The Effect of Different Dosages of TESPT on Metal Friction and Metal Wear in the Mixing Process
Abstract
:1. Introduction
2. Experiment
2.1. Instrument
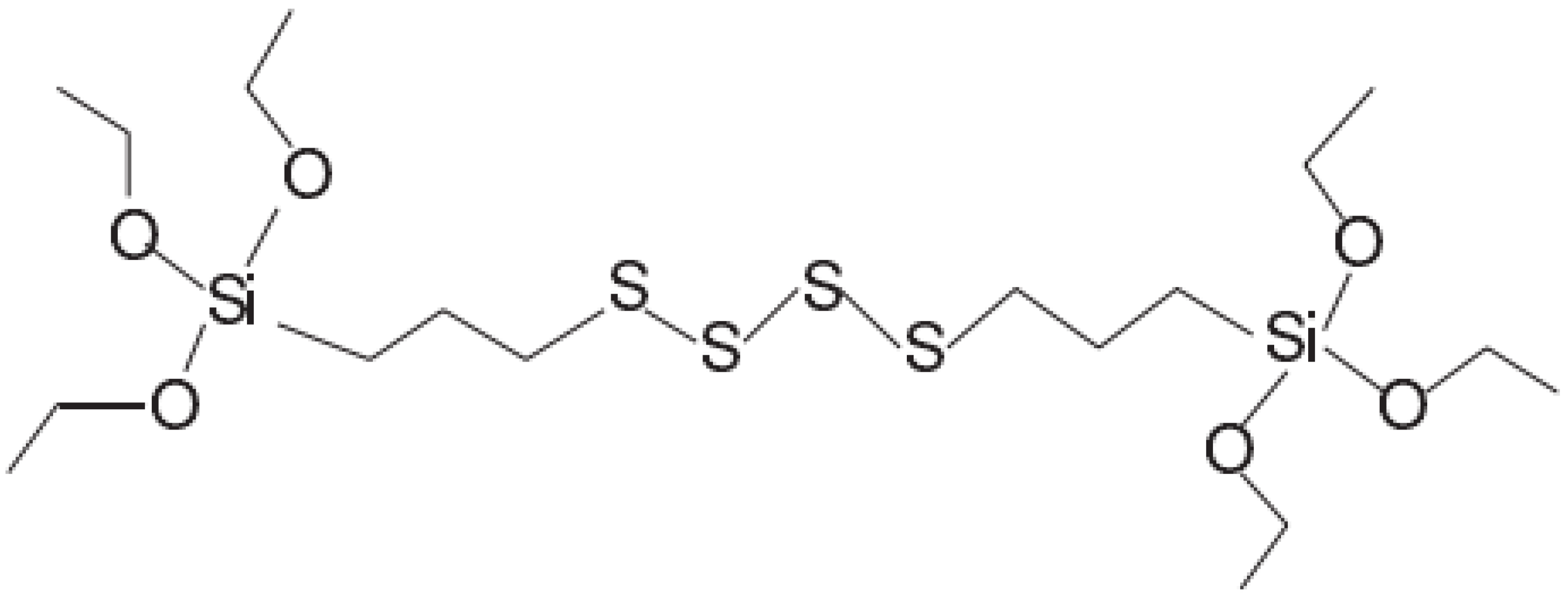
2.2. Chemical Composition
2.3. Mixing Process
2.4. Testing Method
- (1)
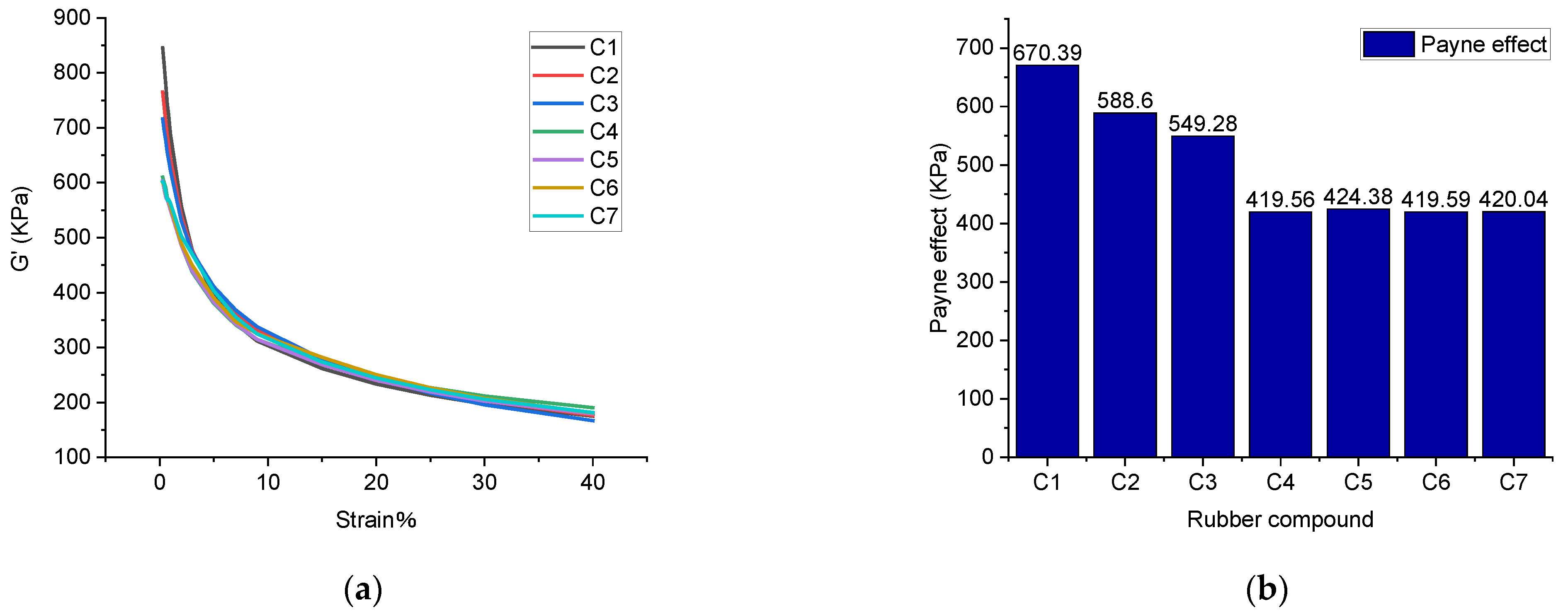
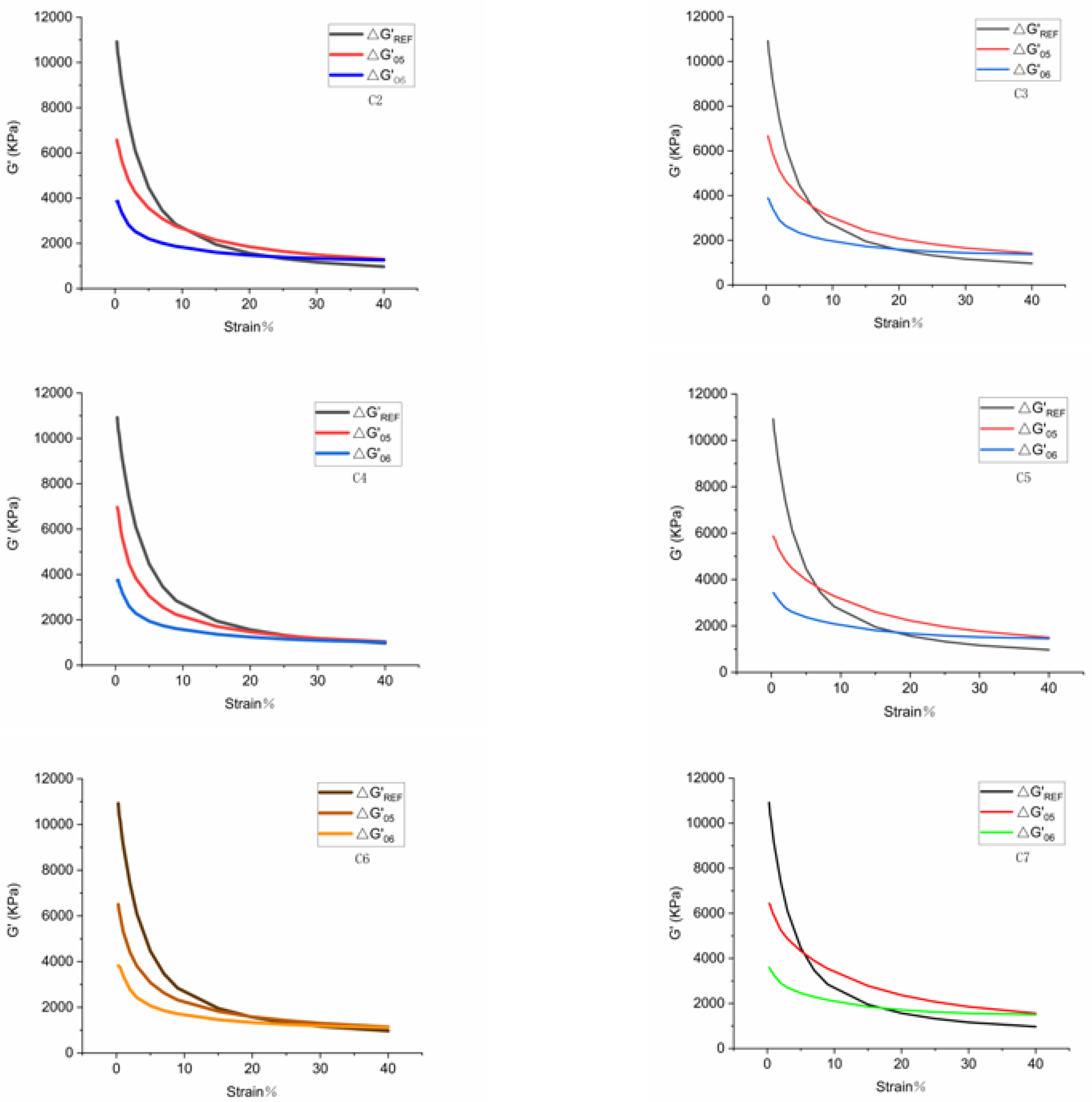
- Payne effect: rubber processing performance analyzer was used to scan deformations on seven rubber compounds. The scanning test was carried out at a scanning frequency of 1 Hz, scanning range of 0.28–40%, and sample temperature of 60 °C. Accordingly, the curve of dynamic modulus Gversus strain was obtained. It is worth noting that the Payne effect originates from the destruction of the network structure between filler and filler. Accordingly, the Payne effect refers to the phenomenon that the dynamic modulus of filled rubber decreases sharply as the strain increases. Generally, the higher the filler aggregation, the worse the dispersibility of the filler, and the more obvious the Payne effect. Therefore, the Payne effect is widely used to reflect the dispersibility of the filler [11,12,13,14,15].
- (2)
- Silylation reaction index: The rubber processing analyzer was utilized to test the silanization reaction index and measure the degree of the silanization reaction. In this regard, the settings are presented in Table 3.
- (3)
- Friction-and-wear test: A CSM was used in the experiment to carry out the friction- and-wear test. After calibrating the CSM, the pressure, rotating speed, and experiment time were set to 5 N, 80 r/min, and 120 min, respectively. To study the wear of the mixing chamber after long-term use, the selected metal grinding head was not coated. To ensure the authenticity of the experiment, the grinding head and the mixing section were made of the same material. Studies showed that the rubber compound had the most serious wear on the metal in the final stage of the investigation [36,37,38]. Accordingly, the CSM temperature was set to 150 °C. The principal diagram of the CSM wear experiment is shown in Figure 2.
