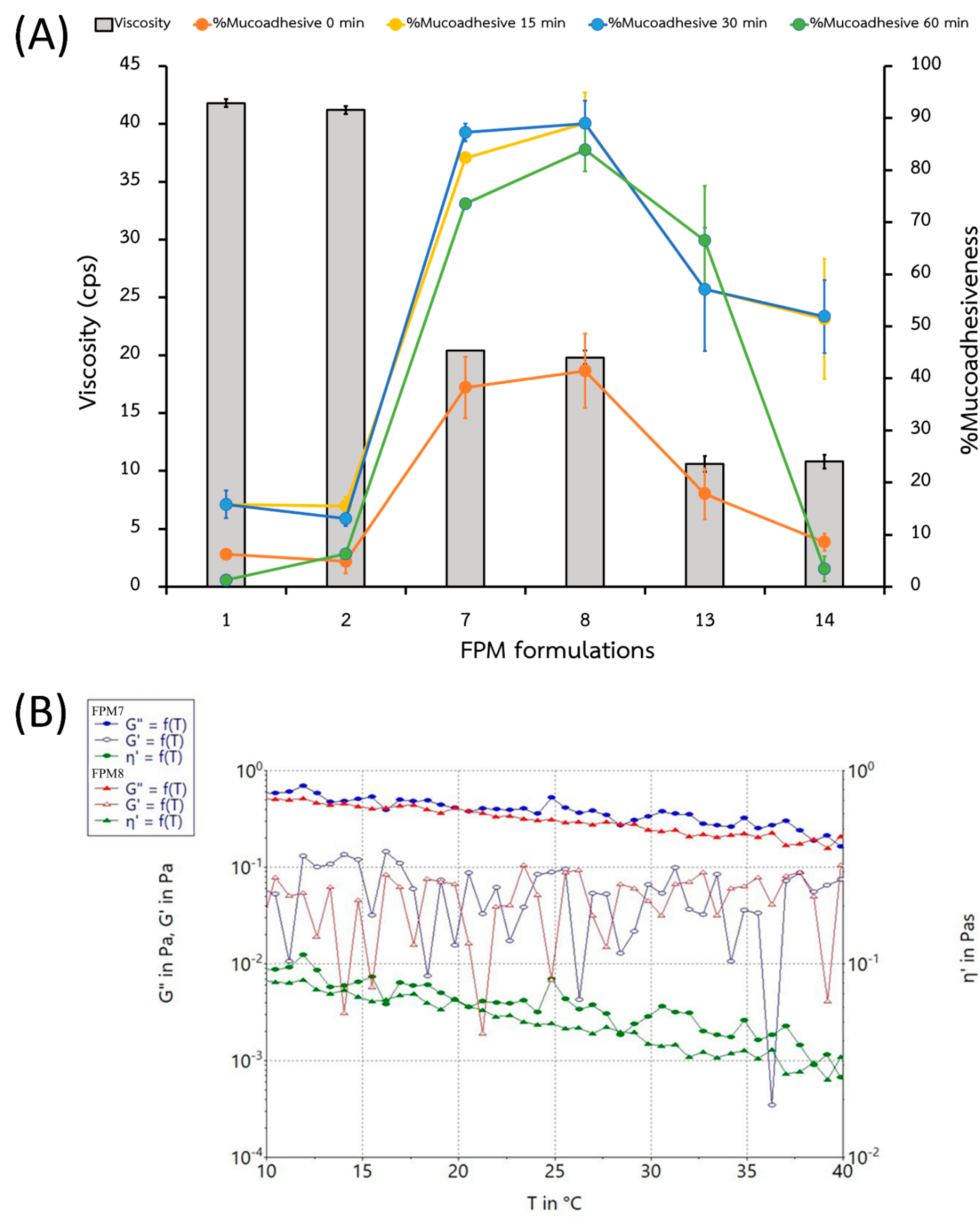
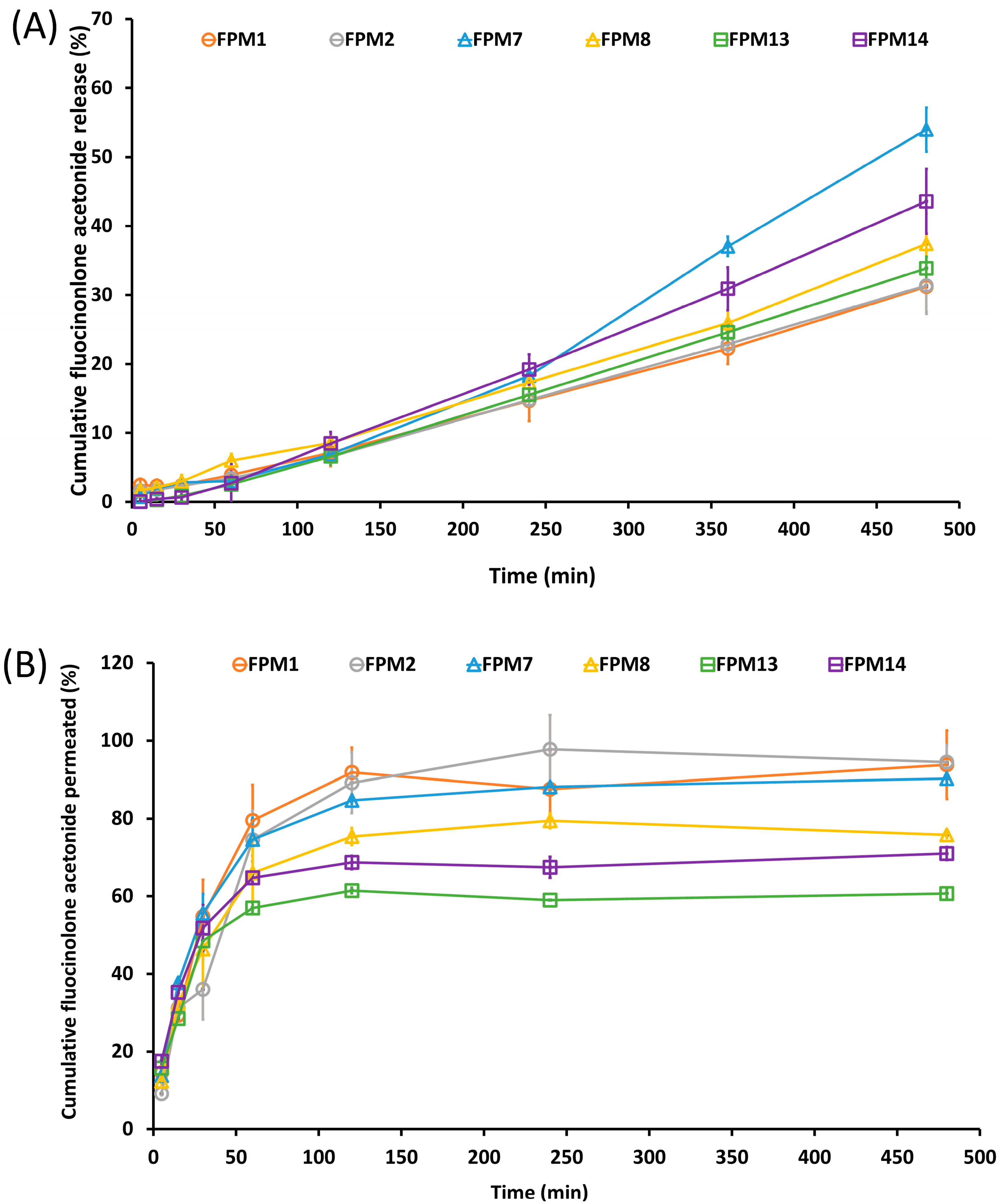


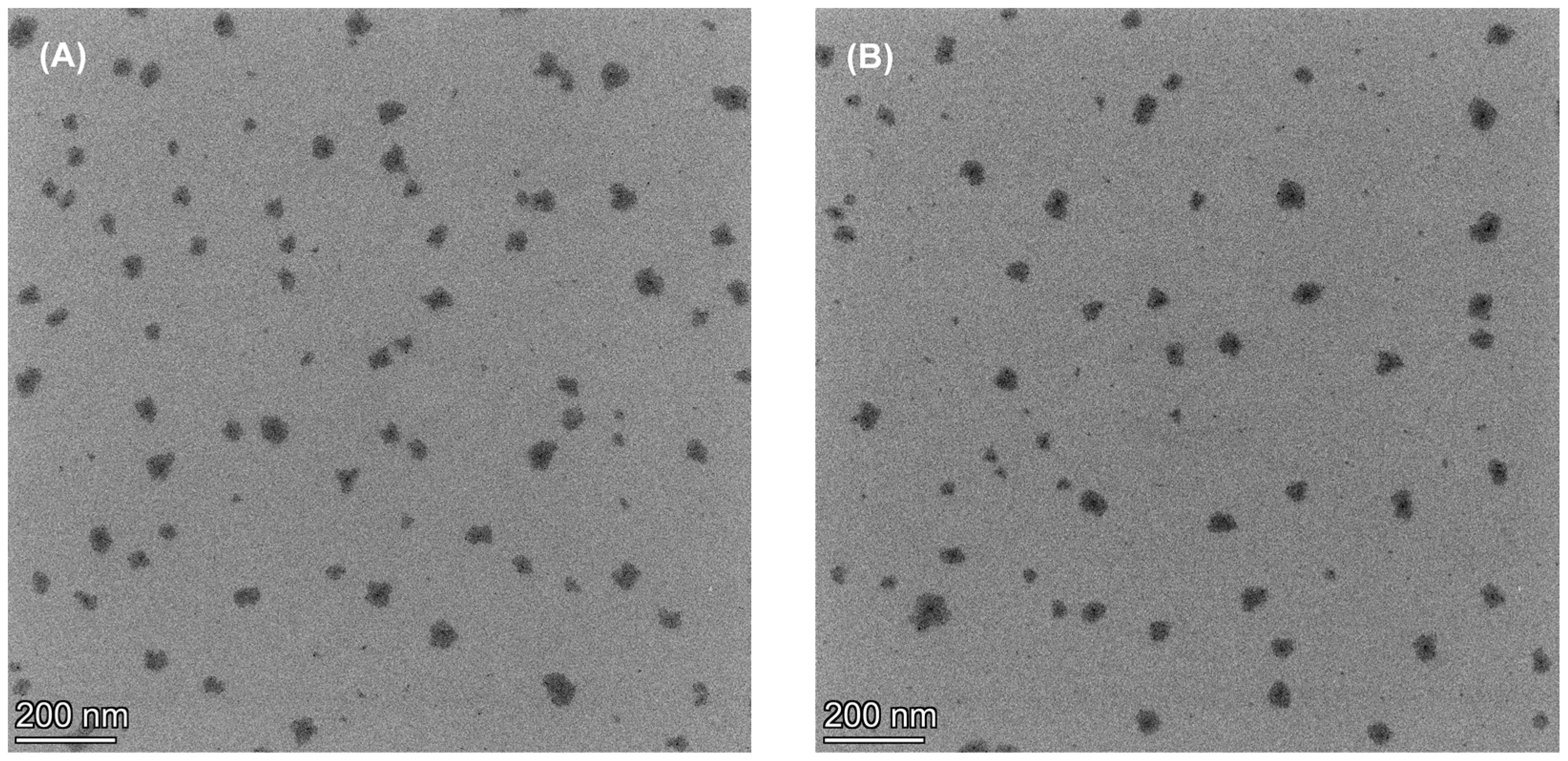
| Rx | P407 (w/v%) | PEG (w/v%) | SPA (w/v%) | Appearance (after Preparation) | Particle Size (nm) | PDI | Zeta potential (mV) | Appearance (at 7 Days) |
|---|---|---|---|---|---|---|---|---|
| FPM1 | 10 | 15 | 0.01 | Suspension | 3827.47 ± 300.36 | 0.52 ± 0.03 | −6.41 ± 1.27 | suspension |
| FPM2 | 10 | 15 | 0 | Suspension | 2080.8 ± 945.62 | 0.44 ± 0.04 | 4.83 ± 9.18 | suspension |
| FPM3 | 10 | 10 | 0.01 | Suspension | 2341.93 ± 635.85 | 0.54 ± 0.02 | −13.28 ± 1.59 | precipitated |
| FPM4 | 10 | 10 | 0 | Suspension | 1212.70 ± 886.53 | 0.42 ± 0.04 | 6.50 ± 1.15 | precipitated |
| FPM5 | 10 | 5 | 0.01 | Suspension | 316.63 ± 158.44 | 0.20 ± 0.01 | ND | precipitated |
| FPM6 | 10 | 5 | 0 | Suspension | 188.80 ± 92.59 | 0.21 ± 0.08 | ND | precipitated |
| FPM7 | 7.5 | 15 | 0.01 | clear solution | 96.20 ± 11.17 | 0.07 ± 0.004 | −21.23 ± 1.70 | clear solution |
| FPM8 | 7.5 | 15 | 0 | clear solution | 93.50 ± 12.98 | 0.08 ± 0.002 | 19.70 ± 1.80 | clear solution |
| FPM9 | 7.5 | 10 | 0.01 | clear solution | 103.22 ± 36.87 | >0.6 | ND | precipitated |
| FPM10 | 7.5 | 10 | 0 | clear solution | 98.62 ± 46.72 | >0.6 | ND | precipitated |
