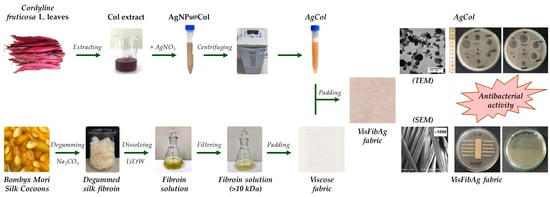
Fabrication of Silver Nanoparticles Using Cordyline fruticosa L. Leave Extract Endowing Silk Fibroin Modified Viscose Fabric with Durable Antibacterial Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
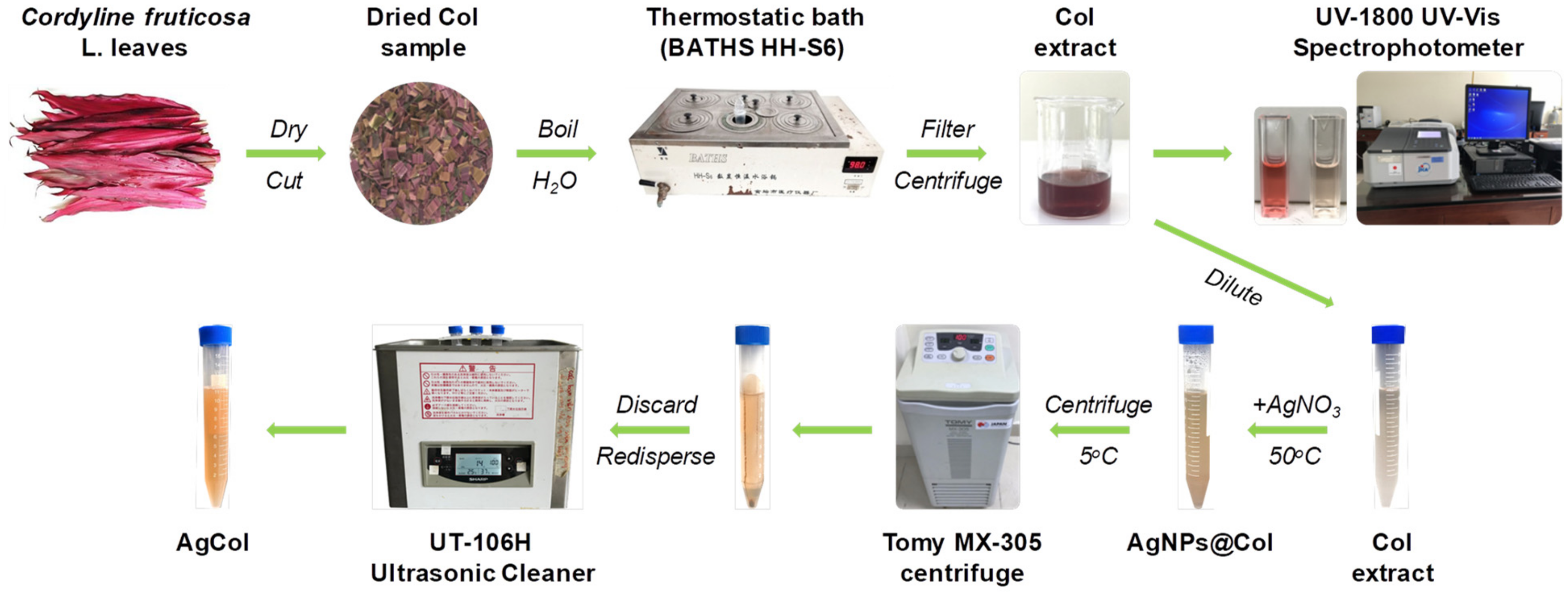
2.2. Preparation of Cordyline fruticosa L. Leaf Extract
2.3. Synthesis of Silver Nanoparticles
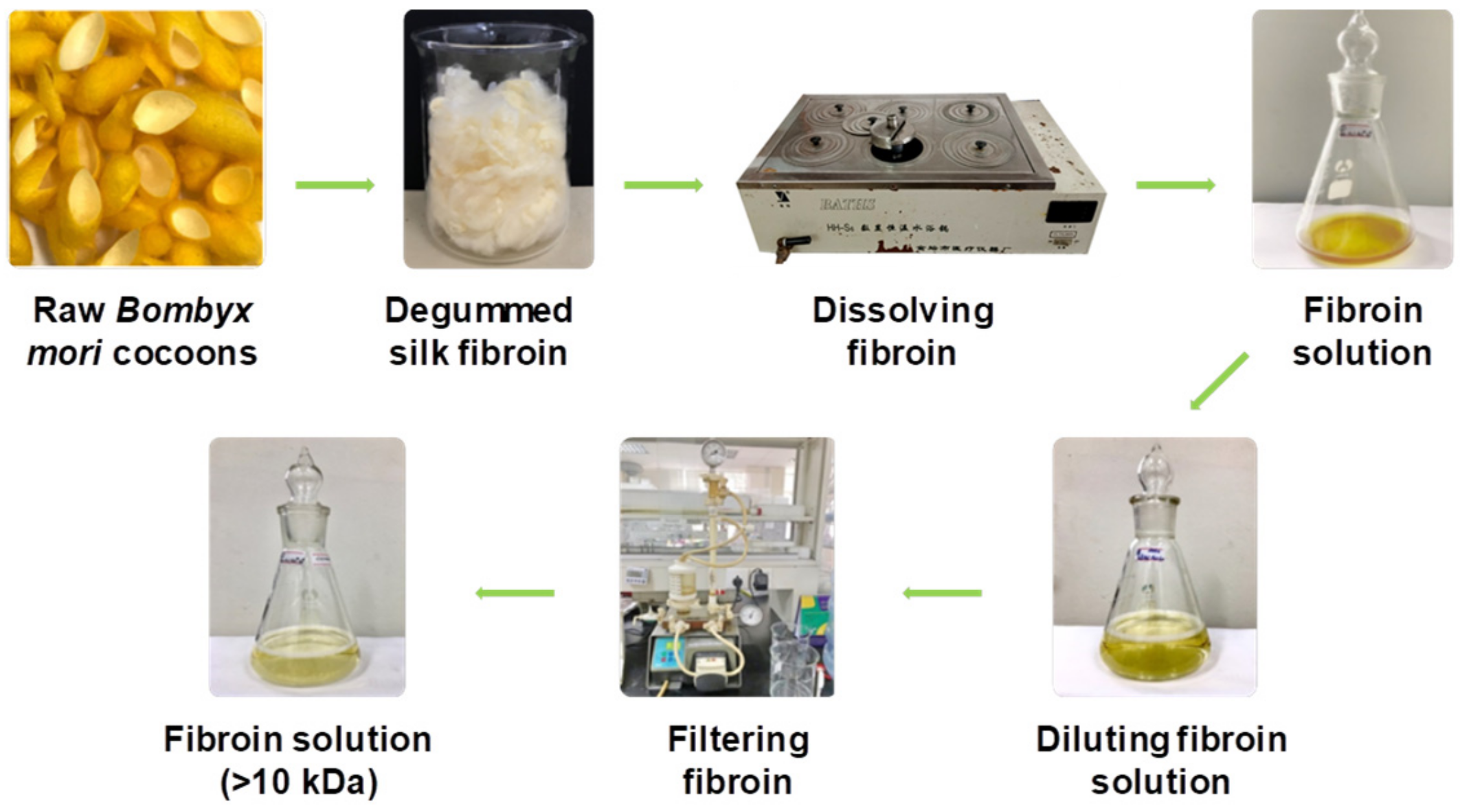
2.4. Dissolution of Silk Fibroin
2.5. Modification of Viscose Fabrics with Silk Fibroin and Silver Nanoparticles
2.6. Analytical Methods
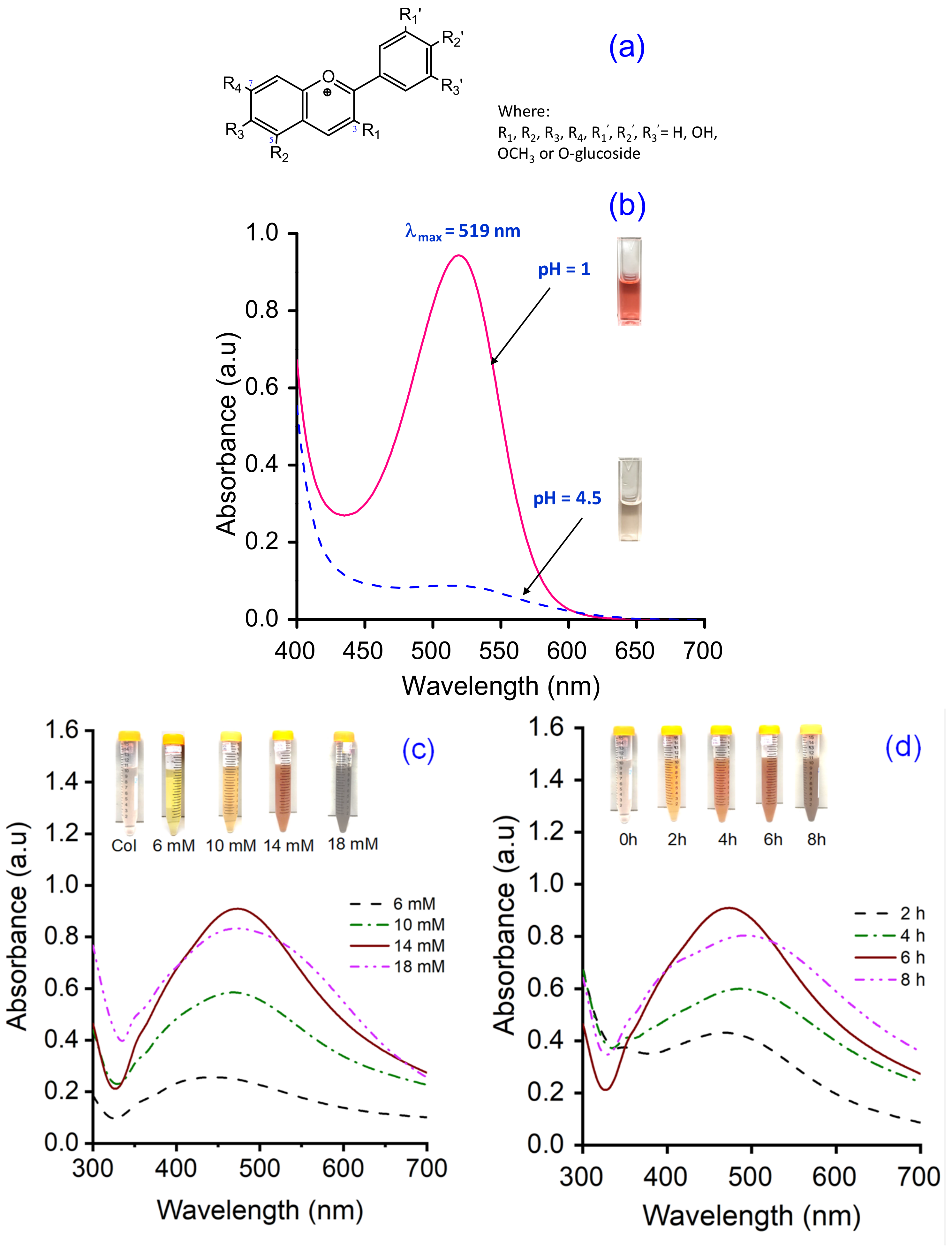
2.6.1. The Total Anthocyanin Content of the Col Extract
2.6.2. Characterization of the AgCol
2.6.3. Characterization of the Modified Viscose Fabrics
2.7. Antibacterial Activities
2.7.1. Bio-Synthesized Silver Nanoparticles (AgCol)
2.7.2. The Modified Viscose Fabrics with AgCol and Silk Fibroin
2.7.3. Durability of the Antimicrobial Treatment against Washing
3. Results and Discussion
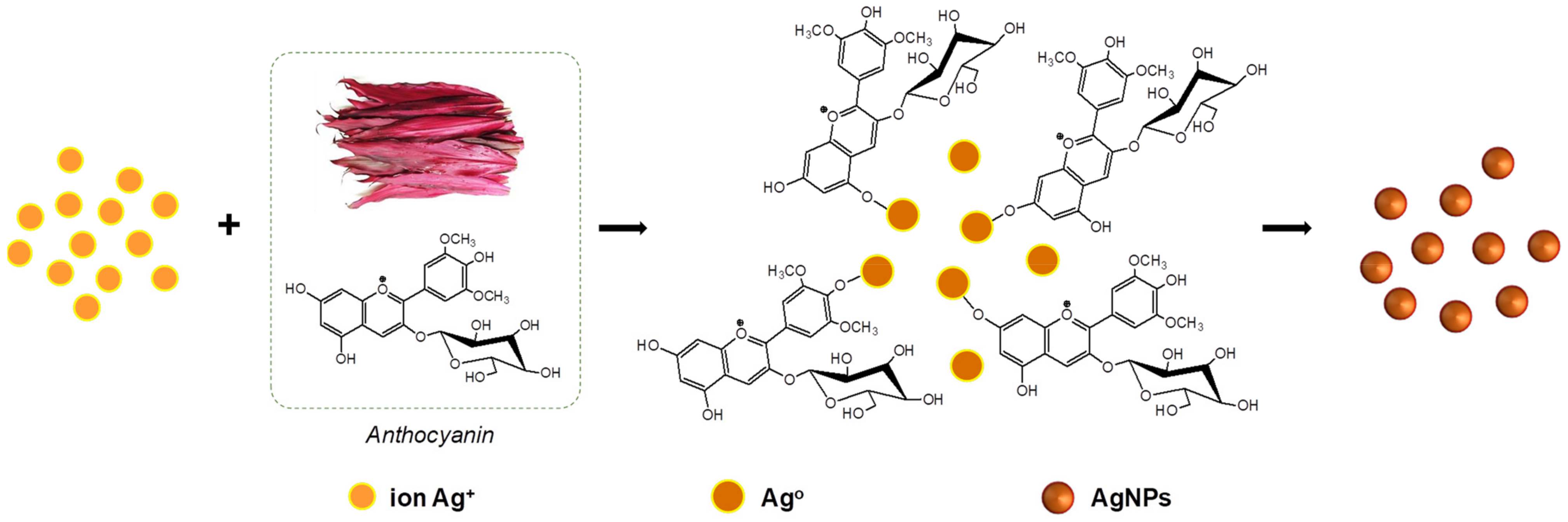
3.1. Synthesis and Optimization of AgCol from Col Extract
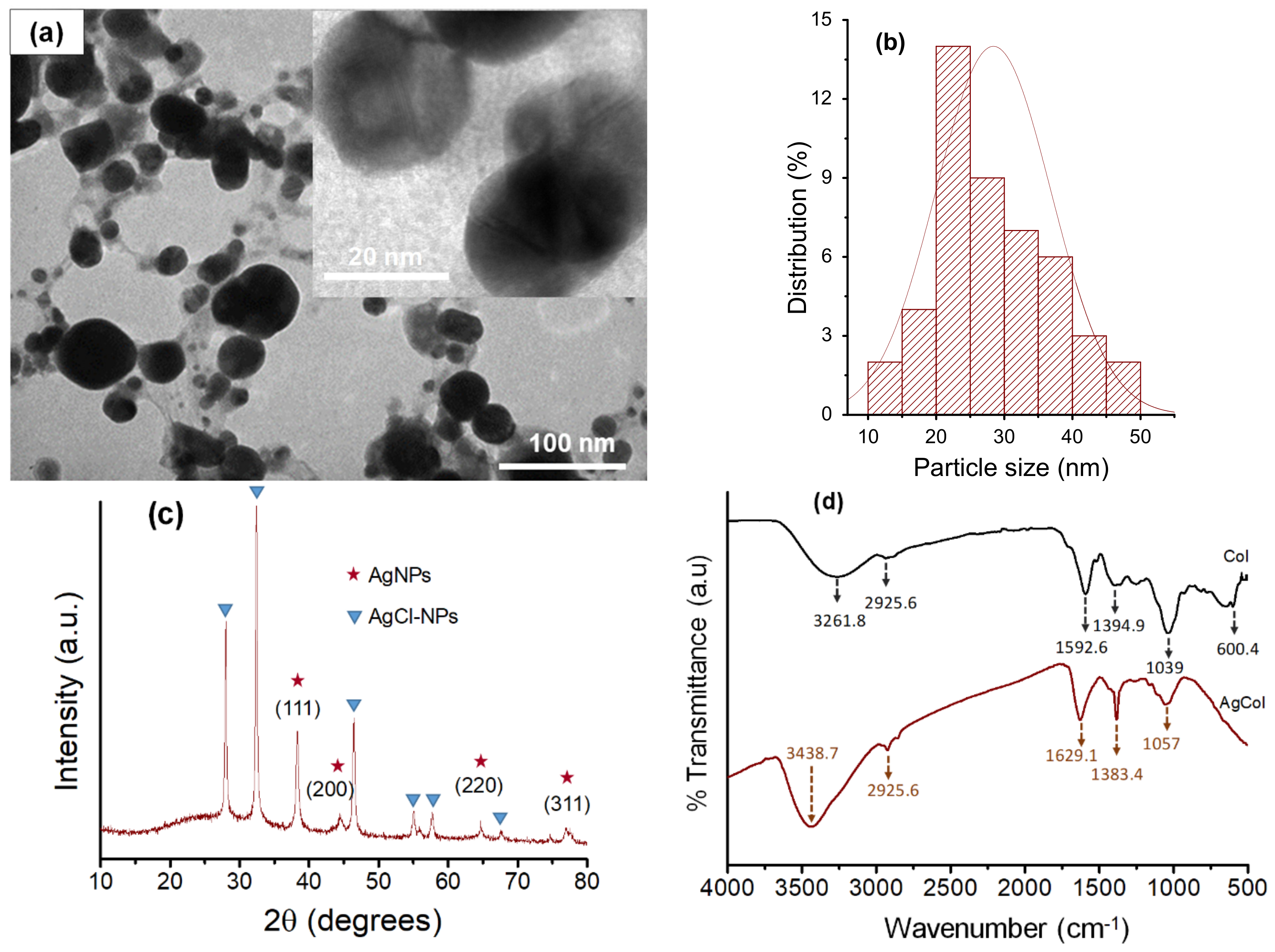
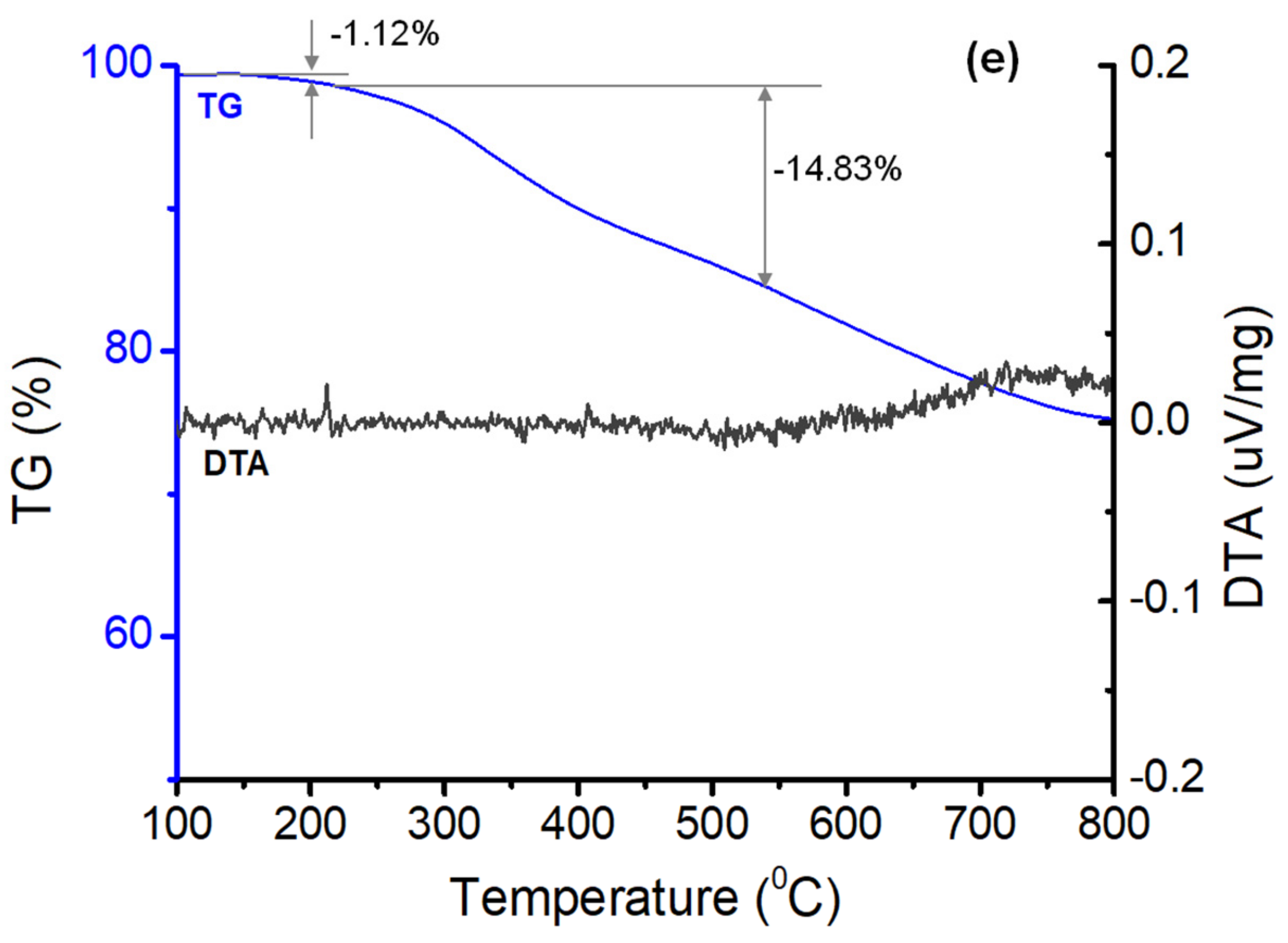
3.2. Morphological and Structural Characterization of AgCol
3.3. Antimicrobial Activity of AgCol
3.4. Coloration of the Modified Viscose Fabrics with AgCol and Silk Fibroin
3.5. Antibacterial Efficacy of the Modified Viscose Fabrics with AgCol and Silk Fibroin
3.6. Characteristics of the Modified Viscose Fabrics with AgCol and Silk Fibroin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Q.; Zheng, W.; Duan, P.; Chen, J.; Zhang, Y.; Fu, F.; Diao, H.; Liu, X. One-pot fabrication of durable antibacterial cotton fabric coated with silver nanoparticles via carboxymethyl chitosan as a binder and stabilizer. Carbohydr. Polym. 2019, 204, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, H.M.; Eid, R.A.A.; Hashem, S.S.; Amr, A. Enhancing some functional properties of viscose fabric. Carbohydr. Polym. 2013, 92, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Xie, L.; Diao, H.; Li, F.; Zhang, Y.; Fu, F.; Liu, X. Antibacterial cotton fabric with enhanced durability prepared using silver nanoparticles and carboxymethyl chitosan. Carbohydr. Polym. 2017, 177, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.A.; El-Zairy, E.M.R.; Eid, B.M. Eco-friendly modification and antibacterial functionalization of viscose fabric. J. Text. Inst. 2017, 108, 1406–1411. [Google Scholar] [CrossRef]
