Photo-Responsive Micelles with Controllable and Co-Release of Carbon Monoxide, Formaldehyde and Doxorubicin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments and Measurements
2.3. Synthesis Procedures
2.3.1. Synthesis of Compound B
2.3.2. Synthesis of FFM Monomer
2.3.3. Synthesis of PEG45-b-PFFM15 Copolymer (PCOFA)
2.4. Self-Assembly of PCOFA Diblock Copolymer
2.5. Characterization of PCOFA Micelles
2.5.1. CMC Test of PCOFA Micelles
2.5.2. Optical Properties and Photolysis of PCOFA Micelles
2.5.3. DOX Loading and Photo-Triggered DOX, FA and CO Release
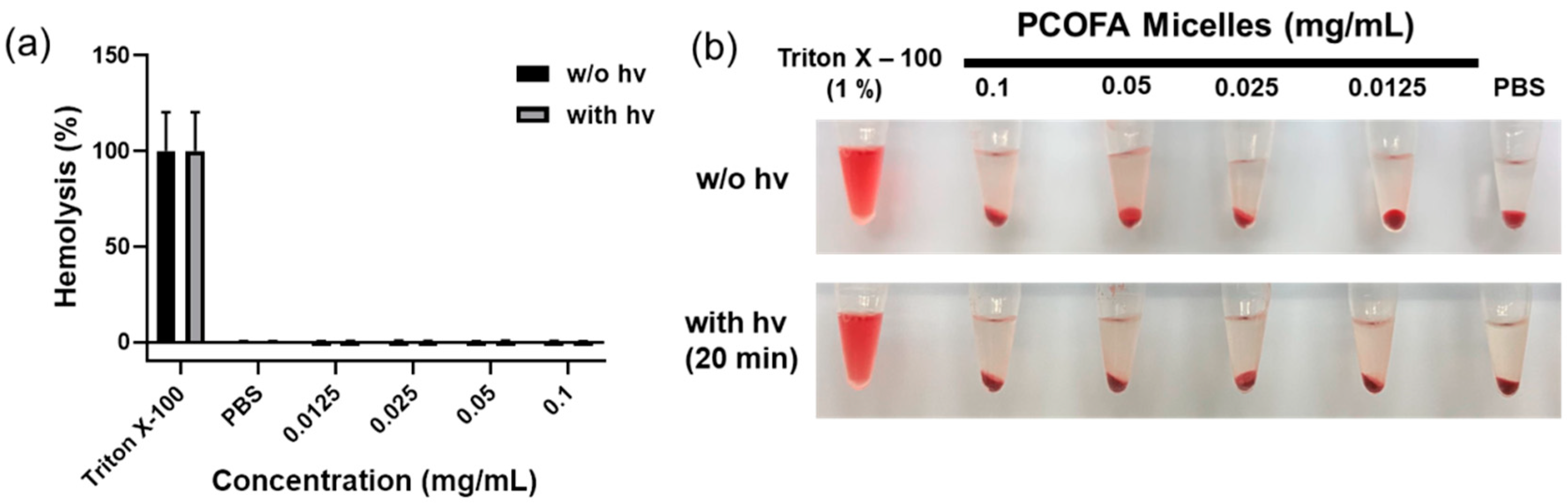
2.5.4. Hemolytic and Cytotoxicity Assessment of DOX-Free and DOX-Loaded Micelles
2.6. Statistical Analysis
3. Results
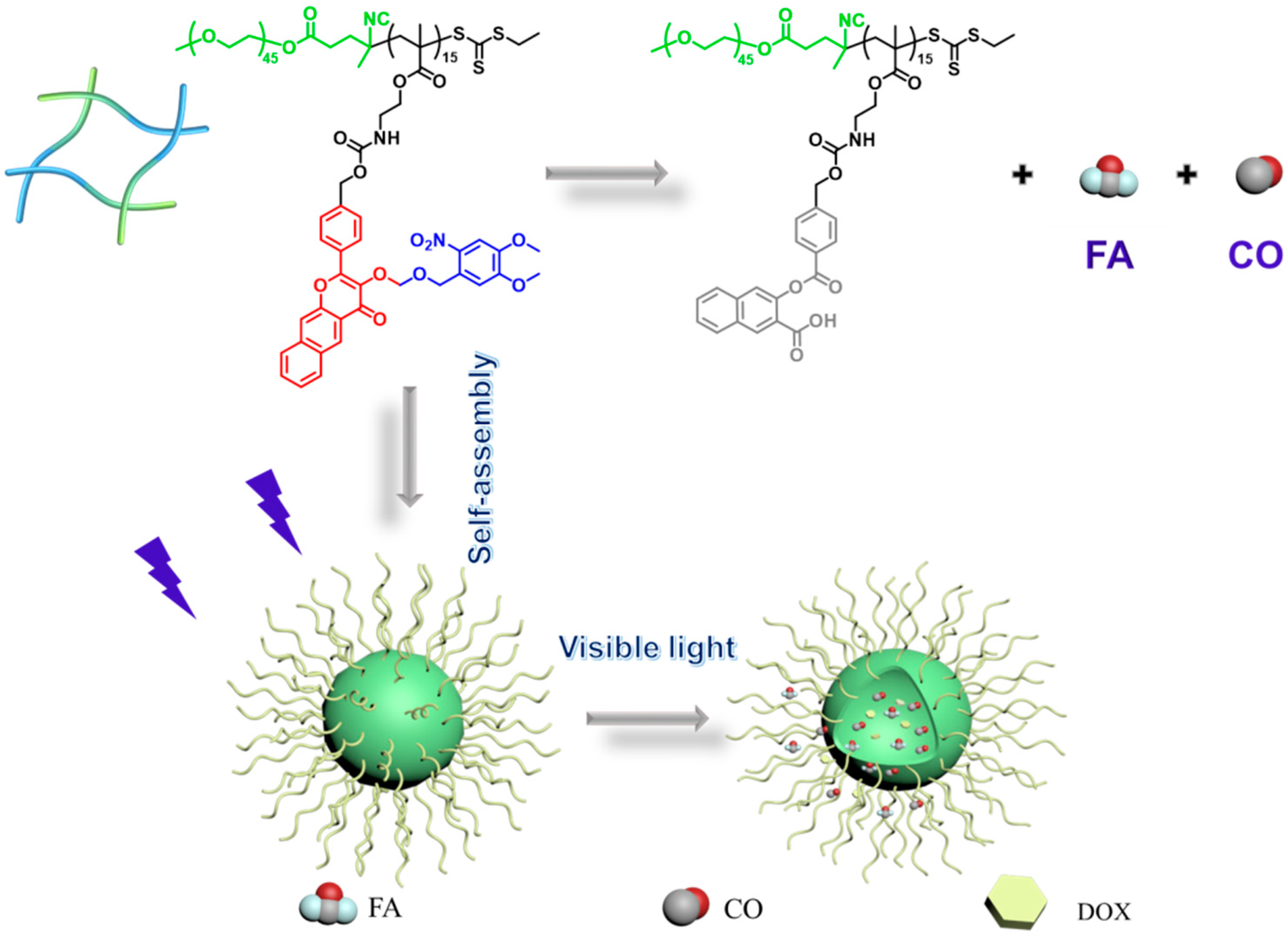
3.1. Design and Characterization of CO, FA, and COX Co-Release Diblock Copolymer

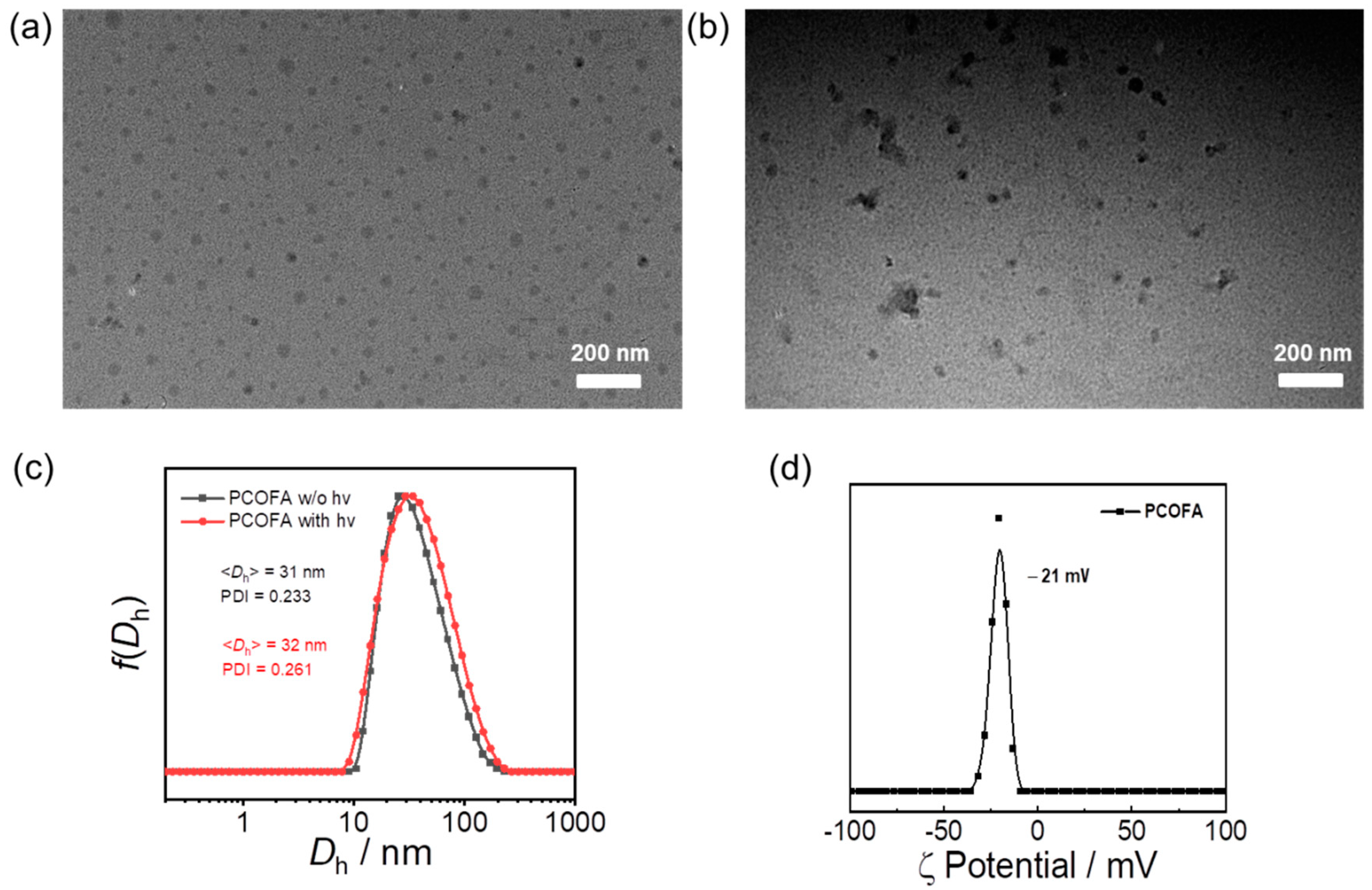
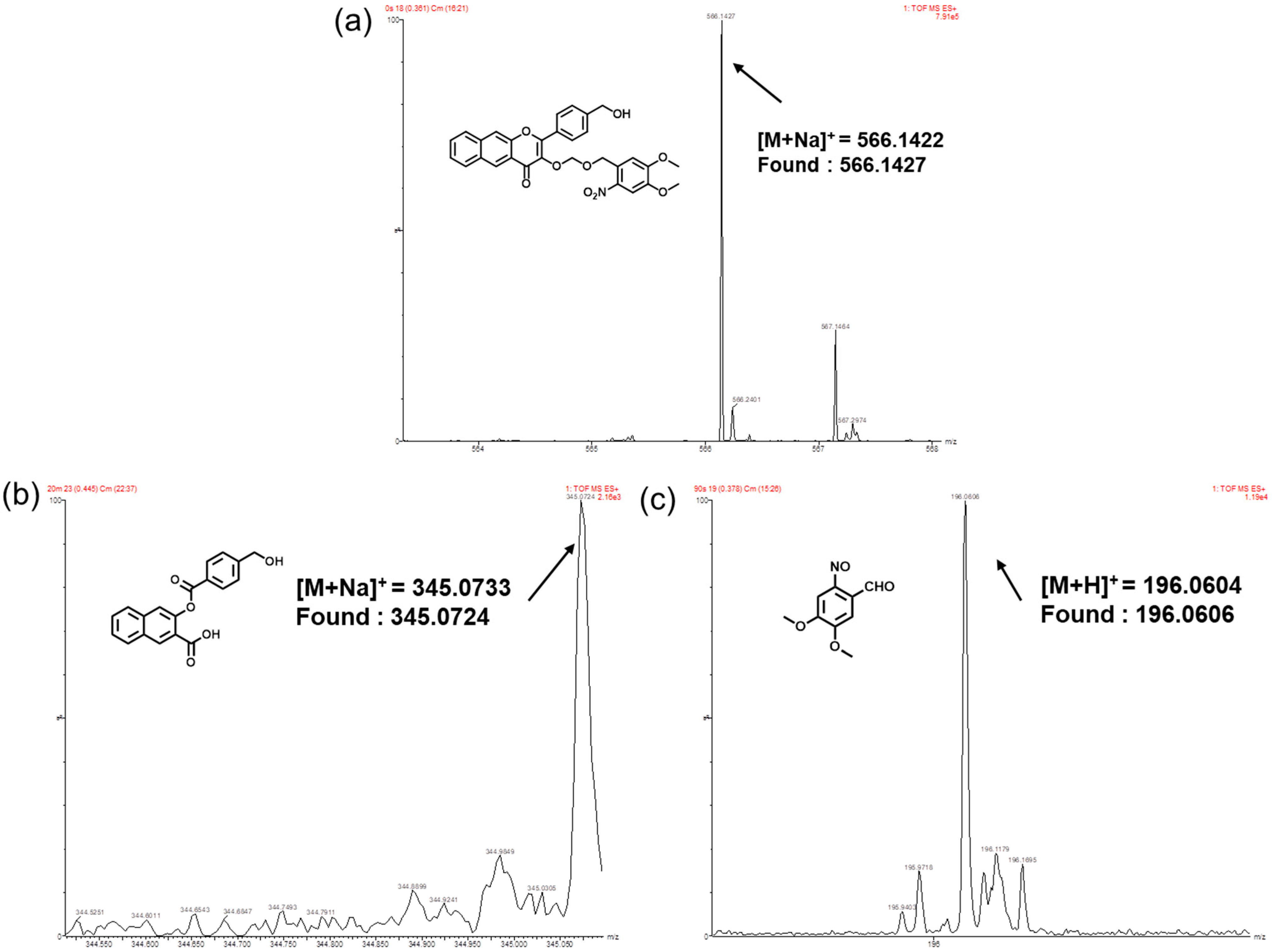
3.2. Visible Light-Triggered CO, FA and Mechanism

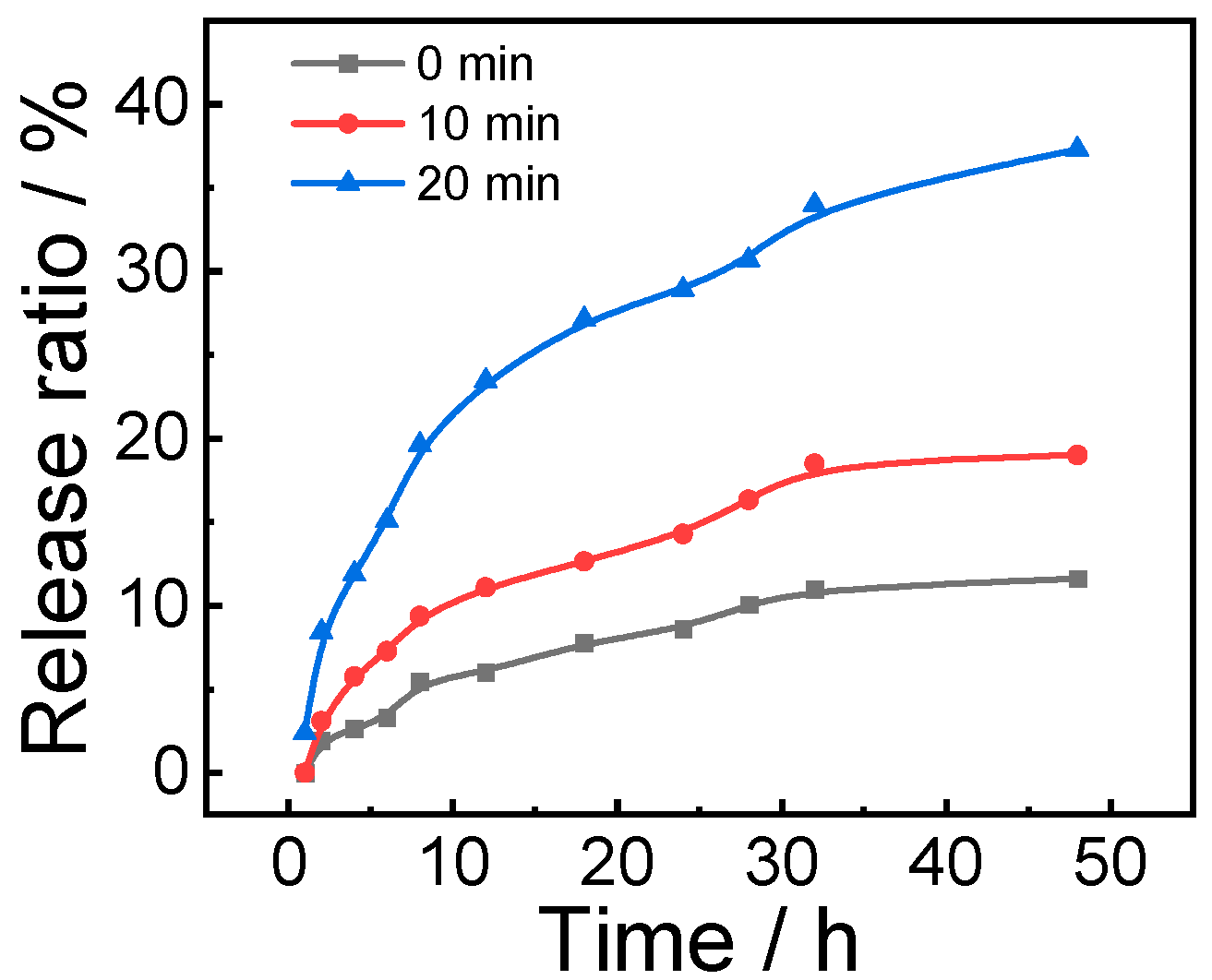
3.3. Co-Release of DOX, CO and FA for Antitumor Attempt
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, L.D.; Hu, P.; Chen, Y. Gas-Generating Nanoplatforms: Material chemistry, multifunctionality, and gas therapy. Adv. Mater. 2018, 30, 1801964. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.; Pischke, S.E.; Zhou, Z.H.; Davis, R.J.; Flavell, R.A.; Loop, T.; Otterbein, S.L.; Otterbein, L.E.; Choi, A.M.K. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J. Biol. Chem. 2003, 278, 36993–36998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuckerbraun, B.S.; Chin, B.Y.; Bilban, M.; d’Avila, J.C.; Rao, J.; Billiar, T.R.; Otterbein, L.E. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007, 21, 1099–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wegiel, B.; Gallo, D.; Csizmadia, E.; Harris, C.; Belcher, J.; Vercellotti, G.M.; Penacho, N.; Seth, P.; Sukhatme, V.; Ahmed, A.; et al. Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer Res. 2013, 73, 7009–7021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manoharan, D.; Li, W.P.; Yeh, C.S. Advances in controlled gas-releasing nanomaterials for therapeutic applications. Nanoscale Horiz. 2019, 4, 557–578. [Google Scholar] [CrossRef]
- Motterlini, R.; Otterbein, L.E. The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 2010, 9, 728–743. [Google Scholar] [CrossRef]
- Szabo, C. Gasotransmitters in cancer: From pathophysiology to experimental therapy. Nat. Rev. Drug Discov. 2016, 15, 185–203. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Gallego, S.; Bernardes, G.J.L. Carbon-Monoxide-Releasing Molecules for the delivery of therapeutic CO in vivo. Angew. Chem. Int. Ed. 2014, 53, 9712–9721. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [Green Version]