| FPM11 | 7.5 | 5 | 0.01 | clear solution | 54.03 ± 32.11 | >0.6 | ND | precipitated |
| FPM12 | 7.5 | 5 | 0 | clear solution | 48.14 ± 24.87 | >0.6 | ND | precipitated |
| FPM13 | 5 | 15 | 0.01 | clear solution | 24.12 ± 0.48 | 0.22 ± 0.08 | −3.23 ± 0.47 | clear solution |
| FPM14 | 5 | 15 | 0 | clear solution | 12.34 ± 0.12 | 0.24 ± 0.11 | 0.11 ± 0.70 | clear solution |
| FPM15 | 5 | 10 | 0.01 | Precipitated | ND | ND | ND | precipitated |
| FPM16 | 5 | 10 | 0 | Precipitated | ND | ND | ND | precipitated |
| FPM17 | 5 | 5 | 0.01 | Precipitated | ND | ND | ND | precipitated |
| FPM18 | 5 | 5 | 0 | Precipitated | ND | ND | ND | precipitated |
| Rx | Viscosity (cps) | %Mucoadhesive at 0 min | %Mucoadhesive at 15 min | %Drug Release | %Drug Permeation |
|---|---|---|---|---|---|
| FPM1 | 41.8 ± 0.35 | 6.2 ± 0.88 | 15.8 ± 2.63 | 2.47 ± 0.001 | 14.58 ± 0.03 |
| FPM2 | 41.2 ± 0.35 | 4.9 ± 2.27 | 15.5 ± 1.62 | 1.64 ± 0.004 | 9.15 ± 0.001 |
| FPM7 | 20.4 ± 0.02 | 38.2 ± 5.88 | 82.4 ± 0.04 | 1.70 ± 0.001 | 17.76 ± 0.001 |
| FPM8 | 19.8 ± 0.60 | 41.4 ± 7.16 | 89.0 ± 5.93 | 0.76 ± 0.003 | 12.37 ± 0.03 |
| FPM13 | 10.6 ± 0.69 | 17.8 ± 4.90 | 57.1 ± 11.84 | 0.07 ± 0.000 | 17.54 ± 0.03 |
| FPM14 | 10.8 ± 0.60 | 8.6 ± 1.69 | 51.4 ± 11.49 | 0.01 ± 0.000 | 15.74 ± 0.04 |