- (4)
- (5)
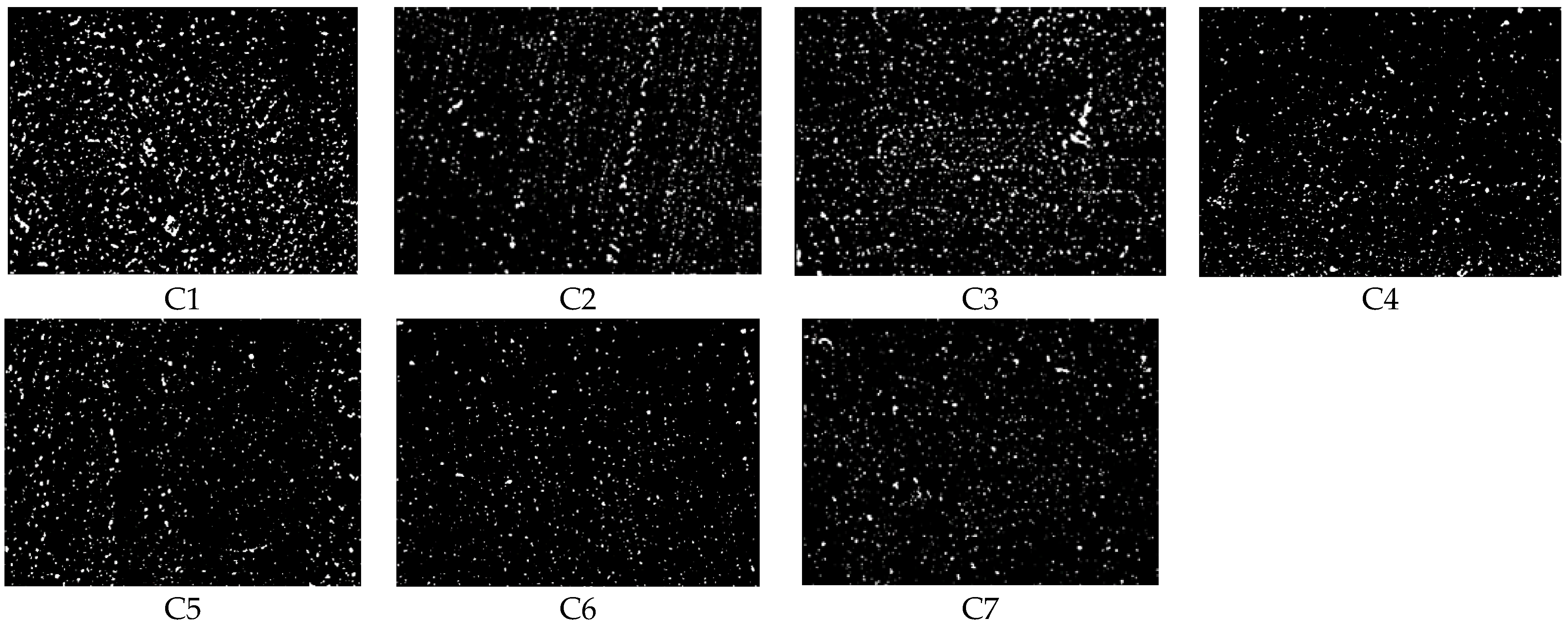
- Dispersion test: A dispersion meter was used to test the degree of dispersion and obtain the dispersion value according to the ASTM D7723 standard.
3. Silanization Reaction Mechanism
4. Experiment Results
4.1. Filler Dispersion Analysis
4.1.1. Payne Effect
4.1.2. Dispersion Comparison
4.2. Silanization Reaction Index
4.3. The Effect of Rubber Compounds with Different Amountsof TESPT on Metal Friction and Wear
4.3.1. Friction Coefficient
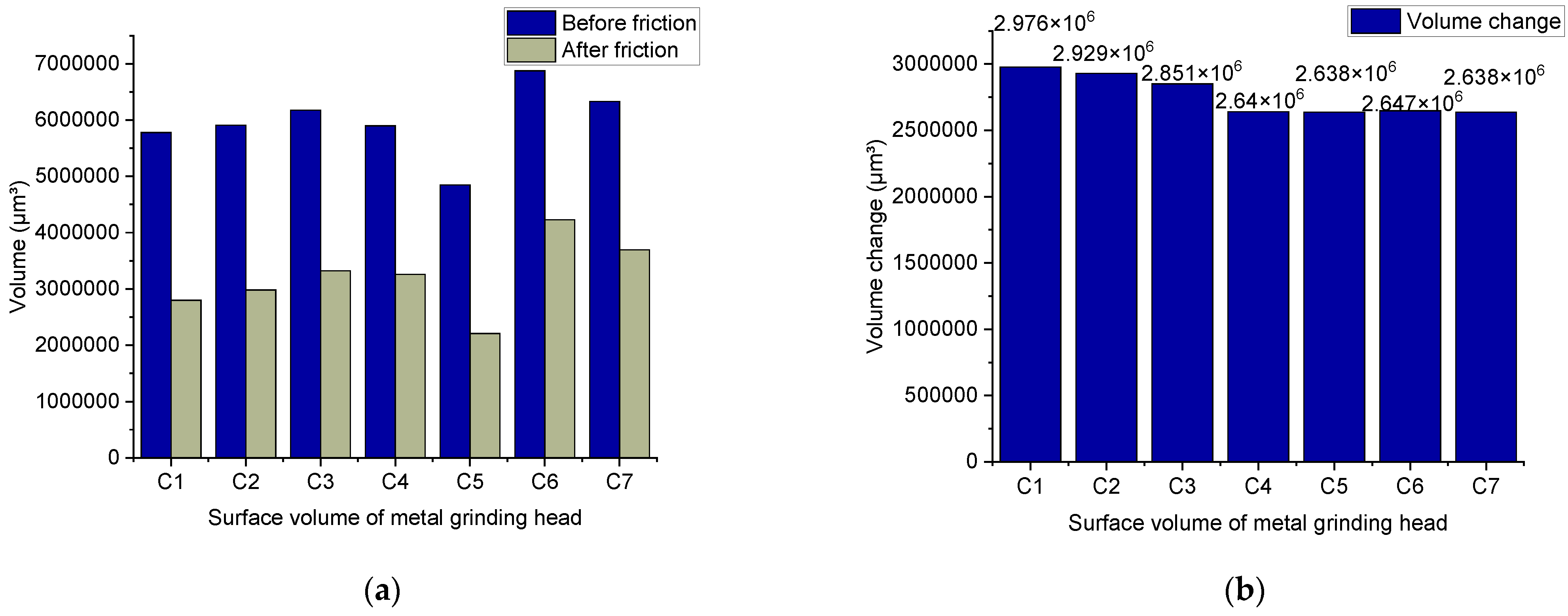
4.3.2. Metal Surface Observation
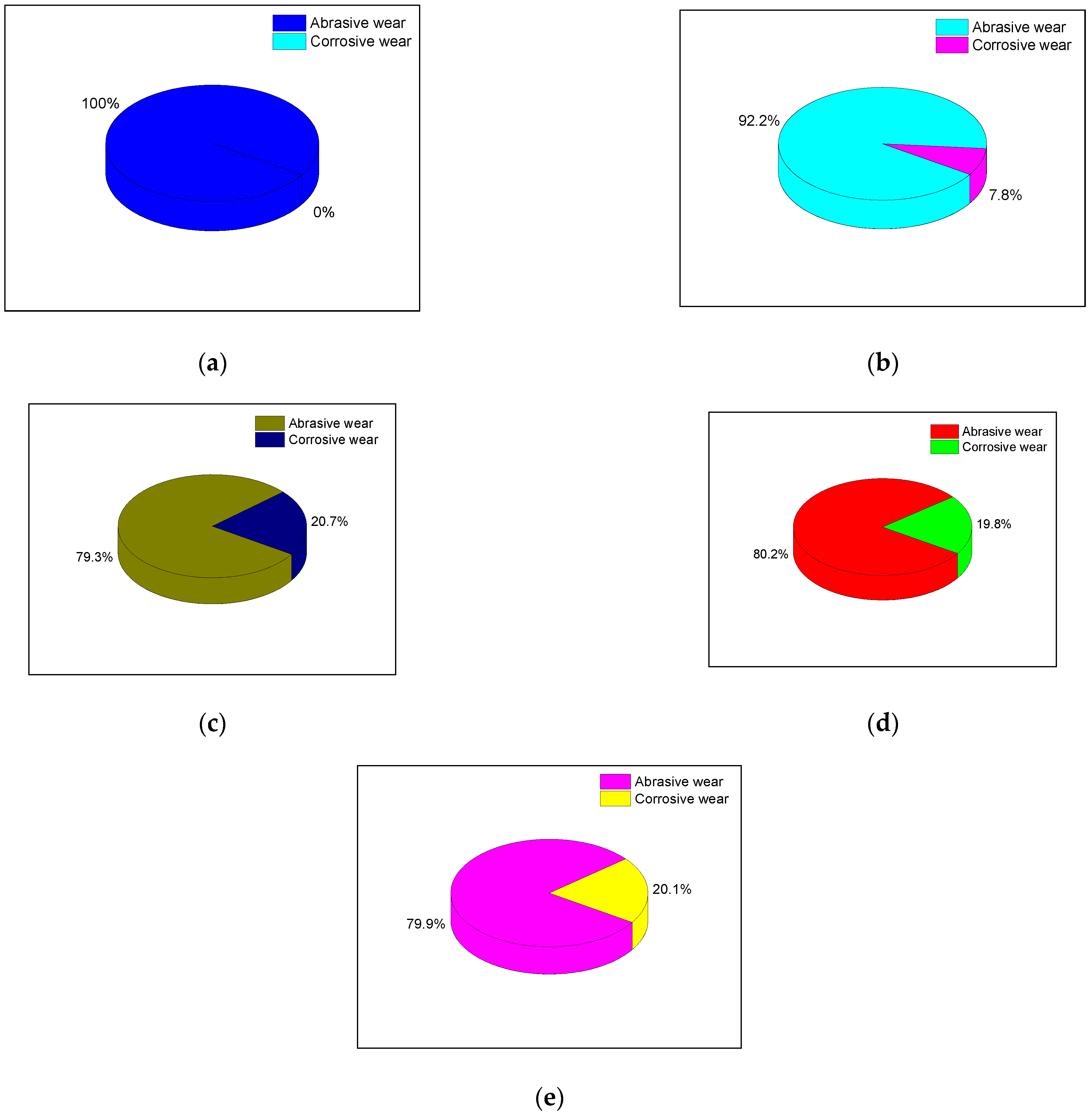
4.3.3. The Proportion of Corrosion Wear and Abrasive Wear
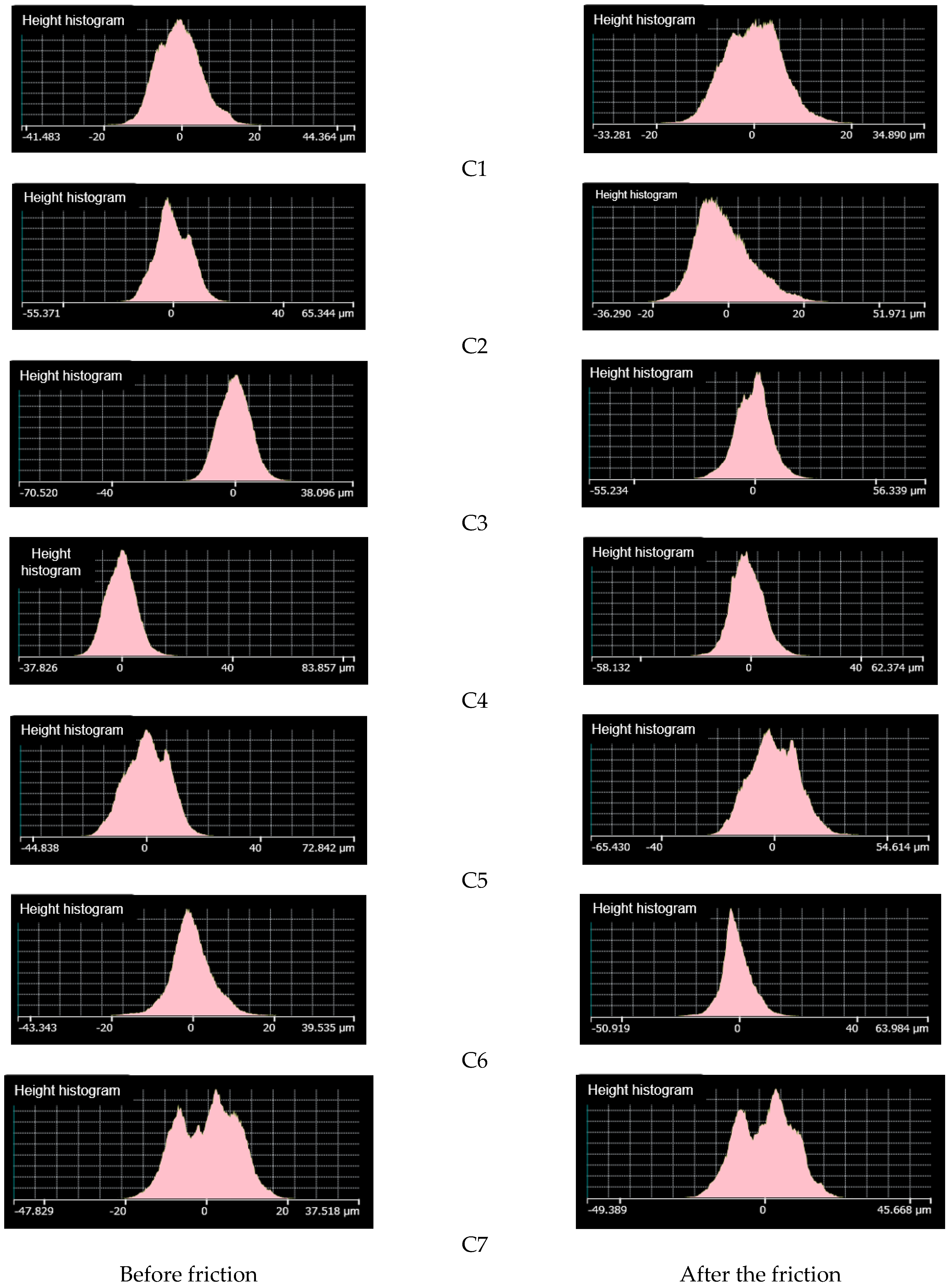
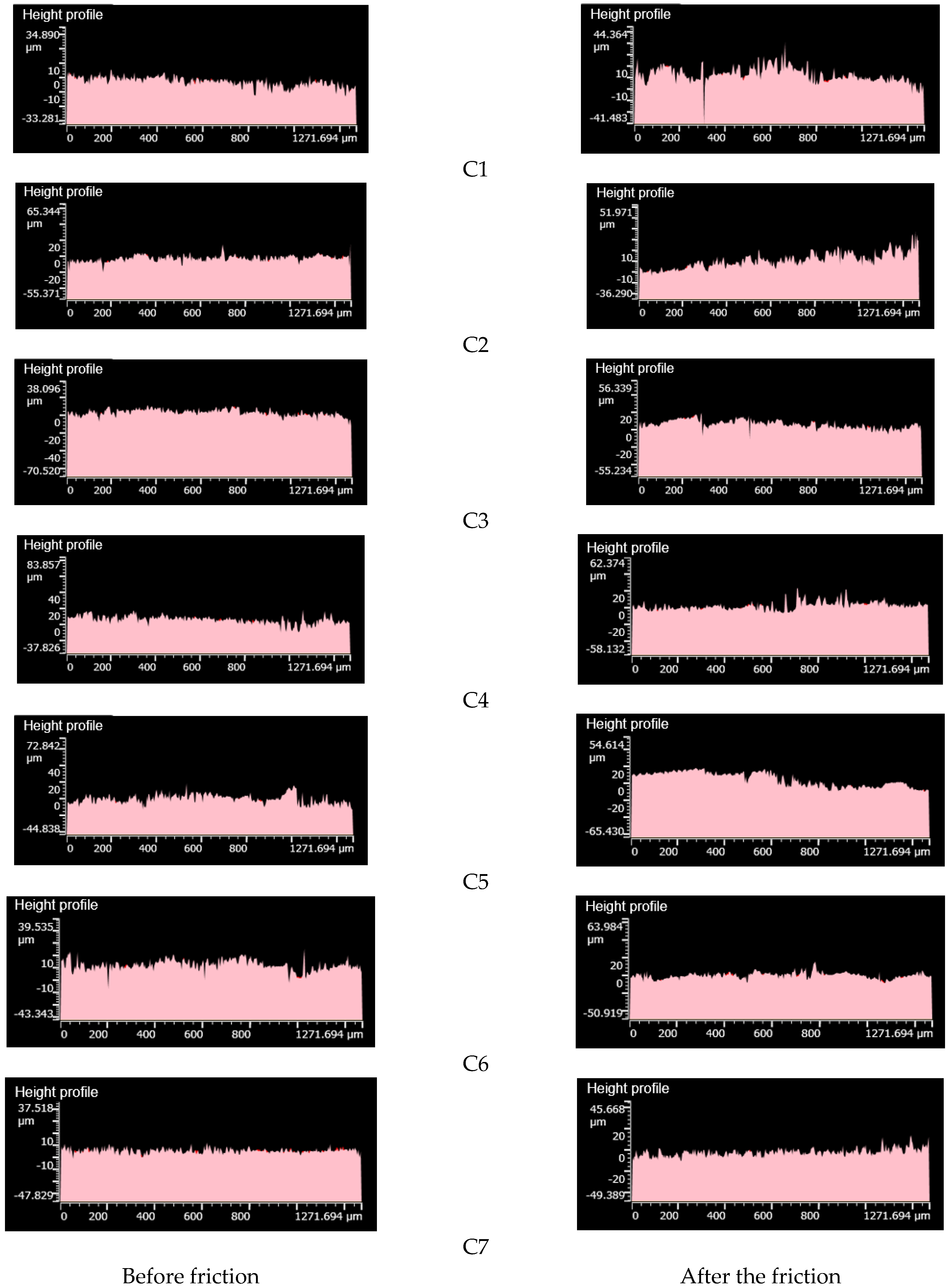
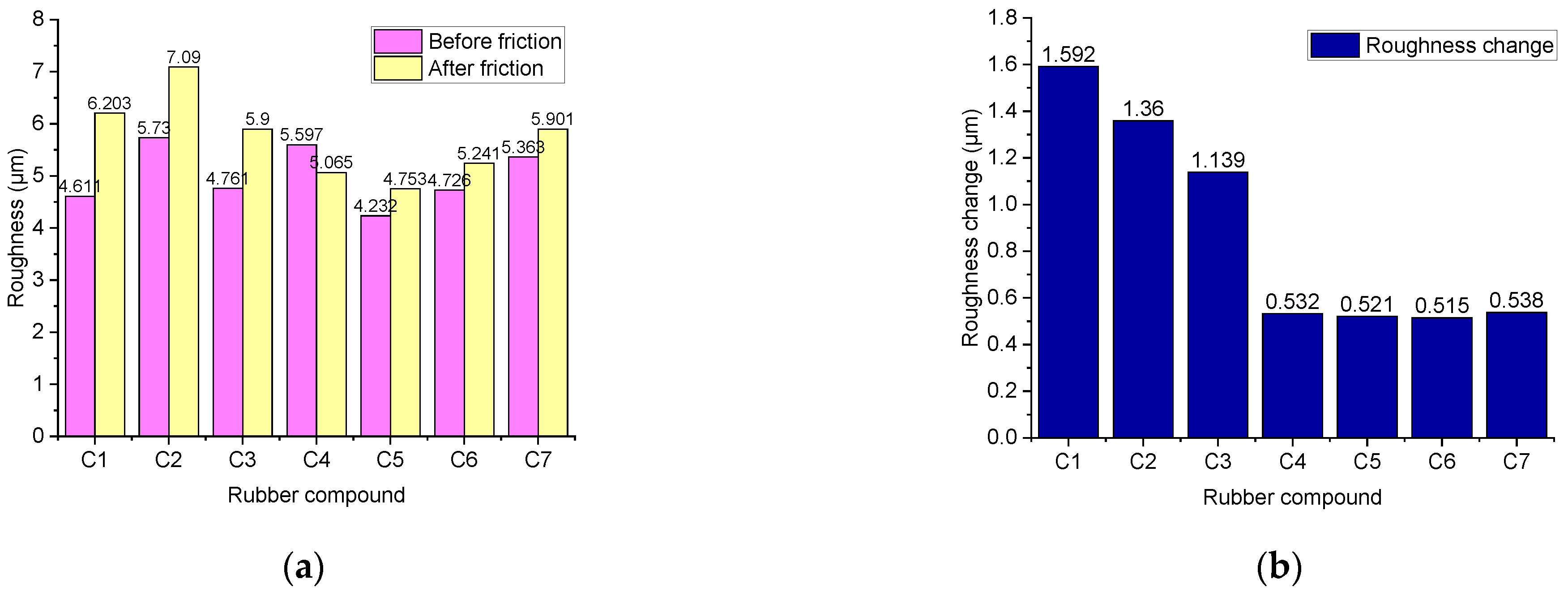
4.3.4. Change in Roughness before and after Friction
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, M.Y.; Ji, X.Y.; Liu, Z. Effect of silane coupling agent content on processability and vulcanization properties of silica filled compound. Chin. Soc. Chem. Eng. 2018, 20, 283–290. [Google Scholar]
- Guo, Y.; Zhang, Z.; Cao, Z.; Wang, D. Wear behavior of hollow glass beads (HGB) reinforced nitrile butadiene rubber: Effects of silane coupling agent and filler content. Mater. Today Commun. 2019, 19, 366–373. [Google Scholar] [CrossRef]
- Sarkawi, S.; Dierkes, W.; Noordermeer, J. The influence of non-rubber constituents on performance of silica reinforced natural rubber compounds. Eur. Polym. J. 2013, 49, 3199–3209. [Google Scholar] [CrossRef]
- Tang, H.; Song, R.; Dong, Y.; Song, X. Measurement of Restitution and Friction Coefficients for Granular Particles and Discrete Element Simulation for the Tests of Glass Beads. Materials 2019, 12, 3170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Hill, C.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Pan, Y.; Han, D.; Zhu, L.; Zhang, M.; Bian, H.; Wang, C.; Han, W. Effect of Adding MoDTC on the Properties of Carbon Black Rubber and the Friction and Wear of Metal during Mixing Process. Materials 2020, 13, 1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, T.; Fujii, S.; Nakamura, Y.; Sasaki, M. Mechanical properties of silica particle-filled styrene-butadiene rubber composites containing polysulfide-type silane coupling agents: Influence of loading method of silane. J. Appl. Polym. Sci. 2013, 130, 322–329. [Google Scholar] [CrossRef]
