Natural Fiber-Reinforced Thermoplastic ENR/PVC Composites as Potential Membrane Technology in Industrial Wastewater Treatment: A Review
Abstract
1. Introduction
2. Membrane Technology
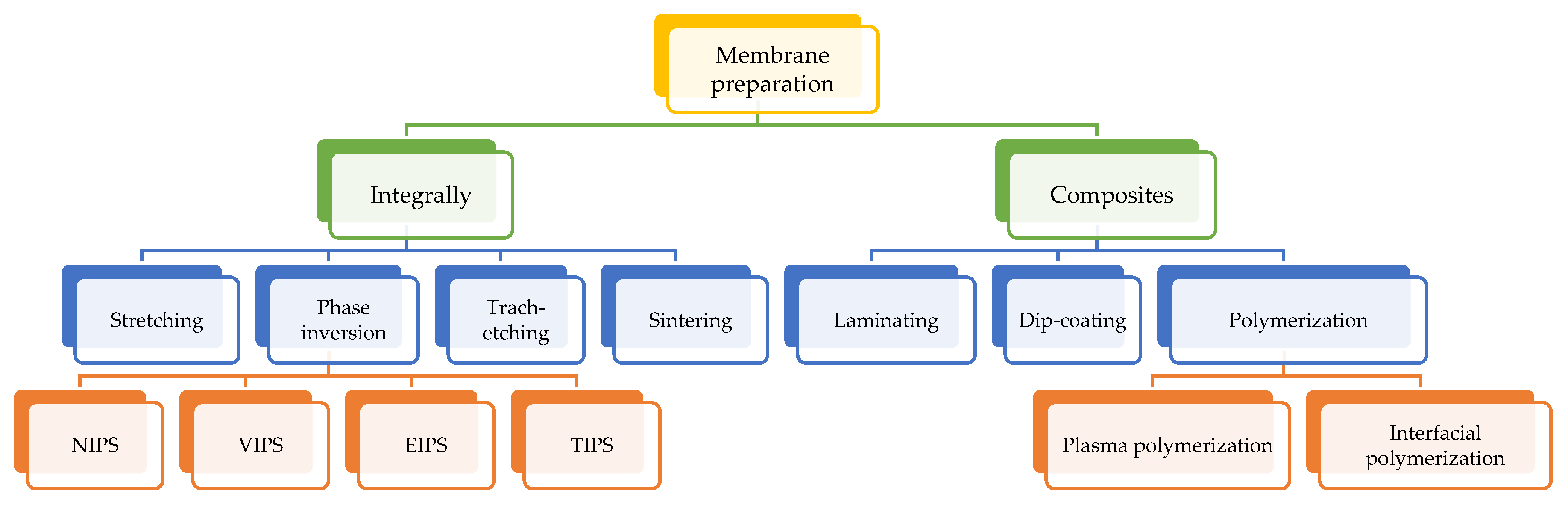
2.1. Membrane Fabrication Techniques
2.1.1. Phase Inversion Method

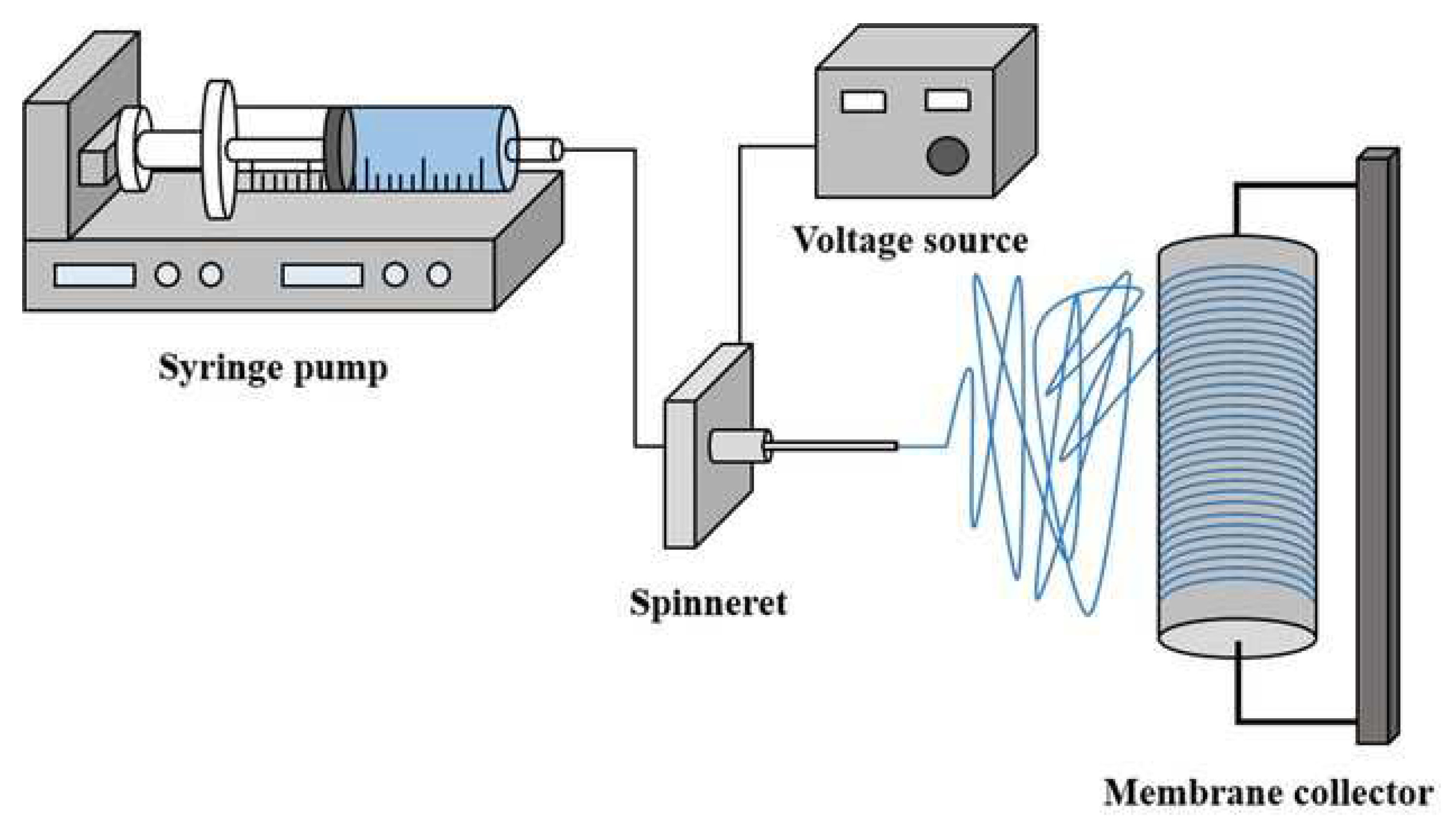
2.1.2. Electrospinning Method
2.2. Applications of Membrane Technology in Wastewater Treatment
3. Rubber-Based Membrane
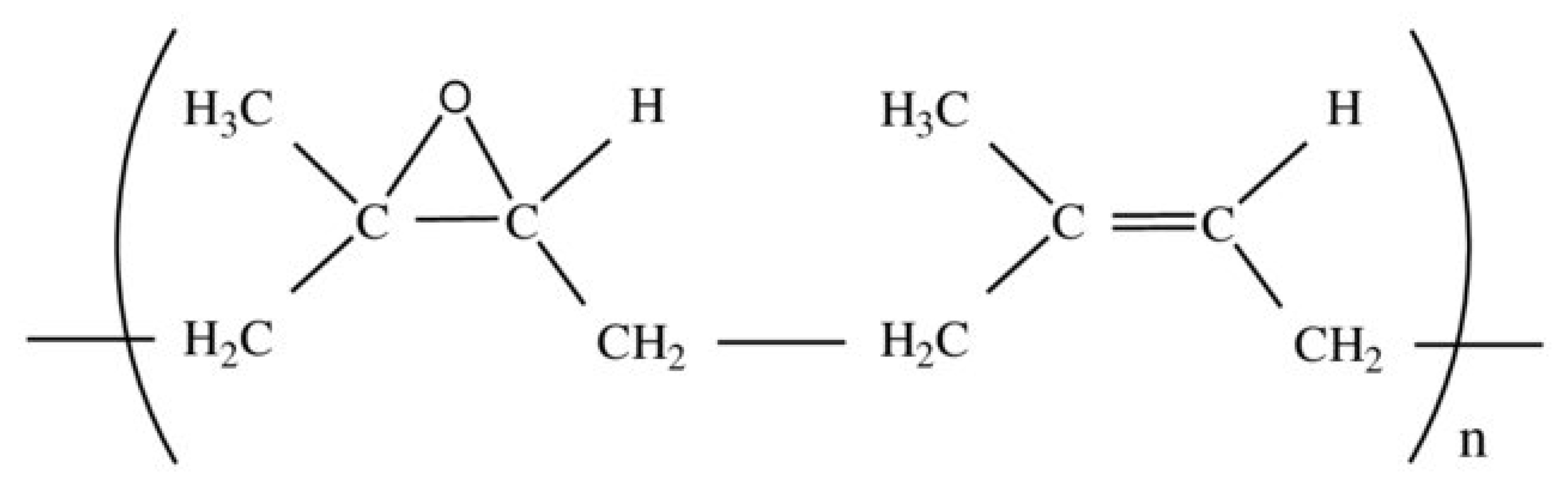
3.1. Epoxidized Natural Rubber Elastomer (ENR)
3.2. Poly(vinyl chloride) (PVC) Thermoplastic
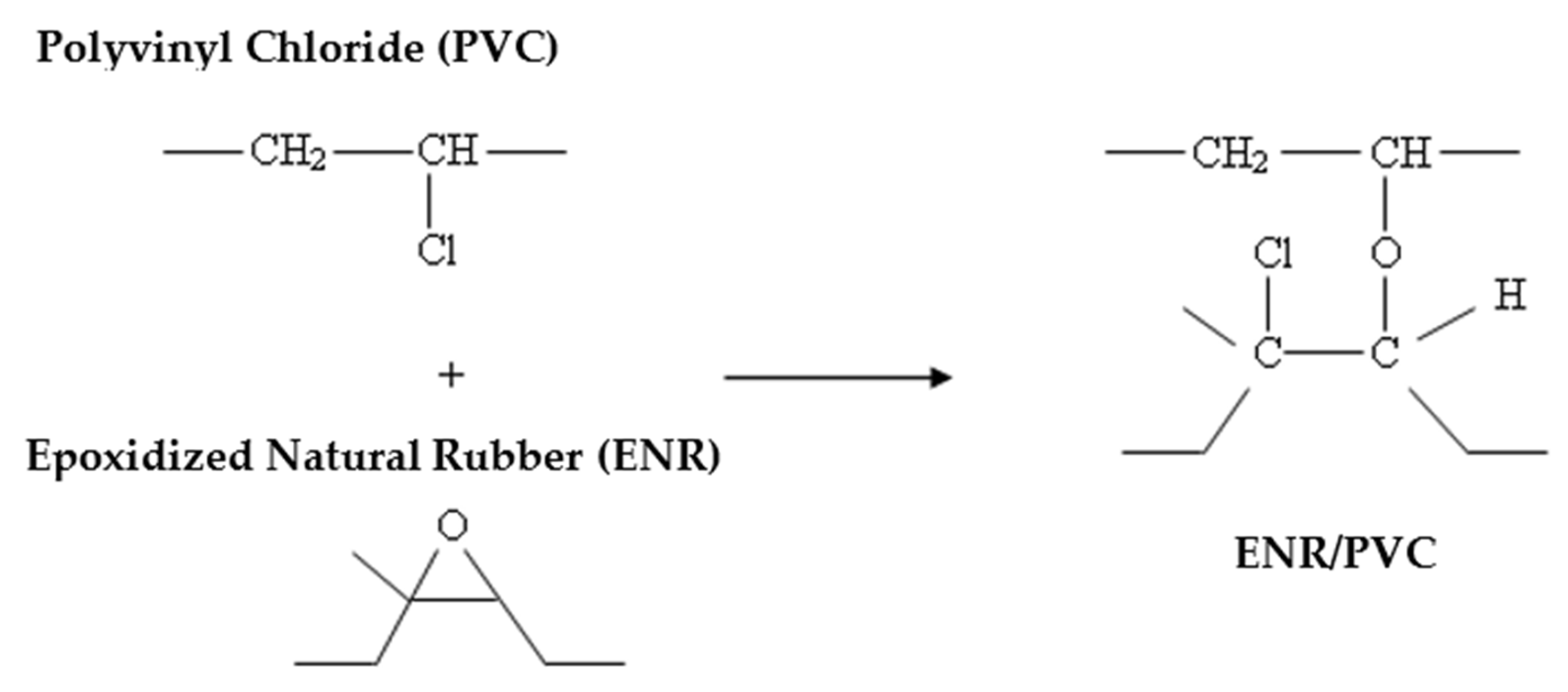
3.3. Thermoplastic Elastomer ENR/PVC Blends
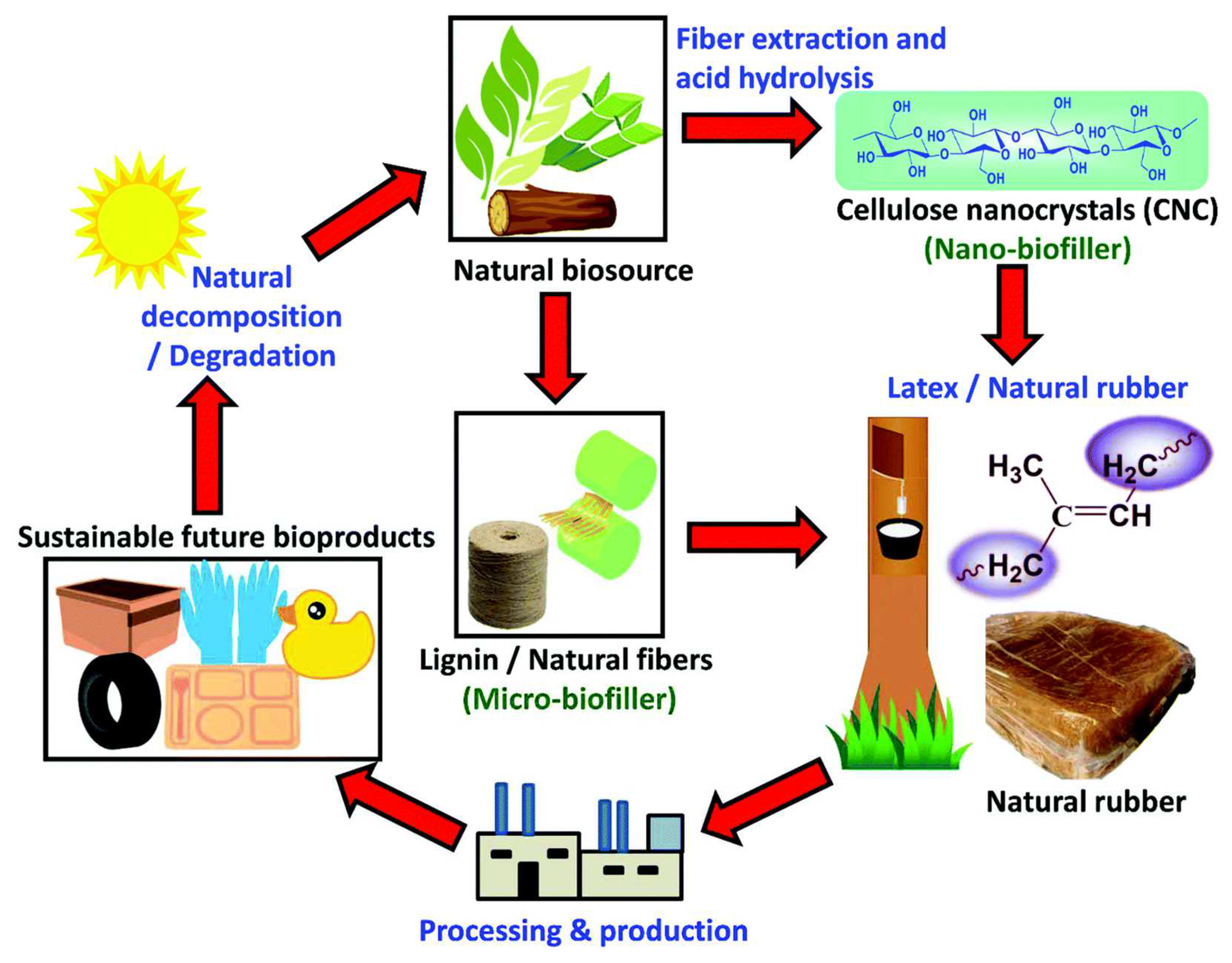
4. Natural Fiber-Reinforced Polymeric Membrane
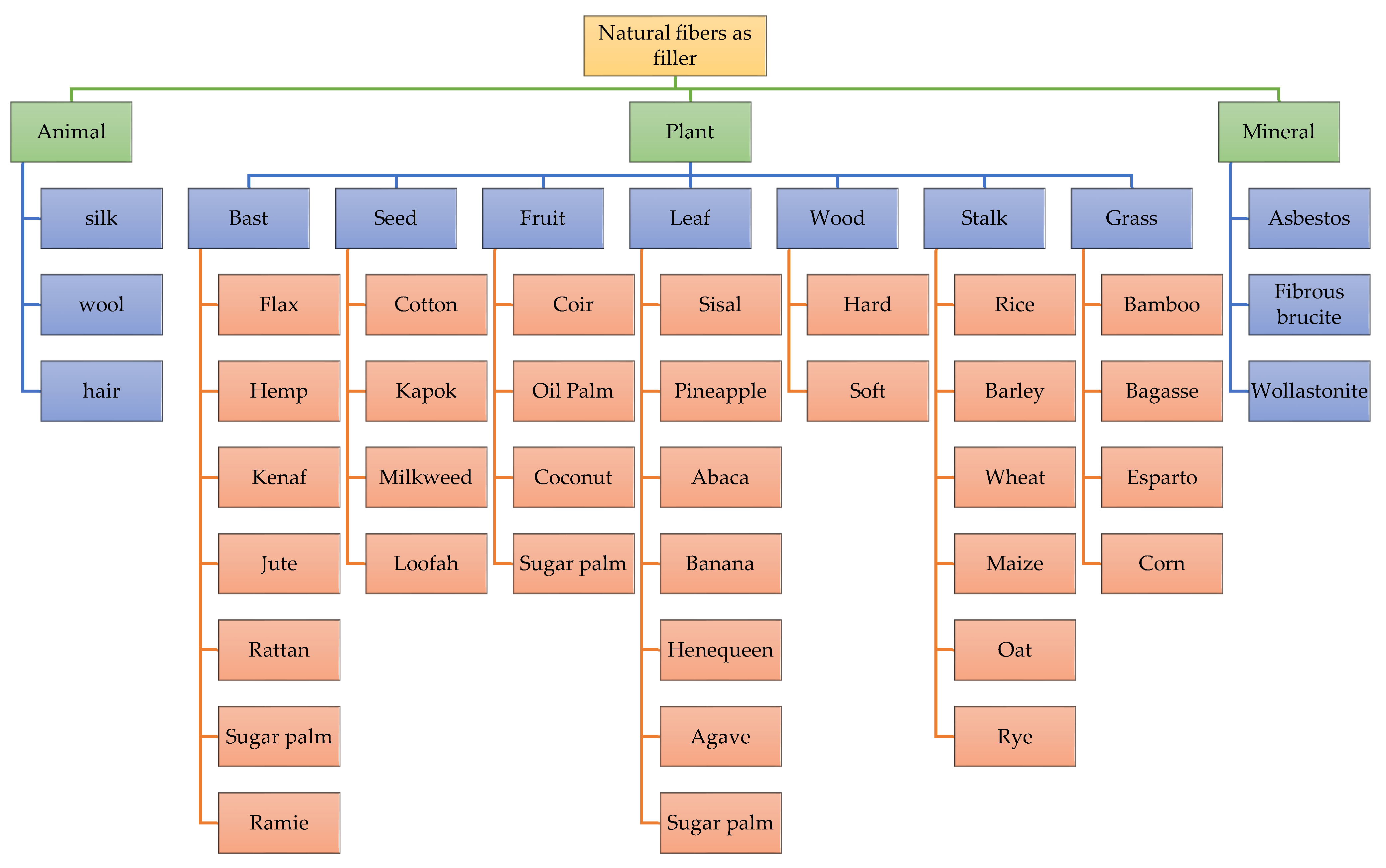
4.1. Matrix Filler
4.2. Pollutant Adsorbent
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schweitzer, L.; Noblet, J. Water Contamination and Pollution. Green Chem. An Incl. Approach 2018, 261–290. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Zhang, Y.; Ma, J.; Huang, L.; Yu, S.; Chen, L.; Song, G.; Qiu, M.; Wang, X. Applications of water-stable metal-organic frameworks in the removal of water pollutants: A review. Environ. Pollut. 2021, 291, 118076. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef] [PubMed]
- Sridevi, M.; Nirmala, C.; Jawahar, N.; Arthi, G.; Vallinayagam, S.; Sharma, V.K. Role of nanomaterial’s as adsorbent for heterogeneous reaction in waste water treatment. J. Mol. Struct. 2021, 1241, 130596. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, J.; Zhou, N.; Zhang, T.; Zeng, H. Does the “10-Point Water Plan” reduce the intensity of industrial water pollution? Quasi-experimental evidence from China. J. Environ. Manag. 2021, 295, 113048. [Google Scholar] [CrossRef]