| Kinetic Models | Zero-Order | First-Order | Higuchi | Korsmeyer-Peppas | |||||
|---|---|---|---|---|---|---|---|---|---|
| K0 | r2 | K1 | r2 | KH | r2 | KKP | n | r2 | |
| Formulation | |||||||||
| FPM1 | 0.0610 | 0.9933 | 0.0025 | 0.9464 | 1.4362 | 0.9191 | 0.058 | 1.015 | 0.9902 |
| FPM2 | 0.0633 | 0.9949 | 0.0028 | 0.9364 | 1.4923 | 0.9243 | 0.058 | 1.014 | 0.9902 |
| FPM7 | 0.1095 | 0.9710 | 0.0035 | 0.9130 | 2.5346 | 0.8694 | 0.074 | 1.003 | 0.9922 |
| FPM8 | 0.0732 | 0.9944 | 0.0027 | 0.8979 | 1.7334 | 0.9323 | 0.007 | 1.459 | 0.9958 |
| FPM13 | 0.0721 | 0.9979 | 0.0038 | 0.8261 | 1.7084 | 0.9368 | 0.024 | 1.173 | 0.9993 |
| FPM14 | 0.0922 | 0.9963 | 0.0049 | 0.7507 | 2.1798 | 0.9300 | 0.024 | 1.216 | 0.9991 |
| Kinetic Models | Zero-Order | First-Order | Higuchi | Korsmeyer-Peppas | |||||
|---|---|---|---|---|---|---|---|---|---|
| K0 | r2 | K1 | r2 | KH | r2 | KKP | n | r2 | |
| Formulation | |||||||||
| FPM1 | 1.1750 | 0.9688 | 0.0125 | 0.8683 | 12.129 | 0.9913 | 5.191 | 0.671 | 0.9918 |
| FPM2 | 1.1113 | 0.9604 | 0.0142 | 0.8073 | 11.266 | 0.9480 | 2.989 | 0.780 | 0.9650 |
| FPM7 | 1.0338 | 0.9132 | 0.0115 | 0.7463 | 10.942 | 0.9824 | 6.293 | 0.578 | 0.9896 |