- King, R.B.; Lancaster, J.K. Wear of metals by elastomers in an abrasive environment. Wear 1980, 61, 341–352. [Google Scholar] [CrossRef]
- Ab-Malek, K.; Stevenson, A. On the lubrication and wear of metal by rubber. J. Mater. Sci. 1984, 19, 585–594. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, A.; Wang, L. Carbon Black Filled Styrene Butadiene Rubber Masterbatch Based on Simple Mixing of Latex and Carbon Black Suspension: Preparation and Mechanical Properties. J. Macromol. Sci. Part B 2015, 54, 1541–1553. [Google Scholar] [CrossRef]
- Xu, Z.; Song, Y. Payne Effect of Carbon Black Filled Natural Rubber Compounds and Their Carbon Black Gels. Polymer 2019, 185, 121953. [Google Scholar] [CrossRef]
- Flambard, J.; Carrette, F.; Monchy-Leroy, C.; Andrieu, E.; Laffont, L. Influence of the transient conditions on release of corrosion products and oxidation of alloy 690 tubes during pressurized water reactor restart after steam generators replacement. J. Nucl. Mater. 2021, 543, 152562. [Google Scholar] [CrossRef]
- Thorhallsson, A.; Csaki, I.; Geambazu, L.; Magnus, F.; Karlsdottir, S. Effect of alloying ratios and Cu-addition on corrosion behaviour of CoCrFeNiMo high-entropy alloys in superheated steam containing CO2, H2S and HCl. Corros. Sci. 2020, 178, 109083. [Google Scholar] [CrossRef]
- Gong, B.; Cai, L.; Lei, P.; Metzger, K.; Lahoda, E.; Boylan, F.; Yang, K.; Fay, J.; Harp, J.; Lian, J. Cr-doped U3Si2 composite fuels under steam corrosion. Corros. Sci. 2020, 177, 109001. [Google Scholar] [CrossRef]
- Ye, X.; Tian, M.; Zhang, L.Q. Some interesting phenomena in silica-filled HNBR with the addition of silane coupling agent. J. Appl. Polym. Sci. 2012, 124, 927–934. [Google Scholar] [CrossRef]
- Abbas, Z.; Tawfilas, M.; Khani, M.; Golian, K.; Marsh, Z.; Jhalaria, M.; Simonutti, R.; Stefik, M.; Kumar, S.; Benicewicz, B. Reinforcement of polychloroprene by grafted silica nanoparticles. Polymer 2019, 171, 96–105. [Google Scholar] [CrossRef]