- Burgos-Barragan, G.; Wit, N.; Meiser, J.; Dingler, F.A.; Pietzke, M.; Mulderrig, L.; Pontel, L.B.; Rosado, I.V.; Brewer, T.F.; Cordell, R.L.; et al. Mammals divert endogenous genotoxic formaldehyde into one-carbon metabolism. Nature 2017, 548, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.Q.; Yu, R.; Hartwell, H.J.; Moeller, B.C.; Bodnar, W.M.; Swenberg, J.A. Measurement of endogenous versus exogenous formaldehyde-induced DNA-protein crosslinks in animal tissues by stable isotope labeling and ultrasensitive mass spectrometry. Cancer Res. 2016, 76, 2652–2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontel, L.B.; Rosado, I.V.; Burgos-Barragan, G.; Garaycoechea, J.I.; Yu, R.; Arends, M.J.; Chandrasekaran, G.; Broecker, V.; Wei, W.; Liu, L.M.; et al. Endogenous formaldehyde is a hematopoietic stem cell genotoxin and metabolic carcinogen. Mol. Cell. 2015, 60, 177–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smaga, L.P.; Pino, N.W.; Ibarra, G.E.; Krishnamurthy, V.; Chan, J. A photoactivatable formaldehyde donor with fluorescence monitoring reveals threshold to arrest cell migration. J. Am. Chem. Soc. 2020, 142, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.Q.; Zhang, J.L.; Luo, W.H.; Wang, W.S.; Li, F.X.; Li, H.; Luo, H.J.; Lu, J.; Zhou, J.N.; Wan, Y.; et al. Urine formaldehyde level is inversely correlated to mini mental state examination scores in senile dementia. Neurobiol. Aging 2011, 32, 31–41. [Google Scholar] [CrossRef]