| FPM8 | 0.9142 | 0.9389 | 0.0115 | 0.7751 | 9.5896 | 0.9921 | 7.694 | 0.562 | 0.9813 |
| FPM13 | 0.7322 | 0.8755 | 0.0094 | 0.7894 | 7.7891 | 0.9515 | 8.189 | 0.485 | 0.9691 |
| FPM14 | 0.8113 | 0.9024 | 0.0092 | 0.7828 | 8.6120 | 0.9764 | 9.570 | 0.475 | 0.9864 |
| Rx | Day | pH | Percentage of Drug Content (%) | ||
|---|---|---|---|---|---|
| 4 ± 2 °C | 25 ± 2 °C | 45 ± 2 °C | |||
| 1 | 0 | 7.42 ± 0.01 | 100.5 ± 0.01 | 100.5 ± 0.01 | 100.5 ± 0.01 |
| 7 | 101.2 ± 0.01 | 100.2 ± 0.01 | 101.2 ± 0.01 | ||
| 30 | 103.0 ± 0.04 | 97.6 ± 0.06 a | 91.8 ± 0.01 a | ||
| 2 | 0 | 7.42 ± 0.01 | 100.9 ± 0.01 | 100.9 ± 0.02 | 100.9 ± 0.02 |
| 7 | 103.2 ± 0.09 | 101.1 ± 0.04 | 99.12 ± 0.01 | ||
| 30 | 97.8 ± 0.07 b | 91.7 ± 0.03 b | 91.0 ± 0.04 b | ||
| 7 | 0 | 7.42 ± 0.01 | 104.1 ± 0.01 | 101.4 ± 0.01 | 104.1 ± 0.01 |
| 7 | 100.6 ± 0.04 | 100.3 ± 0.02 | 102.3 ± 0.02 | ||
| 30 | 100.4 ± 0.03 | 100.0 ± 0.01 | 100.4 ± 0.01 | ||
| 8 | 0 | 7.42 ± 0.01 | 100.3 ± 0.03 | 100.3 ± 0.03 | 100.3 ± 0.03 |
| 7 | 100.2 ± 0.01 | 100.2 ± 0.01 | 100.6 ± 0.03 | ||
| 30 | 100.0 ± 0.04 | 100.1 ± 0.01 | 100.0 ± 0.01 | ||
| 13 | 0 | 7.42 ± 0.01 | 100.4 ± 0.01 | 100.4 ± 0.01 | 100.4 ± 0.01 |
| 7 | 100.2 ± 0.02 | 99.4 ± 0.05 | 95.2 ± 0.07 c | ||
| 30 | 100.1 ± 0.01 | 98.5 ± 0.08 c | 87.4 ± 0.09 c | ||