- Pal, P.; De, S. Studies of Polymer-Filler Interaction, Network Structure, Physical Properties, and Fracture of Silica-and Clay-Filled EPDM Rubber in the Presence of a Silane Coupling Agent. Rubber Chem. Technol. 1983, 56, 737–773. [Google Scholar] [CrossRef]
- Ge, X.; Li, M.-C.; Xiangxu, L.; Cho, U. Effects of silane coupling agents on the properties of bentonite/nitrile butadiene rubber nanocomposites synthesized by a novel green method. Appl. Clay Sci. 2015, 118, 265–275. [Google Scholar] [CrossRef]
- Zhao, Y.; Qi, X.; Ma, J.; Song, L.; Yang, Y.; Yang, Q.-X. Interface of polyimide-silica grafted with different silane coupling agents: Molecular dynamic simulation. J. Appl. Polym. Sci. 2017, 135, 45725. [Google Scholar] [CrossRef]
- Mohd Nor, N.; Muttalib, S.; Othman, N. Synthesis of natural rubber/palygorskite nanocomposites via silylation and cation exchange. In Nanoclay Reinforced Polymer Composites; Jawaid, M., el Kacem Qaiss, A., Bouhfid, R., Eds.; Springer: Cham, Switzerland, 2016; pp. 261–289. [Google Scholar]
- Vinogradov, G.V.; Mustafaev, V.; Podolsky, Y.Y. A study of heavy metal-to-plastic friction duties and of the wear of hardened steel in the presence of polymers. Wear 1965, 8, 358–373. [Google Scholar] [CrossRef]
- Min, K.; Suh, K. Experiments and modeling of flow of elastomers in an internal mixer with intermeshing rotors. Polym. Eng. Sci. 1991, 31, 779–788. [Google Scholar] [CrossRef]
- Gorokhovskii, G.; Chernenko, P.; Smirnov, V. Effect of polymers on the abrasive dispersion of carbon steel. Sov. Mater. Sci. 1974, 8, 557–560. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhang, J.; Chen, S.J. Effects of carbon blacks with various structures on vulcanization and reinforcement of filled ethylene-propylene-diene rubber. Express Polym. Lett. 2008, 2, 695–704. [Google Scholar] [CrossRef]
- Lo Presti, D. Recycled Tyre Rubber Modified Bitumens for road asphalt mixtures: A literature review. Constr. Build. Mater. 2013, 49, 863–881. [Google Scholar] [CrossRef]