- Raza, Z.A.; Bilal, U.; Noreen, U.; Munim, S.A.; Riaz, S.; Abdullah, M.U.; Abid, S. Chitosan Mediated Formation and Impregnation of Silver Nanoparticles on Viscose Fabric in Single Bath for Antibacterial Performance. Fibers Polym. 2019, 20, 1360–1367. [Google Scholar] [CrossRef]
- Mostafa, K.; El-Sanabary, A. Innovative ecological method for producing easy care characteristics and antibacterial activity onto viscose fabric using glutaraldehyde and chitosan nanoparticles. Pigment. Resin Technol. 2020, 49, 11–18. [Google Scholar] [CrossRef]
- Emam, H.E. Antimicrobial cellulosic textiles based on organic compounds. Biotechnology 2019, 9, 29. [Google Scholar] [CrossRef]
- Song, X.; Cvelbar, U.; Strazar, P.; Vossebein, L.; Zille, A. Antimicrobial Efficiency and Surface Interactions of Quaternary Ammonium Compound Absorbed on Dielectric Barrier Discharge (DBD) Plasma Treated Fiber-Based Wiping Materials. ACS Appl. Mater. Interfaces 2020, 12, 298–311. [Google Scholar] [CrossRef]
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial Approaches for Textiles: From Research to Market. Materials 2016, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Hassabo, A.G.; El-Naggar, M.E.; Mohamed, A.L.; Hebeish, A.A. Development of multifunctional modified cotton fabric with tri-component nanoparticles of silver, copper and zinc oxide. Carbohydr. Polym. 2019, 210, 144–156. [Google Scholar] [CrossRef]
- Emam, H.E.; El-Hawary, N.S.; Ahmed, H.B. Green technology for durable finishing of viscose fibers via self-formation of AuNPs. Int. J. Biol. Macromol. 2017, 96, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.-N.; Khan, M.Q.; Nguyen, N.-T.; Phan, T.-T.; Ullah, A.; Khatri, M.; Kien, N.N.; Kim, I.-S. A review on the fabrication of several carbohydrate polymers into nanofibrous structures using electrospinning for removal of metal ions and dyes. Carbohydr. Polym. 2021, 252, 117175. [Google Scholar] [CrossRef] [PubMed]