| 14 | 0 | 7.42 ± 0.01 | 100.3 ± 0.01 | 100.3 ± 0.01 | 100.3 ± 0.01 |
| 7 | 102.2 ± 0.04 | 100.2 ± 0.01 | 87.9 ± 0.07 d | ||
| 30 | 95.3 ± 0.06 d | 97.4 ± 0.08 d | 59.0 ± 0.08 d | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limsitthichaikoon, S.; Soontaranon, S.; Hanpramukkun, N.; Thumanu, K.; Priprem, A. Polymeric Micelles Enhance Mucosal Contact Time and Deposition of Fluocinolone Acetonide. Polymers 2022, 14, 2247. https://doi.org/10.3390/polym14112247
Limsitthichaikoon S, Soontaranon S, Hanpramukkun N, Thumanu K, Priprem A. Polymeric Micelles Enhance Mucosal Contact Time and Deposition of Fluocinolone Acetonide. Polymers. 2022; 14(11):2247. https://doi.org/10.3390/polym14112247
Chicago/Turabian StyleLimsitthichaikoon, Sucharat, Siriwat Soontaranon, Nuntachai Hanpramukkun, Kanjana Thumanu, and Aroonsri Priprem. 2022. "Polymeric Micelles Enhance Mucosal Contact Time and Deposition of Fluocinolone Acetonide" Polymers 14, no. 11: 2247. https://doi.org/10.3390/polym14112247
APA StyleLimsitthichaikoon, S., Soontaranon, S., Hanpramukkun, N., Thumanu, K., & Priprem, A. (2022). Polymeric Micelles Enhance Mucosal Contact Time and Deposition of Fluocinolone Acetonide. Polymers, 14(11), 2247. https://doi.org/10.3390/polym14112247