- Lopez, G.; Alvarez, J.; Amutio, M.; Mkhize, N.; Danon, B.; van der Gryp, P.; Görgens, J.; Bilbao, J.; Olazar, M. Waste truck-tyre processing by flash pyrolysis in a conical spouted bed reactor. Energy Convers. Manag. 2017, 142, 523–532. [Google Scholar] [CrossRef]
- Martinez, J.D.; Puy, N.; Murillo, R.; Garcia, T.; Navarro, M.V.; Mastral, A.M. Waste tyre pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 23, 179–213. [Google Scholar] [CrossRef]
- Bekesi, N.; Varadi, K.; Felhos, D. Wear Simulation of a Reciprocating Seal. J. Tribol. 2011, 133, 031601. [Google Scholar] [CrossRef]
- Blackford, J.R.; Skouvaklis, G.; Purser, M.; Koutsos, V. Friction on ice: Stick and slip. Faraday Discuss. 2012, 156, 243–254. [Google Scholar] [CrossRef]
- Caessa, J.; Vuchkov, T.; Yaqub, T.; Cavaleiro, A. On the Microstructural, Mechanical and Tribological Properties of Mo-Se-C Coatings and Their Potential for Friction Reduction against Rubber. Materials 2021, 14, 1336. [Google Scholar] [CrossRef]
- Cheng, H.M.; Chen, X.Y.; Chen, X.L.; Liu, H.C. Research on Key Factors of Sealing Performance of Combined Sealing Ring. Appl. Sci. 2022, 12, 714. [Google Scholar] [CrossRef]
- Dangnan, F.; Espejo, C.; Liskiewicz, T.; Gester, M.; Neville, A. Friction and wear of additive manufactured polymers in dry contact. J. Manuf. Process. 2020, 59, 238–247. [Google Scholar] [CrossRef]
- Dong, C.; Yuan, C.; Bai, X.; Li, J.; Qin, H.; Yan, X. Coupling mechanism between wear and oxidation processes of 304 stainless steel in hydrogen peroxide environments. Sci. Rep. 2017, 7, 2327. [Google Scholar] [CrossRef]
- Kim, H.; Park, K.; Lee, M.-Y. Biocompatible Dispersion Methods for Carbon Black. Toxicol. Res. 2012, 28, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Liu, Y.; Song, X.; Cheng, J.; Li, J. Influencing Mechanism of Rubber Wheel on Contact Pressure and Metal Removal in Corrugated Rail Grinding by Abrasive Belt. J. Manuf. Sci. Eng. 2018, 140, 124501. [Google Scholar] [CrossRef]
- Feng, D.; Shen, M.-X.; Peng, X.-D.; Meng, X.-K. Surface Roughness Effect on the Friction and Wear Behaviour of Acrylonitrile–Butadiene Rubber (NBR) Under Oil Lubrication. Tribol. Lett. 2017, 65, 10. [Google Scholar] [CrossRef]