- Edgar, K.J.; Zhang, H. Antibacterial modification of Lyocell fiber: A review. Carbohydr. Polym. 2020, 250, 116932. [Google Scholar] [CrossRef] [PubMed]
- McQueen, R.H.; Vaezafshar, S. Odor in textiles: A review of evaluation methods, fabric characteristics, and odor control technologies. Text. Res. J. 2019, 90, 1157–1173. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Liu, J.-H. A green method for in situ synthesis of poly(vinyl alcohol)/chitosan hydrogel thin films with entrapped silver nanoparticles. J. Taiwan Inst. Chem. Eng. 2014, 45, 2827–2833. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Zhang, Z.; Wang, Z.; Zhao, Y.; Sun, L. Fabrication of cotton fabrics with durable antibacterial activities finishing by Ag nanoparticles. Text. Res. J. 2018, 89, 867–880. [Google Scholar] [CrossRef]
- Zhou, Q.; Lv, J.; Ren, Y.; Chen, J.; Gao, D.; Lu, Z.; Wang, C. A green in situ synthesis of silver nanoparticles on cotton fabrics using Aloe vera leaf extraction for durable ultraviolet protection and antibacterial activity. Text. Res. J. 2016, 87, 2407–2419. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Al-Rajhi, A.M.H.; Al Abboud, M.A.; Alawlaqi, M.M.; Ganash Magdah, A.; Helmy, E.A.M.; Mabrouk, A.S. Recent Advances in Green Synthesis of Silver Nanoparticles and Their Applications: About Future Directions. A Review. BioNanoScience 2018, 8, 5–16. [Google Scholar] [CrossRef]
- Thi Lan Huong, V.; Nguyen, N.T. Green synthesis, characterization and antibacterial activity of silver nanoparticles using Sapindus mukorossi fruit pericarp extract. Mater. Today Proc. 2020, 42, 88–93. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Raza, A.; Khan, M.A.; Ahmad, I.; Islam, N.U.; Saravanan, M.; Ubaid, M.F.; Ali, M.; Shinwari, Z.K. Green synthesis of silver nanoparticles via plant extracts: Beginning a new era in cancer theranostics. Nanomedicine 2016, 11, 3157–3177. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.E.M. Effects of Hydrodynamic Diameter of Nanoparticles on Antibacterial Activity and Durability of Ag-treated Cotton Fabrics. Fibers Polym. 2020, 21, 1173–1179. [Google Scholar] [CrossRef]
- Rehan, M.; Mashaly, H.M.; Mowafi, S.; Abou El-Kheir, A.; Emam, H.E. Multi-functional textile design using in-situ Ag NPs incorporation into natural fabric matrix. Dyes Pigments 2015, 118, 9–17. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, L.; Zang, C.; Chen, Y.; Lin, H. Antibacterial cotton fabric grafted with silver nanoparticles and its excellent laundering durability. Carbohydr. Polym. 2013, 92, 2088–2094. [Google Scholar] [CrossRef]
- Zheng, J.; Song, F.; Wang, X.-L.; Wang, Y.-Z. In-situ synthesis, characterization and antimicrobial activity of viscose fiber loaded with silver nanoparticles. Cellulose 2014, 21, 3097–3105. [Google Scholar] [CrossRef]
- Emam, H.E.; Saleh, N.H.; Nagy, K.S.; Zahran, M.K. Functionalization of medical cotton by direct incorporation of silver nanoparticles. Int. J. Biol. Macromol. 2015, 78, 249–256. [Google Scholar] [CrossRef]
- Su, C.-H.; Kumar, G.V.; Adhikary, S.; Velusamy, P.; Pandian, K.; Anbu, P. Preparation of cotton fabric using sodium alginate-coated nanoparticles to protect against nosocomial pathogens. Biochem. Eng. J. 2017, 117, 28–35. [Google Scholar] [CrossRef]
- Zahran, M.; Marei, A.H. Innovative natural polymer metal nanocomposites and their antimicrobial activity. Int. J. Biol. Macromol. 2019, 136, 586–596. [Google Scholar] [CrossRef]
- Hanh, T.T.; Thu, N.T.; Hien, N.Q.; An, P.N.; Loan, T.; Thi, K.; Hoa, P.T. Preparation of silver nanoparticles fabrics against multidrug-resistant bacteria. Radiat. Phys. Chem. 2016, 121, 87–92. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M.; Abdel-Aziz, M.S. Effect of plasma superficial treatments on antibacterial functionalization and coloration of cellulosic fabrics. Appl. Surf. Sci. 2017, 392, 1126–1133. [Google Scholar] [CrossRef]
- Arif, D.; Niazi, M.B.K.; Ul-Haq, N.; Anwar, M.N.; Hashmi, E. Preparation of antibacterial cotton fabric using chitosan-silver nanoparticles. Fibers Polym. 2015, 16, 1519–1526. [Google Scholar] [CrossRef]
- Xu, Q.; Ke, X.; Shen, L.; Ge, N.; Zhang, Y.; Fu, F.; Liu, X. Surface modification by carboxymethy chitosan via pad-dry-cure method for binding Ag NPs onto cotton fabric. Int. J. Biol. Macromol. 2018, 111, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Hoang, T.T.L. Optimization of ultrasound-assisted extraction of natural colorant from Huyet du leaves using ethanol solvent. J. Sci. Technol. HaUI 2019, 51, 109–113. [Google Scholar]
- Lim, T.K. (Ed.) Cordyline fruticosa. In Edible Medicinal and Non Medicinal Plants: Volume 9, Modified Stems, Roots, Bulbs; Lim, T.K. (Ed.) Springer: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Nguyen Thi, D.P.; Tran, D.L.; Le Thi, P.; Park, K.D.; Hoang Thi, T.T. Supramolecular Gels Incorporating Cordyline terminalis Leaf Extract as a Polyphenol Release Scaffold for Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 8759. [Google Scholar] [CrossRef]
- Raslan, M.A.; Taher, R.F.; Al-Karmalawy, A.A.; El-Ebeedy, D.; Metwaly, A.G.; Elkateeb, N.M.; Ghanem, A.; Elghaish, R.A.; Abd El Maksoud, A.I. Cordyline fruticosa (L.) A. Chev. leaves: Isolation, HPLC/MS profiling and evaluation of nephroprotective and hepatoprotective activities supported by molecular docking. New J. Chem. 2021, 45, 22216–22233. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Al-Alwani, M.A.M.; Ludin, N.A.; Mohamad, A.B.; Kadhum, A.A.H.; Sopian, K. Extraction, preparation and application of pigments from Cordyline fruticosa and Hylocereus polyrhizus as sensitizers for dye-sensitized solar cells. Spectrochim. Acta Mol. Biomol. Spectrosc. 2017, 179, 23–31. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2019, 88, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Thang, N.N.; Huong, V.T.L. Enhancement of dye-ability of viscose fabric via modification with fibroin regenerated from waste silk cocoons. Vlak. Text. 2021, 28, 100–107. [Google Scholar]
- Thiex, N.J.; Manson, H.; Anderson, S.; Persson, J.-Å. Determination of Crude Protein in Animal Feed, Forage, Grain, and Oilseeds by Using Block Digestion with a Copper Catalyst and Steam Distillation into Boric Acid: Collaborative Study. J. AOAC Int. 2019, 85, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Wikler, M.A.; Low, D.E.; Cockerill, F.R.; Sheehan, D.J.; Craig, W.A.; Tenover, F.C. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard, 7th ed.; Document M7-A7; CLSI: Wayne, PA, USA, 2006. [Google Scholar]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green nanotechnology: A review on green synthesis of silver nanoparticles-an ecofriendly approach. Int. J. Nanomed. 2019, 14, 5087–5107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interfaces Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Hamidian, K.; Sarani, M.; Barani, M.; Khakbaz, F. Cytotoxic performance of green synthesized Ag and Mg dual doped ZnO NPs using Salvadora persica extract against MDA-MB-231 and MCF-10 cells. Arab. J. Chem. 2022, 15, 103792. [Google Scholar] [CrossRef]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.-T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 033001. [Google Scholar] [CrossRef] [Green Version]
- Kanniah, P.; Radhamani, J.; Chelliah, P.; Muthusamy, N.; Joshua Jebasingh Sathiya Balasingh Thangapandi, E.; Reeta Thangapandi, J.; Balakrishnan, S.; Shanmugam, R. Green Synthesis of Multifaceted Silver Nanoparticles Using the Flower Extract of Aerva lanata and Evaluation of Its Biological and Environmental Applications. ChemistrySelect 2020, 5, 2322–2331. [Google Scholar] [CrossRef]
- Hemmati, S.; Rashtiani, A.; Zangeneh, M.M.; Mohammadi, P.; Zangeneh, A.; Veisi, H. Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron 2019, 158, 8–14. [Google Scholar] [CrossRef]
- Vishwasrao, C.; Momin, B.; Ananthanarayan, L. Green Synthesis of Silver Nanoparticles Using Sapota Fruit Waste and Evaluation of Their Antimicrobial Activity. Waste Biomass Valorization 2019, 10, 2353–2363. [Google Scholar] [CrossRef]
- Srikar, S.K.; Giri, D.D.; Pal, D.B.; Mishra, P.K.; Upadhyay, S.N. Green synthesis of silver nanoparticles: A review. Green Sustain. Chem. 2016, 6, 34–56. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, V.; Vasanthi, S.; Shalini, S.; Shah, S.A.A.; Tripathy, M.; Paliwal, N. Green synthesis, characterization, antibacterial, antioxidant and photocatalytic activity of Parkia speciosa leaves extract mediated silver nanoparticles. Results Phys. 2019, 15, 102565. [Google Scholar] [CrossRef]
- Bagherzade, G.; Tavakoli, M.M.; Namaei, M.H. Green synthesis of silver nanoparticles using aqueous extract of saffron (Crocus sativus L.) wastages and its antibacterial activity against six bacteria. Asian Pac. J. Trop. Biomed. 2017, 7, 227–233. [Google Scholar] [CrossRef]
- Durán, N.; Nakazato, G.; Seabra, A.B. Antimicrobial activity of biogenic silver nanoparticles, and silver chloride nanoparticles: An overview and comments. Appl. Microbiol. Biotechnol. 2016, 100, 6555–6570. [Google Scholar] [CrossRef] [PubMed]
- Asoro, M.; Damiano, J.; Ferreira, P.J. Size Effects on the Melting Temperature of Silver Nanoparticles: In-Situ TEM Observations. Microsc. Microanal. 2009, 15, 706–707. [Google Scholar] [CrossRef] [Green Version]
- Chugh, H.; Sood, D.; Chandra, I.; Tomar, V.; Dhawan, G.; Chandra, R. Role of gold and silver nanoparticles in cancer nano-medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1210–1220. [Google Scholar] [CrossRef]
- Litvin, V.A.; Galagan, R.L.; Minaev, B.F. Kinetic and mechanism formation of silver nanoparticles coated by synthetic humic substances. Colloids Surf. Physicochem. Eng. Asp. 2012, 414, 234–243. [Google Scholar] [CrossRef]
- Alahmad, A.; Feldhoff, A.; Bigall, N.C.; Rusch, P.; Scheper, T.; Walter, J.-G. Hypericum perforatum L.-Mediated Green Synthesis of Silver Nanoparticles Exhibiting Antioxidant and Anticancer Activities. Nanomaterials 2021, 11, 487. [Google Scholar] [CrossRef]
- Tripathi, D.; Modi, A.; Narayan, G.; Rai, S.P. Green and cost effective synthesis of silver nanoparticles from endangered medicinal plant Withania coagulans and their potential biomedical properties. Mater. Sci. Eng. 2019, 100, 152–164. [Google Scholar] [CrossRef]
- Ngo, H.-T.; Bechtold, T. Surface modification of textile material through deposition of regenerated silk fibroin. J. Appl. Polym. Sci. 2017, 134, 45098. [Google Scholar] [CrossRef]
- Wurm, F.; Rietzler, B.; Pham, T.; Bechtold, T. Multivalent Ions as Reactive Crosslinkers for Biopolymers—A Review. Molecules 2020, 25, 1840. [Google Scholar] [CrossRef]







| Sample | Silk Fibroin (%) | AgCol (μg/mL) | Wet Pickup (%) | ||
|---|---|---|---|---|---|
| 70 | 80 | 90 | |||
| Viscose fabric (Vis) | 0 | 80 | VisAg11 | VisAg21 | VisAg31 |
| 0 | 40 | VisAg12 | VisAg22 | VisAg32 | |
| 0 | 20 | VisAg13 | VisAg23 | VisAg33 | |
| Fibroin treated viscose fabric (VisFib) | 2.5 | 80 | VisFibAg11 | VisFibAg21 | VisFibAg31 |
| 2.5 | 40 | VisFibAg12 | VisFibAg22 | VisFibAg32 | |
| 2.5 | 20 | VisFibAg13 | VisFibAg23 | VisFibAg33 | |
| Wet Pickup (%) | AgCol (µg/mL) | L* | a* | b* | ∆E* | K/S | Fabric Images | |
|---|---|---|---|---|---|---|---|---|
| Vis | - | - | 93.54 | −0.96 | 4.32 | 0 | 0.06 |  |
| VisFib | - | - | 93.47 | −0.98 | 4.93 | 0.48 | 0.07 |  |
| VisAg11 | 70 | 80 | 85.16 | 3.86 | 8.95 | 8.39 | 0.19 |  |
| VisAg21 | 80 | 84.32 | 4.55 | 9.08 | 9.17 | 0.2 |  | |
| VisAg31 | 90 | 85.12 | 4.1 | 8.77 | 8.49 | 0.18 |  | |
| VisAg21 | 80 | 80 | 84.32 | 4.55 | 9.08 | 9.17 | 0.20 |  |
| VisAg22 | 40 | 85.65 | 3.15 | 6.34 | 6.33 | 0.15 |  | |
| VisAg23 | 20 | 89.58 | 1.11 | 5.75 | 3.53 | 0.11 |  | |
| VisFibAg21 | 80 | 80 | 79.95 | 4.31 | 6.72 | 8.91 | 0.28 |  |
| VisFibAg22 | 40 | 83.99 | 2.85 | 5.90 | 6.44 | 0.19 |  | |
| VisFibAg23 | 20 | 87.73 | 1.56 | 5.22 | 4.33 | 0.13 |  |
| No | Fabric Sample | Washing Cycles | AgCol (μg/mL) | Fibroin (%) | Silver Content (mg/kg) | Nitrogen Content (%) | Fibroin a Content (%) |
|---|---|---|---|---|---|---|---|
| 1 | Vis | - | - | - | - | 0.019 | - |
| 2 | VisFibW0 | 0 | - | 2.5 | - | 0.244 | 1.406 |
| 3 | VisFibW30 | 30 | - | - | - | 0.121 | 0.638 |
| 4 | VisAgW0 | 0 | 80 | - | 642.02 | - | - |
| 5 | VisAgW30 | 30 | 80 | - | 313.43 | - | - |
| 6 | VisFibAgW0 | 0 | 80 | 2.5 | 734.05 | 0.242 | 1.394 |
| 7 | VisFibAgW30 | 30 | 80 | - | 545.86 | 0.127 | 0.675 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.-T.; Vo, T.-L.-H. Fabrication of Silver Nanoparticles Using Cordyline fruticosa L. Leave Extract Endowing Silk Fibroin Modified Viscose Fabric with Durable Antibacterial Property. Polymers 2022, 14, 2409. https://doi.org/10.3390/polym14122409
Nguyen N-T, Vo T-L-H. Fabrication of Silver Nanoparticles Using Cordyline fruticosa L. Leave Extract Endowing Silk Fibroin Modified Viscose Fabric with Durable Antibacterial Property. Polymers. 2022; 14(12):2409. https://doi.org/10.3390/polym14122409
Chicago/Turabian StyleNguyen, Ngoc-Thang, and Thi-Lan-Huong Vo. 2022. "Fabrication of Silver Nanoparticles Using Cordyline fruticosa L. Leave Extract Endowing Silk Fibroin Modified Viscose Fabric with Durable Antibacterial Property" Polymers 14, no. 12: 2409. https://doi.org/10.3390/polym14122409
APA StyleNguyen, N.-T., & Vo, T.-L.-H. (2022). Fabrication of Silver Nanoparticles Using Cordyline fruticosa L. Leave Extract Endowing Silk Fibroin Modified Viscose Fabric with Durable Antibacterial Property. Polymers, 14(12), 2409. https://doi.org/10.3390/polym14122409