- Yu, P.H.; Zuo, D.M. Oxidative deamination of methylamine by semicarbazide-sensitive amine oxidase leads to cytotoxic damage in endothelial-cells-possible consequences for diabetes. Diabetes 1993, 42, 594–603. [Google Scholar] [CrossRef]
- Baan, R.; Grosse, Y.; Straif, K.; Secretan, B.; Ghissassi, F.E.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens--Part F: Chemical agents and related occupations. Lancet Oncol. 2009, 10, 1143–1144. [Google Scholar] [CrossRef]
- Tao, R.R.; Liao, M.H.; Wang, Y.X.; Wang, H.; Tan, Y.H.; Qin, S.Y.; Wei, W.J.; Tang, C.Z.; Liang, X.G.; Han, Y.F.; et al. In situ imaging of formaldehyde in live mice with high spatiotemporal resolution reveals aldehyde dehydrogenase-2 as a potential target for Alzheimer’s disease treatment. Anal. Chem. 2022, 94, 1308–1317. [Google Scholar] [CrossRef]
- Huang, S.M.; Li, Z.J.; Liu, M.H.; Zhou, M.J.; Weng, J.T.; He, Y.; Jiang, Y.; Zhang, H.T.; Sun, H.Y. Reaction-based fluorescent and chemiluminescent probes for formaldehyde detection and imaging. Chem. Commun. 2022, 58, 1442–1453. [Google Scholar] [CrossRef]
- Du, Y.M.; Zhang, Y.Q.; Huang, M.R.; Wang, S.S.; Wang, J.Z.; Liao, K.K.; Wu, X.J.; Zhou, Q.; Zhang, X.H.; Wu, Y.D. Systematic investigation of the aza-Cope reaction for fluorescence imaging of formaldehyde in vitro and in vivo. Chem. Sci. 2021, 12, 13857–13869. [Google Scholar] [CrossRef]
- Duan, Y.T.; He, K.W.; Zhang, G.Y.; Hu, J.M. Photoresponsive micelles enabling codelivery of nitric oxide and formaldehyde for combinatorial antibacterial applications. Biomacromolecules 2021, 22, 2160–2170. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, R.J.; Zhu, X.Y.; Yan, D.Y. Photo-responsive polymeric micelles. Soft Matter. 2014, 10, 6121–6138. [Google Scholar] [CrossRef] [PubMed]
- Viger, M.L.; Grossman, M.; Fomina, N.; Almutairi, A. Low power upconverted near-IR light for efficient polymeric nanoparticle degradation and cargo release. Adv. Mater. 2013, 25, 3733–3738. [Google Scholar] [CrossRef] [PubMed]
- Palao, E.; Slanina, T.; Muchova, L.; Solomek, T.; Vitek, L.; Klan, P. Transition-metal-free CO-releasing BODIPY derivatives activatable by visible to NIR light as promising bioactive molecules. J. Am. Chem. Soc. 2016, 138, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.R.; Tian, Y.; Wang, Y.J.; Yang, W.L. Near-infrared laser-triggered nitric oxide nanogenerators for the reversal of multidrug resistance in cancer. Adv. Funct. Mater. 2017, 27, 1606398. [Google Scholar] [CrossRef]
- Wang, W.W.; Cheng, D.; Gong, F.M.; Miao, X.M.; Shuai, X.T. Design of Multifunctional micelle for tumor-targeted intracellular drug release and fluorescent imaging. Adv. Mater. 2012, 24, 115–120. [Google Scholar] [CrossRef]
- Dai, J.; Lin, S.D.; Cheng, D.; Zou, S.Y.; Shuai, X.T. Interlayer-crosslinked micelle with partially hydrated core showing reduction and pH dual sensitivity for pinpointed intracellular drug release. Angew. Chem. Int. Ed. 2011, 50, 9404–9408. [Google Scholar] [CrossRef]
- Xiao, H.; Li, X.X.; Zheng, C.J.; Liu, Q.M.; Sun, C.Y.; Huang, J.S.; Wang, Y.; Yuan, Y.Y. Intracellular pH-responsive polymeric micelle for simultaneous chemotherapy and MR imaging of hepatocellular carcinoma. J. Nanopart. Res. 2020, 22, 105. [Google Scholar] [CrossRef]
- Yin, T.H.; Wang, P.; Li, J.G.; Zheng, R.Q.; Zheng, B.W.; Cheng, D.; Li, R.T.; Lai, J.Y.; Shuai, X.T. Ultrasound-sensitive siRNA-loaded nanobubbles formed by hetero-assembly of polymeric micelles and liposomes and their therapeutic effect in gliomas. Biomaterials 2013, 34, 4532–4543. [Google Scholar] [CrossRef]
- Ding, Z.L.; He, K.W.; Duan, Y.T.; Shen, Z.Q.; Cheng, J.; Zhang, G.Y.; Hu, J.M. Photo-degradable micelles for co-delivery of nitric oxide and doxorubicin. J. Mater. Chem. B 2020, 8, 7009–7017. [Google Scholar] [CrossRef]
- Duan, Y.T.; Zhang, M.Y.; Shen, Z.Q.; Zhang, M.D.; Zheng, B.; Cheng, S.; Hu, J.M. Photoresponsive Vesicles Enabling Sequential Release of Nitric Oxide (NO) and Gentamicin for Efficient Biofilm Eradication. Macromol. Rapid Commun. 2021, 42, 2000759. [Google Scholar] [CrossRef]
- Cheng, J.; Zheng, B.; Cheng, S.; Zhang, G.Y.; Hu, J.M. Metal-free carbon monoxide-releasing micelles undergo tandem photochemical reactions for cutaneous wound healing. Chem. Sci. 2020, 11, 4499–4507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, P.K.; Banerjee, A. Photochemistry of 2-Nitrobenzyl Enol Ethers: Oxidative CC Bond Scission. Org. Lett. 2005, 7, 2485–2487. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.A.; Koch, A.A.; Sherman, D.H. Substrate Controlled Divergence in Polyketide Synthase Catalysis. J. Am. Chem. Soc. 2015, 137, 3735–3738. [Google Scholar] [CrossRef] [PubMed]
- Che, H.L.; Cao, S.P.; Van Hest, J.C.M. Feedback-induced temporal control of “breathing” polymersomes to create self-adaptive nanoreactors. J. Am. Chem. Soc. 2018, 140, 5356–5359. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, U.; Van Der Vlies, A.J.; Simeoni, E.; Wandrey, C.; Hubbell, J.A. Carbon monoxide-releasing micelles for immuno-therapy. J. Am. Chem. Soc. 2010, 132, 18273–18280. [Google Scholar] [CrossRef]
- Anderson, S.N.; Richards, J.M.; Esquer, H.J.; Benninghoff, A.D.; Arif, A.M.; Berreau, L.M. A structurally-tunable 3-Hydroxyflavone motif for visible light-induced carbon monoxide-releasing molecules (CORMs). ChemistryOpen 2015, 4, 590–594. [Google Scholar] [CrossRef]
- Soboleva, T.; Berreau, L.M. Tracking CO Release in Cells via the Luminescence of Donor Molecules and/or their By-Products. Isr. J. Chem. 2019, 59, 339–350. [Google Scholar] [CrossRef]
- Popova, M.; Soboleva, T.; Ayad, S.; Benninghoff, A.D.; Berreau, L.M. Visible-light-activated quinolone carbon-monoxide-releasing molecule: Prodrug and albumin-assisted delivery enables anticancer and potent anti-inflammatory effects. J. Am. Chem. Soc. 2018, 140, 9721–9729. [Google Scholar] [CrossRef]
- Soboleva, T.; Esquer, H.J.; Benninghoff, A.D.; Berreau, L.M. Sense and release: A thiol-responsive flavonol-based photonically driven carbon monoxide-releasing molecule that operates via a multiple-input AND logic gate. J. Am. Chem. Soc. 2017, 139, 9435–9438. [Google Scholar]
- Soboleva, T.; Benninghoff, A.D.; Berreau, L.M. An H2S-sensing/CO-releasing flavonol that operates via logic gates. Chempluschem 2017, 82, 1408–1412. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, B.; Yu, L.; Dong, H.; Zhu, J.; Yang, L.; Yuan, X. Photo-Responsive Micelles with Controllable and Co-Release of Carbon Monoxide, Formaldehyde and Doxorubicin. Polymers 2022, 14, 2416. https://doi.org/10.3390/polym14122416
Zheng B, Yu L, Dong H, Zhu J, Yang L, Yuan X. Photo-Responsive Micelles with Controllable and Co-Release of Carbon Monoxide, Formaldehyde and Doxorubicin. Polymers. 2022; 14(12):2416. https://doi.org/10.3390/polym14122416
Chicago/Turabian StyleZheng, Bin, Lulu Yu, Huaze Dong, Jinmiao Zhu, Liang Yang, and Xinsong Yuan. 2022. "Photo-Responsive Micelles with Controllable and Co-Release of Carbon Monoxide, Formaldehyde and Doxorubicin" Polymers 14, no. 12: 2416. https://doi.org/10.3390/polym14122416
APA StyleZheng, B., Yu, L., Dong, H., Zhu, J., Yang, L., & Yuan, X. (2022). Photo-Responsive Micelles with Controllable and Co-Release of Carbon Monoxide, Formaldehyde and Doxorubicin. Polymers, 14(12), 2416. https://doi.org/10.3390/polym14122416