- Han, D.S.; Li, S.; Xiao, X.X.; Xu, M.H.; Chen, Y.H.; Wang, C.S.; Bian, H. Effect of different dosages of carbon nanotubes on the metal friction and metal wear in the mixing process. Polym. Compos. 2021, 42, 6051–6064. [Google Scholar] [CrossRef]
- Han, D.; Pan, Y.; Xue, J.; Yu, B.; Yan, G.; Wang, C.; Wang, K.; Pan, Y. Effect of adding different silane coupling agents on metal friction and wear in mixing process. J. Appl. Polym. Sci. 2021, 138, 51408. [Google Scholar] [CrossRef]
- Han, D.S.; Wang, K.S.; Bian, H.G.; Yan, G.; Wang, C.S. Effect of different amounts of graphene on metal friction and wear during the mixing process. Polym. Compos. 2021, 42, 5075–5089. [Google Scholar] [CrossRef]
- Han, D.; Yan, G.; Li, S.; Pan, Y.; Chen, Y.; Wang, C.; Liu, H. Study on mechanical properties and antifriction of calcium powder filled rubber. Polym. Compos. 2022, 43, 874–888. [Google Scholar] [CrossRef]
- Han, D.; Zhang, S.; Wang, K.; Pan, Y.; Zhu, D.; Wang, C.; Pan, Y. A comparison of the effects of traditional and wet mixing processes of rubber on metal friction and wear. Appl. Polym. Sci. 2021, 138, 50761. [Google Scholar] [CrossRef]
- Han, D.; Wang, K.; Yan, G.; Pan, Y.; Xue, J.; Wang, C.; Bian, H. Effect of the ratio of graphene oxide(GO) and multi-walled carbon nanotubes(MWCNTs) on metal friction and wear during mixing. Polym. Test. 2022, 106, 107441. [Google Scholar] [CrossRef]
- Han, D.; Pan, Y.; Li, S.; Xu, M.; Xiao, X.; Huang, E.; Wang, C.; Bian, H. Friction and wear of Stellite 6B during sliding against rubber compound with varied carbon fiber content. Polym. Test. 2022, 106, 107470. [Google Scholar] [CrossRef]
- Han, D.; Zhang, S.; Pan, Y.; Wang, C. Simulation and experimental research on the wear of synchronous quadruple rotor of an internal mixer during the mixing process. Eng. Fail. Anal. 2022, 139, 106450. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Mousa, A.; Major, Z.; Bekesi, N. Dry friction and sliding wear of EPDM rubbers against steel as a function of carbon black content. Wear 2008, 264, 357–365. [Google Scholar] [CrossRef]






| Component (phr) | C1 | C2 | C3 | C4 | C5 | C6 | C7 |
|---|---|---|---|---|---|---|---|
| NR | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Silica115MP | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
| ZnO | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 4020 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| SAD | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| TESPT | 0 | 2 | 4 | 6 | 8 | 10 | 12 |
| DPG | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 |
| S | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 |
| CZ | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 |
| 1.6L Hake Mixer, 80rpm, 75% FF | ||
|---|---|---|
| Time | T (°C) | Ingredients |
| Masterbatch | ||
| 0:00 | 70 | Polymers |
| 0:40 | Chemical, Silica | |
| 1:10 | Silica | |
| 2:30 | 120 | Sweep |
| 4:00 | 135 | Sweep, Sampleing |
| 5:00 | 145 | Discharge |
| Stage | Frequency/hz | Temperature/°C | Time/Min | Strain | Test Items |
|---|---|---|---|---|---|
| 1 | 0.1 | 60 | 5 | 0.28% | - |
| 2 | 1 | 60 | - | 0.28–40% | |
| 3 | 1 | 60 | - | 0.28–40% | |
| 4 | 0.1 | 60/160/160 | 0/2.5/5 | 0.28% | - |
| 5 | 1 | 60 | - | 0.28–40% | |
| 6 | 1 | 60 | - | 0.28–40% |
| Rubber Compounds | C1 | C2 | C3 | C4 | C5 | C6 | C7 |
|---|---|---|---|---|---|---|---|
| Dispersion | 5.42 | 5.98 | 6.51 | 7.47 | 7.56 | 7.49 | 7.53 |
| Rubber Compound | C1 | C2 | C3 | C4 | C5 | C6 | C7 |
|---|---|---|---|---|---|---|---|
| Silanization reaction index | 0 | 0.16249 | 0.30586 | 0.55879 | 0.54589 | 0.55969 | 0.56328 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Wang, K.; Wang, C.; Han, W. The Effect of Different Dosages of TESPT on Metal Friction and Metal Wear in the Mixing Process. Polymers 2022, 14, 2314. https://doi.org/10.3390/polym14122314
Han D, Wang K, Wang C, Han W. The Effect of Different Dosages of TESPT on Metal Friction and Metal Wear in the Mixing Process. Polymers. 2022; 14(12):2314. https://doi.org/10.3390/polym14122314
Chicago/Turabian StyleHan, Deshang, Kongshuo Wang, Chuansheng Wang, and Wenwen Han. 2022. "The Effect of Different Dosages of TESPT on Metal Friction and Metal Wear in the Mixing Process" Polymers 14, no. 12: 2314. https://doi.org/10.3390/polym14122314