- Yang, Z.; Li, S.; Ma, S.; Liu, P.; Peng, D.; Ouyang, Z.; Guo, X. Characteristics and removal efficiency of microplastics in sewage treatment plant of Xi’an City, northwest China. Sci. Total Environ. 2021, 771, 145377. [Google Scholar] [CrossRef]
- Radelyuk, I.; Tussupova, K.; Klemeš, J.J.; Persson, K.M. Oil refinery and water pollution in the context of sustainable development: Developing and developed countries. J. Clean. Prod. 2021, 302, 126987. [Google Scholar] [CrossRef]
- Koulini, G.V.; Laiju, A.R.; Ramesh, S.T.; Gandhimathi, R.; Nidheesh, P.V. Effective degradation of azo dye from textile wastewater by electro-peroxone process. Chemosphere 2022, 289, 133152. [Google Scholar] [CrossRef]
- Aghilesh, K.; Mungray, A.; Agarwal, S.; Ali, J.; Chandra Garg, M. Performance optimisation of forward-osmosis membrane system using machine learning for the treatment of textile industry wastewater. J. Clean. Prod. 2021, 289, 125690. [Google Scholar] [CrossRef]
- Pascoal, P.V.; Ribeiro, D.M.; Cereijo, C.R.; Santana, H.; Nascimento, R.C.; Steindorf, A.S.; Calsing, L.C.G.; Formighieri, E.F.; Brasil, B.S.A.F. Biochemical and phylogenetic characterization of the wastewater tolerant Chlamydomonas biconvexa Embrapa|LBA40 strain cultivated in palm oil mill effluent. PLoS ONE 2021, 16, e0249089. [Google Scholar] [CrossRef]
- Mohamad, N.A.; Hamzah, S.; Che Harun, M.H.; Ali, A.; Rasit, N.; Awang, M.; Rahman, W.R.W.A.; Azmi, A.A.A.R.; Abu Habib, A.A.; Amri Zahid, M.S.; et al. Integration of copperas and calcium hydroxide as a chemical coagulant and coagulant aid for efficient treatment of palm oil mill effluent. Chemosphere 2021, 281, 130873. [Google Scholar] [CrossRef] [PubMed]
- Nqombolo, A.; Mpupa, A.; Moutloali, R.M.; Nomngongo, P.N. Wastewater Treatment Using Membrane Technology. Wastewater Water Qual. 2018, 29, 29–40. [Google Scholar]
- Staudt-Bickel, C.; Koros, W.J. Improvement of CO2/CH4 separation characteristics of polyimides by chemical crosslinking. J. Memb. Sci. 1999, 155, 145–154. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Othman, N.H.; Alias, N.H.; Fuzil, N.S.; Marpani, F.; Shahruddin, M.Z.; Chew, C.M.; Ng, K.M.D.; Lau, W.J.; Ismail, A.F. A review on the use of membrane technology systems in developing countries. Membranes 2022, 12, 30. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Hussain, S.; Wan, X.; Li, Z.; Peng, X. Cu-TCPP nanosheets blended polysulfone ultrafiltration membranes with enhanced antifouling and photo-tunable porosity. Sep. Purif. Technol. 2021, 268, 118688. [Google Scholar] [CrossRef]
- Twibi, M.F.; Othman, M.H.D.; Hubadillah, S.K.; Alftessi, S.A.; Kurniawan, T.A.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Raji, Y.O. Development of high strength, porous mullite ceramic hollow fiber membrane for treatment of oily wastewater. Ceram. Int. 2021, 47, 15367–15382. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Fan, Z.; Wu, G.; Liu, L.; Huang, Y. Aramid nanofiber-based porous membrane for suppressing dendrite growth of metal-ion batteries with enhanced electrochemistry performance. Chem. Eng. J. 2021, 426, 131924. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, F.; Yue, X.; Tian, Q.; Yang, D.; Zhang, T. Eco-friendly self-crosslinking cellulose membrane with high mechanical properties from renewable resources for oil/water emulsion separation. J. Environ. Chem. Eng. 2021, 9, 105857. [Google Scholar] [CrossRef]
- Yin, Z.; Yuan, F.; Li, M.; Xue, M.; Zhou, D.; Chen, Y.; Liu, X.; Luo, Y.; Hong, Z.; Xie, C.; et al. Self-cleaning, underwater writable, heat-insulated and photocatalytic cellulose membrane for high-efficient oil/water separation and removal of hazardous organic pollutants. Prog. Org. Coat. 2021, 157, 106311. [Google Scholar] [CrossRef]
- Zhou, Z.; Dong, Z.; Wang, L.; Song, R.; Mei, N.; Chen, T.; Luo, L.; Ding, Q.; Wang, X.; Tang, S. Cellulose membrane modified with LED209 as an antibacterial and anti-adhesion material. Carbohydr. Polym. 2021, 252, 117138. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Pîrțac, A.; Albu, P.C.; Grosu, A.R.; Dumitru, F.; Dimulescu, I.A.; Oprea, O.; Pașcu, D.; Nechifor, G.; Bungău, S.G. Recuperative amino acids separation through cellulose derivative membranes with microporous polypropylene fiber matrix. Membranes 2021, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Fu, L.; Wang, Y.; Chen, W.; Chen, F.; Zhang, S.; Wang, J. Co-MOF-74 based Co3O4/cellulose derivative membrane as dual-functional catalyst for colorimetric detection and degradation of phenol. Carbohydr. Polym. 2021, 273, 118548. [Google Scholar] [CrossRef] [PubMed]
- El-Shafai, N.M.; Ramadan, M.S.; Amin, M.A.; El-Mehasseb, I.M. Graphene oxide/cellulose derivative nanohybrid membrane with Yttrium oxide: Upgrading the optical and electrochemical properties for removing organic pollutants and supercapacitors implementations. J. Energy Storage 2021, 44, 103344. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Al-Naqshabandi, M.A.; Larijani, B.; Badiei, A.; Vatanpour, V.; Rajabi, H.R.; Rezania, H.; Paziresh, S.; Mahmodi, G.; Kim, S.J.; et al. Improvement of dye and protein filtration efficiency using modified PES membrane with 2-mercaptoethanol capped zinc sulfide quantum dots. Chem. Eng. Res. Des. 2021, 168, 109–121. [Google Scholar] [CrossRef]
- Khosravi, M.J.; Hosseini, S.M.; Vatanpour, V. Performance improvement of PES membrane decorated by Mil-125(Ti)/chitosan nanocomposite for removal of organic pollutants and heavy metal. Chemosphere 2022, 290, 133335. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, J.; Liu, Y.; Xu, Y.; Li, R.; Hong, H.; Shen, L.; Lin, H.; Liao, B.Q. Enhanced permeability and antifouling performance of polyether sulfone (PES) membrane via elevating magnetic Ni@MXene nanoparticles to upper layer in phase inversion process. J. Memb. Sci. 2021, 623, 119080. [Google Scholar] [CrossRef]
- Azizi, A.; Feijani, E.A.; Ghorbani, Z.; Tavasoli, A. Fabrication and characterization of highly efficient three component CuBTC/graphene oxide/PSF membrane for gas separation application. Int. J. Hydrogen Energy 2021, 46, 2244–2254. [Google Scholar] [CrossRef]
- Kusworo, T.D.; Dalanta, F.; Aryanti, N.; Othman, N.H. Intensifying separation and antifouling performance of PSf membrane incorporated by GO and ZnO nanoparticles for petroleum refinery wastewater treatment. J. Water Process Eng. 2021, 41, 102030. [Google Scholar] [CrossRef]
- Li, N.; Wang, W.; Ma, C.; Zhu, L.; Chen, X.; Zhang, B.; Zhong, C. A novel conductive rGO/ZnO/PSF membrane with superior water flux for electrocatalytic degradation of organic pollutants. J. Memb. Sci. 2022, 641, 119901. [Google Scholar] [CrossRef]
- Behboudi, A.; Ghiasi, S.; Mohammadi, T.; Ulbricht, M. Preparation and characterization of asymmetric hollow fiber polyvinyl chloride (PVC) membrane for forward osmosis application. Sep. Purif. Technol. 2021, 270, 118801. [Google Scholar] [CrossRef]
- Shokri, E.; Shahed, E.; Hermani, M.; Etemadi, H. Towards enhanced fouling resistance of PVC ultrafiltration membrane using modified montmorillonite with folic acid. Appl. Clay Sci. 2021, 200, 105906. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Qin, Y.; Huang, Y.; Xiao, C. Oriented structure design and evaluation of Fe3O4/o-MWCNTs/PVC composite membrane assisted by magnetic field. J. Taiwan Inst. Chem. Eng. 2021, 120, 278–290. [Google Scholar] [CrossRef]
- Peng, G.; Yaoqin, W.; Changmei, S.; Chunnuan, J.; Ying, Z.; Rongjun, Q.; Ying, W. Preparation and properties of PVC-based ultrafiltration membrane reinforced by in-situ synthesized p-aramid nanoparticles. J. Memb. Sci. 2022, 642, 119993. [Google Scholar] [CrossRef]
- Shamsuddin, M.R.; Abdullah, I.; Othaman, R. Celluloses filled ENR/PVC membranes for palm oil mill effluent (POME) treatment. AIP Conf. Proc. 2013, 1571, 897–903. [Google Scholar] [CrossRef]
- Tanjung, F.A.; Hassan, A.; Hasan, M. Use of epoxidized natural rubber as a toughening agent in plastics. J. Appl. Polym. Sci. 2015, 132, 1–9. [Google Scholar] [CrossRef]
- Arman Alim, A.A.; Othaman, R. Epoxidized Natural Rubber/Polyvinyl Chloride/Microcrystalline Cellulose (ENR/PVC/MCC) Composite Membrane for Palm Oil Mill Effluent (POME) Treatment. Sains Malaysiana 2018, 47, 1517–1525. [Google Scholar] [CrossRef]
- Siekierka, A.; Smolińska-Kempisty, K.; Wolska, J. Enhanced specific mechanism of separation by polymeric membrane modification—a short review. Membranes 2021, 11, 942. [Google Scholar] [CrossRef]
- Tan, X.M.; Rodrigue, D. A review on porous polymeric membrane preparation. Part II: Production techniques with polyethylene, polydimethylsiloxane, polypropylene, polyimide, and polytetrafluoroethylene. Polymers 2019, 11, 1310. [Google Scholar] [CrossRef]
- Alharbi, A. Methods Synthetic Membrane Polymeric Membrane. Int. J. Adv. Sci. Eng. Technol. 2018, 6, 32–35. [Google Scholar]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egypt. J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Harussani, M.M.; Sapuan, S.M.; Rashid, U.; Khalina, A.; Ilyas, R.A. Pyrolysis of polypropylene plastic waste into carbonaceous char: Priority of plastic waste management amidst COVID-19 pandemic. Sci. Total Environ. 2021, 803, 149911. [Google Scholar] [CrossRef]
- Awais, H.; Nawab, Y.; Amjad, A.; Anjang, A.; Akil, H.; Abidin, M.S.Z. Environmental benign natural fibre reinforced thermoplastic composites: A review. Compos. Part C Open Access 2020, 100082. [Google Scholar] [CrossRef]
- Azman, M.A.; Asyraf, M.R.M.; Khalina, A.; Petrů, M.; Ruzaidi, C.M.; Sapuan, S.M.; Wan Nik, W.B.; Ishak, M.R.; Ilyas, R.A.; Suriani, M.J. Natural Fiber Reinforced Composite Material for Product Design: A Short Review. Polymers 2021, 13, 1917. [Google Scholar] [CrossRef]
- Gaba, E.W.; Asimeng, B.O.; Kaufmann, E.E.; Katu, S.K.; Foster, E.J.; Tiburu, E.K. Mechanical and structural characterization of pineapple leaf fiber. Fibers 2021, 9, 51. [Google Scholar] [CrossRef]
- De Azeredo, H.M.C. Nanocomposites for food packaging applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Kundie, F.; Azhari, C.H.; Muchtar, A.; Ahmad, Z.A. Effects of filler size on the mechanical properties of polymer-filled dental composites: A review of recent developments. J. Phys. Sci. 2018, 29, 141–165. [Google Scholar] [CrossRef]
- Jogur, G.; Nawaz Khan, A.; Das, A.; Mahajan, P.; Alagirusamy, R. Impact properties of thermoplastic composites. Text. Prog. 2018, 50, 109–183. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Chuah, C.H.; Hai, N.D.; Singh, R.; Nor, A.R.M. Preparation and characterization of starch-based bioplastic composites with treated oil palm empty fruit bunch fibers and citric acid. Cellulose 2021, 28, 4191–4210. [Google Scholar] [CrossRef]
- Suriani, M.J.; Radzi, F.S.M.; Ilyas, R.A.; Petru, M.; Sapuan, S.M.; Ruzaidi, C.M. Flammability, Tensile, and Morphological Properties of Oil. Polymers 2021, 13, 1282. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Raja, V.M. Processing and determination of mechanical properties of Prosopis juliflora bark, banana and coconut fiber reinforced hybrid bio composites for an engineering field. Compos. Sci. Technol. 2021, 208, 108695. [Google Scholar] [CrossRef]
- Sujita, S. Characterization of mechanical properties of composite materials with filler coconut shell powder and sawdust with coconut fiber reinforcement as an alternative to low loaded brake friction materials. Glob. J. Eng. Technol. Adv. 2021, 09, 017–023. [Google Scholar] [CrossRef]
- Senthilnathan, K.; Ravi, R.; Stephen Leon, J.; Suresh, G.; Manikandan, K.; Lavanya, R. Analysing the effect of mechanical properties of various proportions of filler material on jute fibre/epoxy reinforced composites. J. Phys. Conf. Ser. 2021, 1921, 012089. [Google Scholar] [CrossRef]
- Dewi, R.; Oktaviani, O.; Ginting, Z.; Sylvia, N. Mechanical Characteristic and Water Absorption Property of Bio Composite from Sago Starch and Jute Fiber (Boehmeria Nivea) as the filler. Int. J. Eng. Sci. Inf. Technol. 2021, 2, 94–99. [Google Scholar] [CrossRef]
- Chawalitsakunchai, W.; Dittanet, P.; Loykulnant, S.; Sae-oui, P.; Tanpichai, S.; Seubsai, A.; Prapainainar, P. Properties of natural rubber reinforced with nano cellulose from pineapple leaf agricultural waste. Mater. Today Commun. 2021, 28, 102594. [Google Scholar] [CrossRef]
- Reddy, M.; Adaveesh, B.; Mohankumar, T.S.; Nagaral, M. Tensile and flexural behaviour of graphite filler particles and pineapple leaf fiber (palf) reinforced polymer composites. Metall. Mater. Eng. 2021. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Palm fiber reinforced starch. BioResources 2016, 11, 4134–4145. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ibrahim, R.; Abral, H.; Ishak, M.R.; