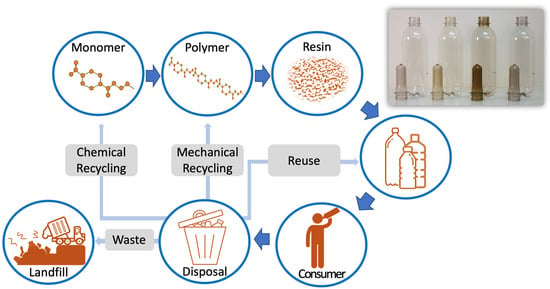
Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review
Abstract
:1. Introduction
2. PET and the Processes for Post-Consumer Recycling
2.1. Collection of PET Bottles
2.2. PET Value Recovery
2.2.1. Primary Recycling (Re-Extrusion)
2.2.2. Secondary Recycling (Mechanical)
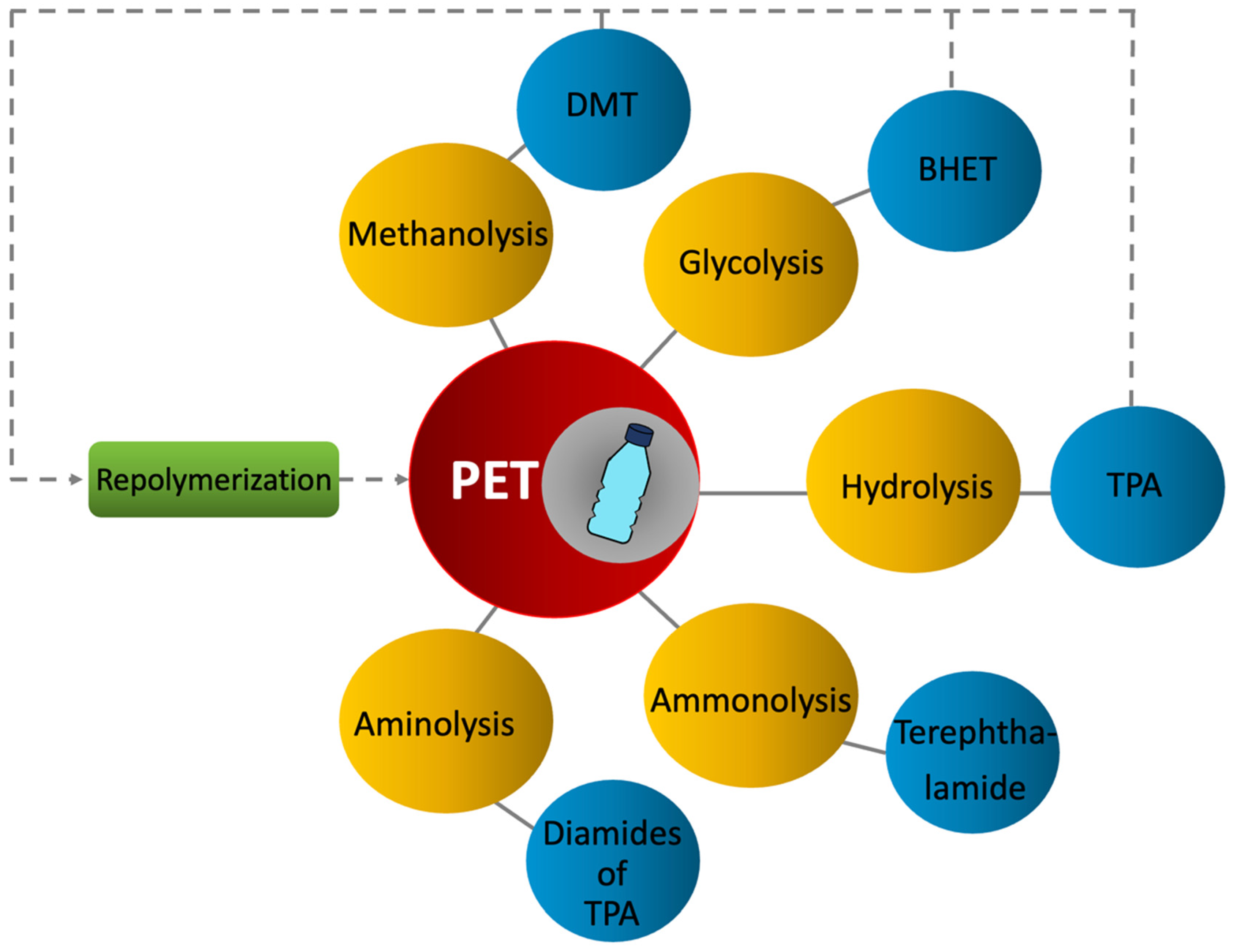
2.2.3. Tertiary Recycling (Chemical)
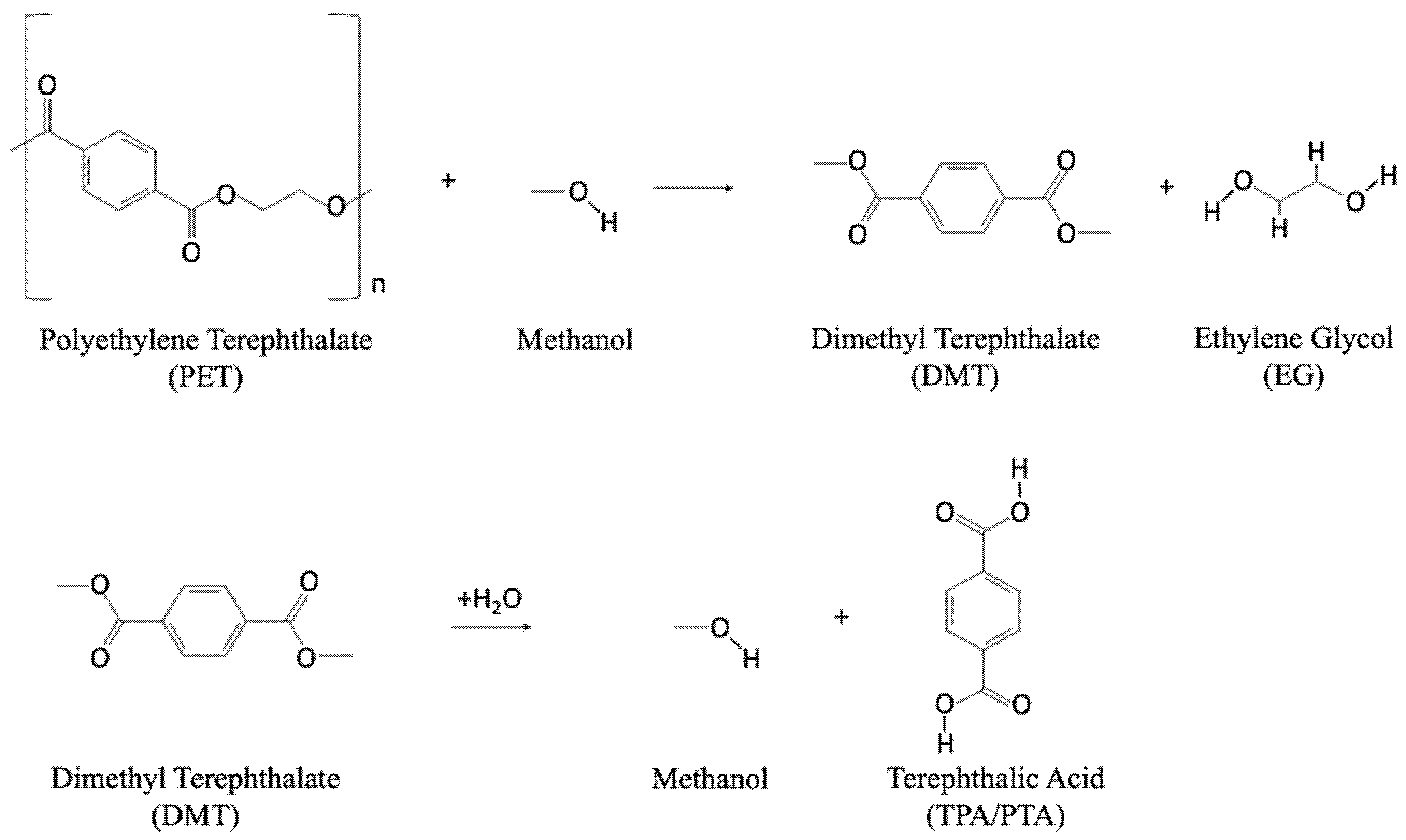
Methanolysis
Glycolysis
- Catalyzed glycolysis
- 2.
- Solvent-Assisted Glycolysis
- 3.
- Supercritical Glycolysis
- 4.
- Microwave-assisted glycolysis
Hydrolysis
- (1)
- Acid hydrolysis: Several acid substances can be employed in the process of acid hydrolysis, including concentrated sulfuric acid (H2SO4) (which is most commonly used), nitric acid (HNO3), and phosphoric acid (H3PO4). The use of concentrated sulfuric acid can help to reduce the energy consumption of the process via lowering of the requirement of high pressure and temperature in the reaction vessel [84,85]. TPA can be recovered from the PET feed scrap within several minutes under operating conditions of 60–93 °C in 87 wt% H2SO4 solution and 85–90 °C in 90 wt% H2SO4 solution [86]. The chemical reaction for the acid hydrolysis is shown in Figure 6.
- (2)
- Alkaline hydrolysis: For the alkaline hydrolysis process (Figure 7), a 4–20 wt% aqueous alkaline solution of sodium hydroxide (NaOH) or potassium hydroxide (KOH) is used [38]. The process is conducted under a pressure of 1.4–2 MPa and at temperatures of 210–250 °C for 3–5 h. This reaction results in disodium or dipotassium terephthalate, which is further treated with sulfuric acid to separate TPA [88].
- (3)
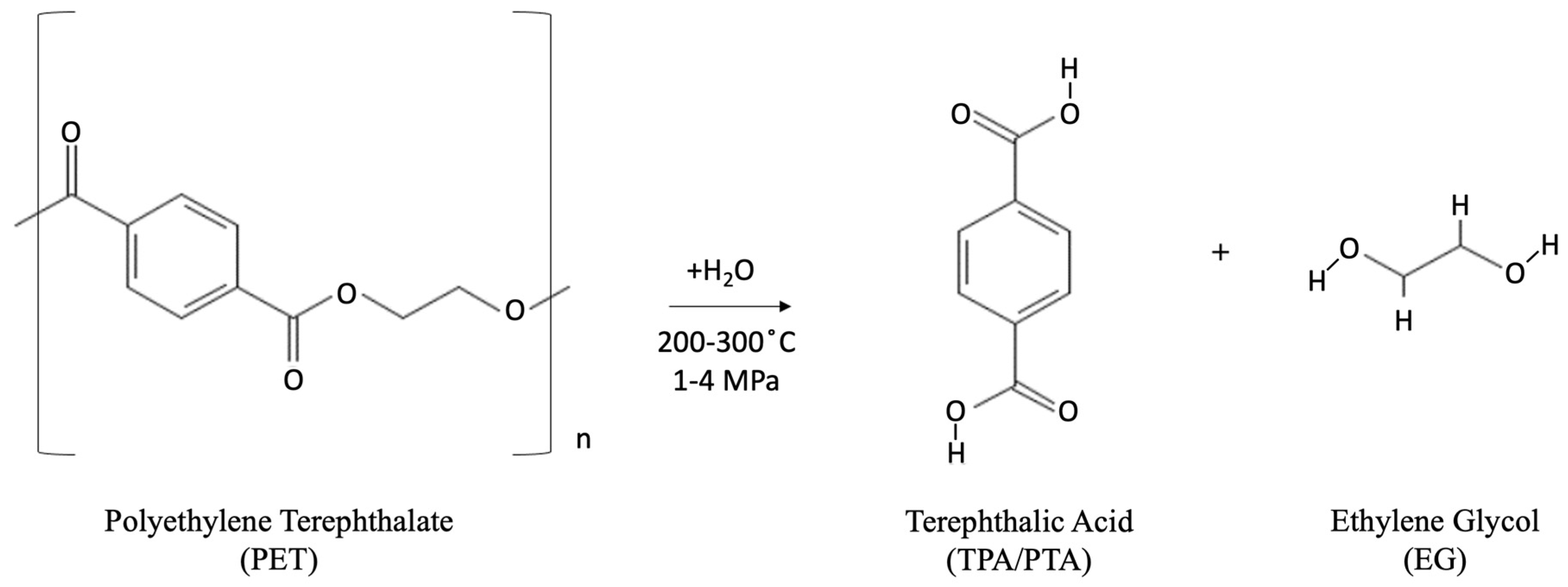
- Neutral hydrolysis: Hot water or steam at temperatures of 200–300 °C is used for the neutral hydrolysis reaction, with pressures of 1–4 MPa mainly used in this process. This hydrolysis method has been shown to produce high-purity TPA and EG monomers [46,91] and high yield at temperatures greater than 250 °C [92]. As with alkaline hydrolysis, the yield of TPA and EG directly correlates with increases in reaction temperature. The yield of TPA has been shown to reach 90% at reaction temperatures of 420 °C. The chemical reactions taking place during the neutral hydrolysis process are shown in Figure 8.
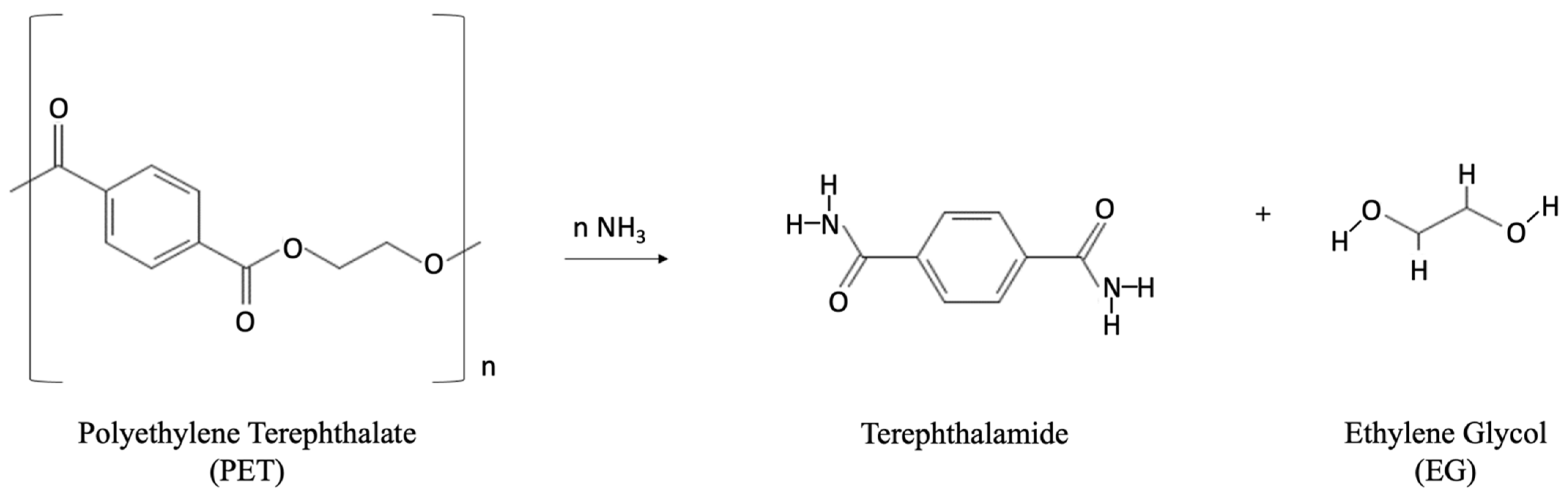
Ammonolysis
Aminolysis
2.2.4. Quaternary (Energy Recovery)
3. Recycling Operation for PCR PET
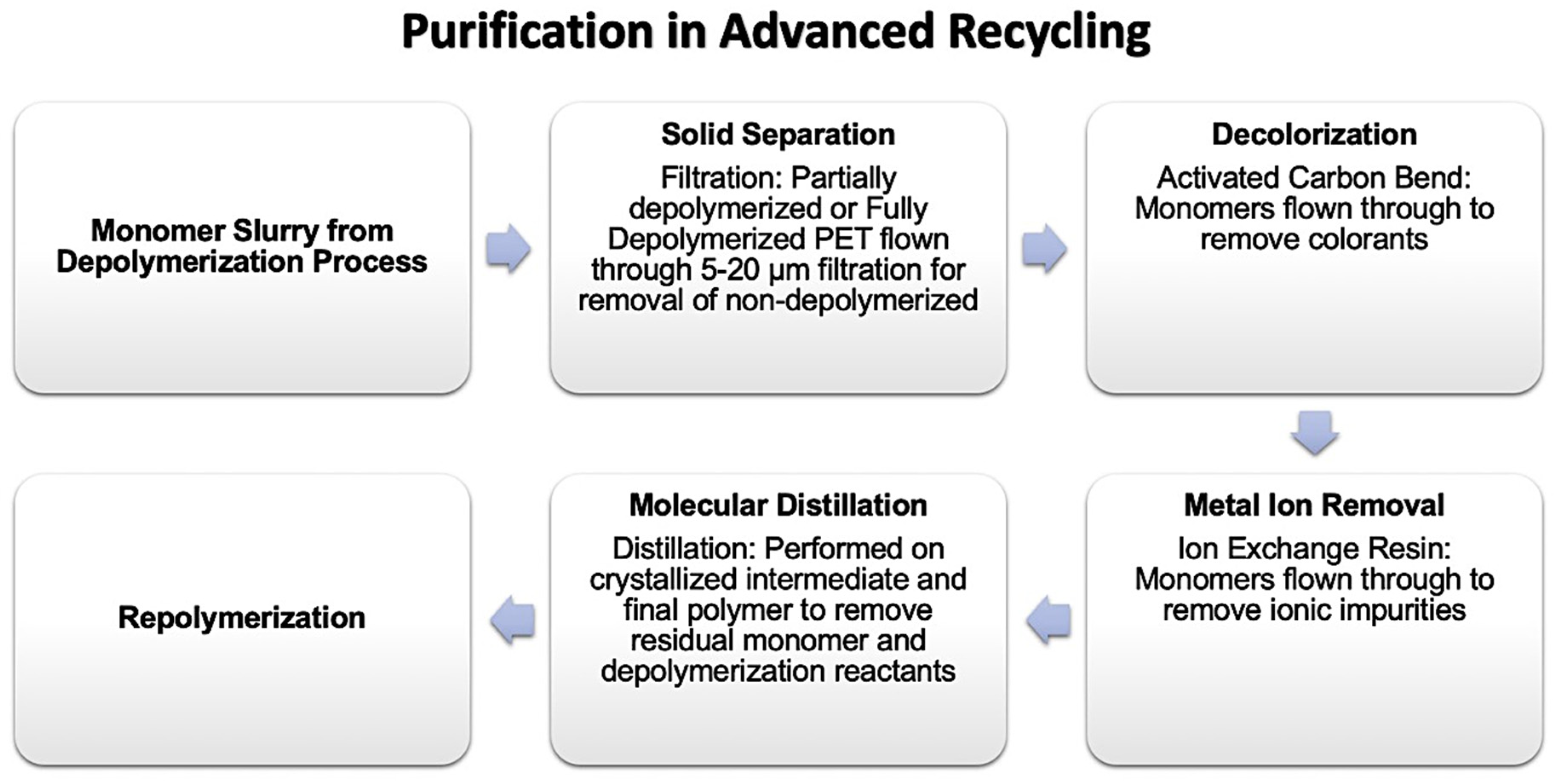
3.1. Sortation and Purification
3.2. Super Cleaning Process
4. Processing and Performance Differences between Virgin PET and Post-Consumer Recycled PET
- (1)
- Water grade (low IV, acetaldehyde suppression, could have additives to enable ultrathin bottle walls);
- (2)
- Heat-set grade (higher IV, DEG suppression, co-monomers to suppress hot-fill shrinkage, could have additives to aid reheating and crystallization);
- (3)
- Carbonated soft drink (CSD) grade (highest IV, co-monomers to resist expansion).
4.1. Injection
4.1.1. Drying of Material and Injection Pressure
4.1.2. AA Generation
4.1.3. Haze Due to Additional Nucleation Sites
4.1.4. Degradation of IV
4.2. Preform and Container Appearance
4.3. Blow Molding
4.3.1. Preform Heating (Energy Requirements)
4.3.2. Material Distribution Variation
4.3.3. Scrap Rate
4.4. Container Performance
4.4.1. Bottle Shrinkage When Exposed to Heat
4.4.2. UV Light Performance
4.4.3. Topload
4.4.4. Burst Strength and Thermal Expansion
5. Application and Limitations
5.1. Cold-Fill Applications
5.2. Heat-Set Bottles for Hot-Filled and Aseptic Products
5.3. Pressurized Containers
6. Regulatory Requirements
- (1)
- Description of the controls that are in place to prevent non-PET plastics from entering the PCR production stream.
- (2)
- Documentation and evidence of efficient contaminant removal. If requested, a surrogate contaminant will be used to validate the recycling process and demonstrate the effective removal of the contaminant. Additional migration modeling and testing can be used to demonstrate contaminant reduction to below 0.5 ppb (the dietary concentration that assumes negligible exposure for use with food products).
- (3)
- Description of how the plastic will be used. With food contact materials, these descriptors include temperature range for use, type of food, duration of contact, and if the plastic will be used in a single-use or repeated application.
7. Conclusions and Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- Berlinet, C.; Brat, P.; Ducruet, V. Quality of orange juice in barrier packaging material. Packag. Technol. Sci. Int. J. 2008, 21, 279–286. [Google Scholar] [CrossRef]
- Ghoshal, G. Recent development in beverage packaging material and its adaptation strategy. Trends Beverage Packag. 2019, 16, 21–50. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Selke, S.E. Recycling of plastic packaging in the United States. Packag. Technol. Sci. 1988, 1, 93–98. [Google Scholar] [CrossRef]
- EPA. Facts and Figures about Materials, Waste and Recycling: Plastics: Material-Specific Data. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/plastics-material-specific-data (accessed on 3 January 2022).
- EEA. EU Recycled 41% of Plastic Packaging Waste in 2019. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20211027-2 (accessed on 3 January 2022).
- European Commission. Communication from the Commission to the Council, the European Parliament, the European Economic and Social Committee and the Committee of the Regions: Civil Society Dialogue Between the EU and Candidate Countries; European Commission: Brussels, Belgium, 2005; Volume 290. [Google Scholar]
- EPA. Sustainable Materials Management: Non-Hazardous Materials and Waste Management Hierarchy. Available online: https://www.epa.gov/smm/sustainable-materials-management-non-hazardous-materials-and-waste-management-hierarchy#Source_Reduction (accessed on 3 January 2022).
- Schwanse, E. Recycling policies and programmes for PET drink bottles in Mexico. Waste Manag. Res. 2011, 29, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Franz, R.; Welle, F. Recycling of post-consumer packaging materials into new food packaging applications—Critical review of the European approach and future perspectives. Sustainability 2022, 14, 824. [Google Scholar] [CrossRef]
- Coelho, T.M.; Castro, R.; Gobbo, J., Jr. PET containers in Brazil: Opportunities and challenges of a logistics model for post-consumer waste recycling. Resour. Conserv. Recycl. 2011, 55, 291–299. [Google Scholar] [CrossRef]
- Mishra, M. Encyclopedia of Polymer Applications, 3 Volume Set; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Lin, C.C. Recycling technology of poly (ethylene terephthalate) materials. In Macromolecular Symposia; Wiley-VCH: Weinheim, Germany, 1998; pp. 129–135. [Google Scholar]
- Awaja, F.; Pavel, D. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Mackey, G. A review of advanced recycling technology. In Plastics, Rubber and Paper Recycling; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Welle, F. Twenty years of PET bottle to bottle recycling—An overview. Resour. Conserv. Recycl. 2011, 55, 865–875. [Google Scholar] [CrossRef]
- Nkwachukwu, O.I.; Chima, C.H.; Ikenna, A.O.; Albert, L. Focus on potential environmental issues on plastic world towards a sustainable plastic recycling in developing countries. Int. J. Ind. Chem. 2013, 4, 34. [Google Scholar] [CrossRef]
- Selke, S.E.; Culter, J.D.; Auras, R.A.; Rabnawaz, M. Plastics Packaging: Properties, Processing, Applications, and Regulations; Carl Hanser Verlag GmbH Co KG: Weinheim, Germany, 2021. [Google Scholar]
- Groeninckx, G.; Berghmans, H.; Overbergh, N.; Smets, G. Crystallization of poly (ethylene terephthalate) induced by inorganic compounds. I. Crystallization behavior from the glassy state in a low-temperature region. J. Polym. Sci. Polym. Phys. Ed. 1974, 12, 303–316. [Google Scholar] [CrossRef]
- Apotheker, S. Curbside collection: Complete separation versus commingled collection. Resour. Recycl. 1990, 9, 58–63. [Google Scholar]
- CRI. Bottle Bills. Available online: https://www.container-recycling.org/index.php/issues/bottle-bills (accessed on 3 January 2022).
- Cheng, X.; Yeh, C.-N. An economic analysis of the effectiveness of bottle bills. ASBBS E-J. 2013, 9, 30. [Google Scholar]
- Suter, M. Beyond PET: An Extended Deposit-Return System for Plastic Packaging in Sweden: A Qualitative Investigation of Challenges and Lessons from future and earlier Deposit-Return Systems. Master’s Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2019. [Google Scholar]
- Polygalov, S.; Ilinykh, G.; Korotaev, V.; Stanisavljevic, N.; Batinic, B. Determination of the composition and properties of PET bottles: Evidence of the empirical approach from Perm, Russia. Waste Manag. Res. 2021, 39, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Stanisavljevic, N.; Brunner, P.H. Quantity AND quality: New priorities for waste management. Waste Manag. Res. 2019, 37, 665–666. [Google Scholar] [CrossRef]
- Al-Salem, S.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Sheel, A.; Pant, D. Chemical depolymerization of PET bottles via glycolysis. In Recycling of Polyethylene Terephthalate Bottles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 61–84. [Google Scholar]
- Sinha, V.; Patel, M.R.; Patel, J.V. PET waste management by chemical recycling: A review. J. Polym. Environ. 2010, 18, 8–25. [Google Scholar] [CrossRef]
- Neale, C.; Hilyard, N.; Barber, P. Observations on the economics of recycling industrial scrap plastic in new products. Conserv. Recycl. 1983, 6, 91–105. [Google Scholar] [CrossRef]
- Al-Salem, S. Establishing an integrated databank for plastic manufacturers and converters in Kuwait. Waste Manag. 2009, 29, 479–484. [Google Scholar] [CrossRef]
- Jensen, J.; Holman, J.; Stephenson, J. Recycling and disposal of waste plastics. Ann. Arbor Sci. 1974, 219–249. [Google Scholar]
- Frounchi, M. Studies on degradation of PET in mechanical recycling. In Macromolecular Symposia; Wiley-VCH: Weinheim, Germany, 1999; pp. 465–469. [Google Scholar]
- Al-Salem, S.; Lettieri, P.; Baeyens, J. The valorization of plastic solid waste (PSW) by primary to quaternary routes: From re-use to energy and chemicals. Prog. Energy Combust. Sci. 2010, 36, 103–129. [Google Scholar] [CrossRef]
- Das, S.K.; Eshkalak, S.K.; Chinnappan, A.; Ghosh, R.; Jayathilaka, W.; Baskar, C.; Ramakrishna, S. Plastic recycling of polyethylene terephthalate (PET) and polyhydroxybutyrate (PHB)—A comprehensive review. Mater. Circ. Econ. 2021, 3, 9. [Google Scholar] [CrossRef]
- Mastellone, M.; Perugini, F.; Ponte, M.; Arena, U. Fluidized bed pyrolysis of a recycled polyethylene. Polym. Degrad. Stab. 2002, 76, 479–487. [Google Scholar] [CrossRef]
- Achilias, D.; Karayannidis, G. The chemical recycling of PET in the framework of sustainable development. Water Air Soil Pollut. Focus 2004, 4, 385–396. [Google Scholar] [CrossRef]
- Shojaei, B.; Abtahi, M.; Najafi, M. Chemical recycling of PET: A stepping-stone toward sustainability. Polym. Adv. Technol. 2020, 31, 2912–2938. [Google Scholar] [CrossRef]
- Paszun, D.; Spychaj, T. Chemical recycling of poly (ethylene terephthalate). Ind. Eng. Chem. Res. 1997, 36, 1373–1383. [Google Scholar] [CrossRef]
- Michel, R. Recovery of methyl esters of aromatic acids and glycols from thermoplastic polyester scrap using methanol vapor. Eur. Pat. 1992, 484, 963. [Google Scholar]
- Marathe, M.; Dabholkar, D.; Jain, M. Process for the recovery of dimethyl terephthalate from polyethylene terephthalate. GB Pat. 1980, 2, 916. [Google Scholar]
- Dimov, K.; Terlemezyan, E. Catalytic action of calcium acetate and the manganese acetate–sodium acetate mixture in the pre-esterification of dimethyl terephthalate with ethylene glycol. J. Polym. Sci. Part A-1 Polym. Chem. 1972, 10, 3133–3141. [Google Scholar] [CrossRef]
- Baliga, S.; Wong, W.T. Depolymerization of poly (ethylene terephthalate) recycled from post-consumer soft-drink bottles. J. Polym. Sci. Part A Polym. Chem. 1989, 27, 2071–2082. [Google Scholar] [CrossRef]
- Cornell, D. Depolymerization of PET for food packaging. In Proceedings of the Society of Plastics Engineers RETEC Conference, Bethel, CT, USA, 22–23 September 1993. [Google Scholar]
- Kurokawa, H.; Ohshima, M.-A.; Sugiyama, K.; Miura, H. Methanolysis of polyethylene terephthalate (PET) in the presence of aluminium tiisopropoxide catalyst to form dimethyl terephthalate and ethylene glycol. Polym. Degrad. Stab. 2003, 79, 529–533. [Google Scholar] [CrossRef]
- Genta, M.; Iwaya, T.; Sasaki, M.; Goto, M.; Hirose, T. Depolymerization mechanism of poly (ethylene terephthalate) in supercritical methanol. Ind. Eng. Chem. Res. 2005, 44, 3894–3900. [Google Scholar] [CrossRef]
- Scheirs, J. Polymer Recycling: Science, Technology and Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1998. [Google Scholar]
- Han, M. Depolymerization of PET bottle via methanolysis and hydrolysis. In Recycling of Polyethylene Terephthalate Bottles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–108. [Google Scholar]
- Bartolome, L.; Imran, M.; Cho, B.G.; Al-Masry, W.A.; Kim, D.H. Recent developments in the chemical recycling of PET. Mater. Recycl.-Trends Perspect. 2012, 406, 576–596. [Google Scholar]
- Wang, H.; Liu, Y.; Li, Z.; Zhang, X.; Zhang, S.; Zhang, Y. Glycolysis of poly (ethylene terephthalate) catalyzed by ionic liquids. Eur. Polym. J. 2009, 45, 1535–1544. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Duque-Ingunza, I.; de Rivas, B.; Flores-Giraldo, L.; Gutiérrez-Ortiz, J.I. Kinetics of catalytic glycolysis of PET wastes with sodium carbonate. Chem. Eng. J. 2011, 168, 312–320. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, X.; Tang, S.; Lu, X.; Zhang, X.; Zhang, S. Urea as an efficient and reusable catalyst for the glycolysis of poly (ethylene terephthalate) wastes and the role of hydrogen bond in this process. Green Chem. 2012, 14, 2559–2566. [Google Scholar] [CrossRef]
- Zhu, M.; Li, S.; Li, Z.; Lu, X.; Zhang, S. Investigation of solid catalysts for glycolysis of polyethylene terephthalate. Chem. Eng. J. 2012, 185, 168–177. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, X.; Wang, Q.; Zhu, M.; Li, Z. Effective catalysis of poly (ethylene terephthalate)(PET) degradation by metallic acetate ionic liquids. Pure Appl. Chem. 2012, 84, 789–801. [Google Scholar] [CrossRef]
- López-Fonseca, R.; González-Marcos, M.; González-Velasco, J.; Gutiérrez-Ortiz, J. A kinetic study of the depolymerisation of poly (ethylene terephthalate) by phase transfer catalysed alkaline hydrolysis. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2009, 84, 92–99. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, J.; Zou, J.; Yi, F. Hydrolysis of poly (ethylene terephthalate) waste bottles in the presence of dual functional phase transfer catalysts. J. Appl. Polym. Sci. 2013, 130, 2790–2795. [Google Scholar] [CrossRef]
- Yue, Q.F.; Xiao, L.F.; Zhang, M.L.; Bai, X.F. The glycolysis of poly (ethylene terephthalate) waste: Lewis acidic ionic liquids as high efficient catalysts. Polymers 2013, 5, 1258–1271. [Google Scholar] [CrossRef]
- Campanelli, J.; Kamal, M.; Cooper, D. Kinetics of glycolysis of poly (ethylene terephthalate) melts. J. Appl. Polym. Sci. 1994, 54, 1731–1740. [Google Scholar] [CrossRef]
- Bakar, D.A.; Ahmad, I.; Ramli, A. Chemical recycling of PET waste from soft drink bottles to produce a thermosetting polyester resin. Malays. J. Chem. 2006, 8, 22–26. [Google Scholar]
- Chen, C.H. Study of glycolysis of poly (ethylene terephthalate) recycled from postconsumer soft-drink bottles. III. Further investigation. J. Appl. Polym. Sci. 2003, 87, 2004–2010. [Google Scholar] [CrossRef]
- Pingale, N.D.; Palekar, V.S.; Shukla, S. Glycolysis of postconsumer polyethylene terephthalate waste. J. Appl. Polym. Sci. 2010, 115, 249–254. [Google Scholar] [CrossRef]
- Shukla, S.; Kulkarni, K. Depolymerization of poly (ethylene terephthalate) waste. J. Appl. Polym. Sci. 2002, 85, 1765–1770. [Google Scholar] [CrossRef]
- Shukla, S.; Harad, A.M.; Jawale, L.S. Recycling of waste PET into useful textile auxiliaries. Waste Manag. 2008, 28, 51–56. [Google Scholar] [CrossRef]
- Troev, K.; Grancharov, G.; Tsevi, R.; Gitsov, I. A novel catalyst for the glycolysis of poly (ethylene terephthalate). J. Appl. Polym. Sci. 2003, 90, 1148–1152. [Google Scholar] [CrossRef]
- Xin, J.; Zhang, Q.; Huang, J.; Huang, R.; Jaffery, Q.Z.; Yan, D.; Zhou, Q.; Xu, J.; Lu, X. Progress in the catalytic glycolysis of polyethylene terephthalate. J. Environ. Manag. 2021, 296, 113267. [Google Scholar] [CrossRef]
- Wi, R.; Imran, M.; Lee, K.G.; Yoon, S.H.; Cho, B.G.; Kim, D.H. Effect of support size on the catalytic activity of metal-oxide-doped silica particles in the glycolysis of polyethylene terephthalate. J. Nanosci. Nanotechnol. 2011, 11, 6544–6549. [Google Scholar] [CrossRef]
- Imran, M.; Lee, K.G.; Imtiaz, Q.; Kim, B.-K.; Han, M.; Cho, B.G.; Kim, D.H. Metal-oxide-doped silica nanoparticles for the catalytic glycolysis of polyethylene terephthalate. J. Nanosci. Nanotechnol. 2011, 11, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Fernando, S.D. Reaction kinetics of soybean oil transesterification using heterogeneous metal oxide catalysts. Chem. Eng. Technol. Ind. Chem.-Plant Equip.-Process Eng.-Biotechnol. 2007, 30, 1716–1720. [Google Scholar] [CrossRef]
- Heiz, U.; Landman, U. Nanocatalysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Niederberger, M.; Pinna, N. Metal Oxide Nanoparticles in Organic Solvents: Synthesis, Formation, Assembly and Application; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Wang, X.; Li, F.; Chen, Y. Polyoxometalates supporting cyclopendienylzirconium CpZrXW11O39n-: A new kind of olefin polymerization catalyst. Inorg. Chem. Commun. 2005, 8, 70–71. [Google Scholar] [CrossRef]
- Aouissi, A.; Al-Deyab, S.S.; Al-Shahri, H. The cationic ring-opening polymerization of tetrahydrofuran with 12-tungstophosphoric acid. Molecules 2010, 15, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Alsalme, A.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Heteropoly acids as catalysts for liquid-phase esterification and transesterification. Appl. Catal. A Gen. 2008, 349, 170–176. [Google Scholar] [CrossRef]
- Geng, Y.; Dong, T.; Fang, P.; Zhou, Q.; Lu, X.; Zhang, S. Fast and effective glycolysis of poly (ethylene terephthalate) catalyzed by polyoxometalate. Polym. Degrad. Stab. 2015, 117, 30–36. [Google Scholar] [CrossRef]
- Wang, H.; Yan, R.; Li, Z.; Zhang, X.; Zhang, S. Fe-containing magnetic ionic liquid as an effective catalyst for the glycolysis of poly (ethylene terephthalate). Catal. Commun. 2010, 11, 763–767. [Google Scholar] [CrossRef]
- Yue, Q.; Wang, C.; Zhang, L.; Ni, Y.; Jin, Y. Glycolysis of poly (ethylene terephthalate)(PET) using basic ionic liquids as catalysts. Polym. Degrad. Stab. 2011, 96, 399–403. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, X.; Zhou, X.; Zhu, M.; He, H.; Zhang, X. 1-allyl-3-methylimidazolium halometallate ionic liquids as efficient catalysts for the glycolysis of poly (ethylene terephthalate). J. Appl. Polym. Sci. 2013, 129, 3574–3581. [Google Scholar] [CrossRef]
- Güçlü, G.; Kas¸ göz, A.; Özbudak, S.; Özgümüs, S.; Orbay, M. Glycolysis of poly (ethylene terephthalate) wastes in xylene. J. Appl. Polym. Sci. 1998, 69, 2311–2319. [Google Scholar] [CrossRef]
- Imran, M.; Kim, B.-K.; Han, M.; Cho, B.G. Sub-and supercritical glycolysis of polyethylene terephthalate (PET) into the monomer bis (2-hydroxyethyl) terephthalate (BHET). Polym. Degrad. Stab. 2010, 95, 1686–1693. [Google Scholar] [CrossRef]
- Al-Sabagh, A.; Yehia, F.; Eshaq, G.; Rabie, A.; ElMetwally, A. Greener routes for recycling of polyethylene terephthalate. Egypt. J. Pet. 2016, 25, 53–64. [Google Scholar] [CrossRef]
- Pingale, N.; Shukla, S. Microwave assisted ecofriendly recycling of poly (ethylene terephthalate) bottle waste. Eur. Polym. J. 2008, 44, 4151–4156. [Google Scholar] [CrossRef]
- Cho, J.Y.; Hong, C.-J.; Choi, H.-M. Microwave-assisted glycolysis for PET with highly hydrophilic surface. Ind. Eng. Chem. Res. 2013, 52, 2309–2315. [Google Scholar] [CrossRef]
- Chaudhary, S.; Surekha, P.; Kumar, D.; Rajagopal, C.; Roy, P.K. Microwave assisted glycolysis of poly (ethylene terepthalate) for preparation of polyester polyols. J. Appl. Polym. Sci. 2013, 129, 2779–2788. [Google Scholar] [CrossRef]
- Alnaqbi, M.A.; Mohsin, M.A.; Busheer, R.M.; Haik, Y. Microwave assisted glycolysis of poly (ethylene terephthalate) catalyzed by 1-butyl-3-methylimidazolium bromide ionic liquid. J. Appl. Polym. Sci. 2015, 132, 12. [Google Scholar] [CrossRef]
- Sharma, N.; Vaidya, A.; Sharma, P. Recovery of pure terephthalic acid from polyester materials. Ind Patent 163,385, 1985. [Google Scholar]
- Pusztaszeri, S.F. Method for Recovery of Terephthalic Acid from Polyester Scrap. U.S. Patent No. 4,355,175, 19 October 1982. [Google Scholar]
- Dutt, K.; Soni, R. A review on synthesis of value added products from polyethylene terephthalate (PET) waste. Polym. Sci. Ser. B 2013, 55, 430–452. [Google Scholar] [CrossRef]
- Brown, G.E., Jr.; O’Brien, R.C. Method for Recovering Terephthalic Acid and Ethylene Glycol from Polyester Materials. U.S. Patent No. 3,952,053, 20 April 1976. [Google Scholar]
- Karayannidis, G.; Chatziavgoustis, A.; Achilias, D. Poly (ethylene terephthalate) recycling and recovery of pure terephthalic acid by alkaline hydrolysis. Adv. Polym. Technol. J. Polym. Processing Inst. 2002, 21, 250–259. [Google Scholar] [CrossRef]
- Spaseska, D.; Civkaroska, M. Alkaline hydrolysis of poly (ethylene terephthalate) recycled from the postconsumer soft-drink bottles. J. Univ. Chem. Technol. Metall. 2010, 45, 379–384. [Google Scholar]
- Datye, K.; Raje, H.; Sharma, N. Poly (ethylene terephthalate) waste and its utilisation: A review. Resour. Conserv. 1984, 11, 117–141. [Google Scholar] [CrossRef]
- Campanelli, J.; Cooper, D.; Kamal, M. Catalyzed hydrolysis of polyethylene terephthalate melts. J. Appl. Polym. Sci. 1994, 53, 985–991. [Google Scholar] [CrossRef]
- Sato, O.; Arai, K.; Shirai, M. Hydrolysis of poly (ethylene terephthalate) and poly (ethylene 2, 6-naphthalene dicarboxylate) using water at high temperature: Effect of proton on low ethylene glycol yield. Catal. Today 2006, 111, 297–301. [Google Scholar] [CrossRef]
- Michalski, A. Hydrolysis of poly (ethylene terephthalate) waste to obtain terephthalic acid. Wl. Chem. 1987, 49, 144. [Google Scholar]
- Michalski, A. Purification of Terephthalic Acid Obtained by Hydrolysis of Waste PET. Polish Patent No. 140015, 1987. [Google Scholar]
- Blackmon, K.P.; Fox, D.W.; Shafer, S.J. Process for Converting Pet Scrap to Diamine Monomers. U.S. Patent No. 4,973,746, 27 November 1990. [Google Scholar]
- Spychaj, T. Chemical recycling of PET: Methods and products. In Handbook of Thermoplastic Polyesters: Homopolymers, Copolymers, Blends, and Composites; Wiley-VCH: Weinheim, Germany, 2002; pp. 1252–1290. [Google Scholar]
- Collins, M.J.; Zeronian, S.H.; Marshall, M.L. Analysis of the molecular weight distributions of aminolyzed poly (ethylene terephthalate) by using gel permeation chromatography. J. Macromol. Sci. Chem. 1991, 28, 775–792. [Google Scholar] [CrossRef]
- Overton, J.; Haynes, S. Determination of the crystalline fold period in poly (ethylene terephthalate). J. Polym. Sci. Polym. Symp. 1973, 43, 9–17. [Google Scholar] [CrossRef]
- Ellison, M.; Fisher, L.; Alger, K.; Zeronian, S. Physical properties of polyester fibers degraded by aminolysis and by alkalin hydrolysis. J. Appl. Polym. Sci. 1982, 27, 247–257. [Google Scholar] [CrossRef]
- Awodi, Y.; Johnson, A.; Peters, R.; Popoola, A. The aminolysis of poly (ethylene terephthalate). J. Appl. Polym. Sci. 1987, 33, 2503–2512. [Google Scholar] [CrossRef]
- Popoola, V. Polyester formation: Aminolytic degradation and proposed mechanisms of the reaction. J. Appl. Polym. Sci. 1988, 36, 1677–1683. [Google Scholar] [CrossRef]
- Spychaj, T.; Paszun, D. New trends in chemical recycling of poly (ethylene terephthalate). In Macromolecular Symposia; Wiley-VCH: Weinheim, Germany, 1998; pp. 137–145. [Google Scholar]
- Fabrycy, E.; Leistner, A.; Spychaj, T. New epoxy resin hardeners from PET scrap. Adhesion 2000, 44, 35–39. [Google Scholar]
- Mangovska, B.; Bogoeva-Gaceva, G.; Pohlers, A. Structure and basic properties of aminated PET. J. Appl. Polym. Sci. 1996, 62, 605–612. [Google Scholar] [CrossRef]
- Holmes, S. Aminolysis of poly (ethylene terephthalate) in aqueous amine and amine vapor. J. Appl. Polym. Sci. 1996, 61, 255–260. [Google Scholar] [CrossRef]
- Shukla, S.; Harad, A.M. Aminolysis of polyethylene terephthalate waste. Polym. Degrad. Stab. 2006, 91, 1850–1854. [Google Scholar] [CrossRef]
- Padhan, R.K.; Sreeram, A. Chemical depolymerization of PET bottles via combined chemolysis methods. In Recycling of Polyethylene Terephthalate Bottles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–147. [Google Scholar]
- Vollmer, I.; Jenks, M.J.; Roelands, M.C.; White, R.J.; van Harmelen, T.; de Wild, P.; van der Laan, G.P.; Meirer, F.; Keurentjes, J.T.; Weckhuysen, B.M. Beyond mechanical recycling: Giving new life to plastic waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef] [PubMed]
- EPA. Commercial and Industrial Solid Waste Incineration Units (CISWI): New Source Performance Standards (NSPS) and Emission Guidelines (EG) for Existing Sources. Available online: https://www.epa.gov/stationary-sources-air-pollution/commercial-and-industrial-solid-waste-incineration-units-ciswi-new (accessed on 2 May 2022).
- Pinter, E.; Welle, F.; Mayrhofer, E.; Pechhacker, A.; Motloch, L.; Lahme, V.; Grant, A.; Tacker, M. Circularity study on PET bottle-to-bottle recycling. Sustainability 2021, 13, 7370. [Google Scholar] [CrossRef]
- Fritsch, K.; Welle, F. Polyethylene terephthalate (PET)-For packaging. Kunstst.-Plast Eur. 2002, 92, 111. [Google Scholar]
- Welle, F. Investigation into the decontamination efficiency of a new PET recycling concept. Food Addit. Contam. 2008, 25, 123–131. [Google Scholar] [CrossRef]
- Franz, R.; Welle, F. Recycled poly (ethylene terephthalate) for direct food contact applications: Challenge test of an inline recycling process. Food Addit. Contam. 2002, 19, 502–511. [Google Scholar] [CrossRef]
- NAPCOR. Postconsumer PET Recycling Activity in 2018. Available online: https://napcor.com/wp-content/uploads/2021/07/Postconsumer-PET-Recycling-Activity-in-2018.pdf (accessed on 26 February 2022).
- Venkatachalam, S.; Nayak, S.G.; Labde, J.V.; Gharal, P.R.; Rao, K.; Kelkar, A.K. Degradation and recyclability of poly (ethylene terephthalate). In Polyester; InTech: Rijeka, Croatia, 2012; pp. 75–98. [Google Scholar]
- United States Food and Drug Administration (US FDA). Use of Recycled Plastics in Food Packaging (Chemistry Considerations): Guidance for Industry; United States Food and Drug Administration (US FDA): Silver Spring, MD, USA, 2021.
- EFSA. Scientific opinion on the criteria to be used for safety evaluation of a mechanical recycling process to produce recycled PET intended to be used for manufacture of materials and articles in contact with food. Eur. Food Saf. Auth. 2011, 9, 2184. [Google Scholar]
- Franz, R.; Welle, F. Contamination levels in recollected PET bottles from non-food applications and their impact on the safety of recycled PET for food contact. Molecules 2020, 25, 4998. [Google Scholar] [CrossRef]
- Tournier, V.; Topham, C.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.-L.; Texier, H.; Gavalda, S. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef] [PubMed]
- APR. PET (Polyethylene Terephthalate, Resin Identification Code #1). Available online: https://plasticsrecycling.org/pet-design-guidance (accessed on 5 March 2022).
- Arnold, M. Recycling Food Packaging: Discussion Paper; VTT Technical Research Centre of Finland: Espoo, Finland, 2022. [Google Scholar]
- Mangold, H.; von Vacano, B. The frontier of plastics recycling: Rethinking waste as a resource for high-value applications. Macromol. Chem. Phys. 2022, 2100488, 1–17. [Google Scholar] [CrossRef]
- Berg, H.; Kulinna, R.; Stöcker, C.; Guth-Orlowski, S.; Thiermann, R.; Porepp, N. Overcoming Information Asymmetry in the Plastics Value Chain with Digital Product Passports: How Decentralised Identifiers and Verifiable Credentials Can Enable a Circular Economy for Plastics; Wuppertal Institut für Klima, Umwelt, Energie: Wuppertal, Germany, 2022. [Google Scholar]













| PET Type | L* | a* | b* | % Haze | Yellowness Index |
|---|---|---|---|---|---|
| Virgin | 94.61 | −0.04 | 0.60 | 0.74 | 1.13 |
| PCR Grade A | 94.83 | −0.10 | 0.77 | 2.34 | 1.39 |
| PCR Grade B | 94.88 | −0.14 | 1.32 | 1.92 | 2.42 |
| PCR Grade C | 93.19 | −0.17 | 2.64 | 6.20 | 4.99 |
| PCR Grade D | 94.65 | −0.14 | 0.82 | 2.59 | 1.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. https://doi.org/10.3390/polym14122366
Benyathiar P, Kumar P, Carpenter G, Brace J, Mishra DK. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers. 2022; 14(12):2366. https://doi.org/10.3390/polym14122366
Chicago/Turabian StyleBenyathiar, Patnarin, Pankaj Kumar, Gregory Carpenter, John Brace, and Dharmendra K. Mishra. 2022. "Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review" Polymers 14, no. 12: 2366. https://doi.org/10.3390/polym14122366
APA StyleBenyathiar, P., Kumar, P., Carpenter, G., Brace, J., & Mishra, D. K. (2022). Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers, 14(12), 2366. https://doi.org/10.3390/polym14122366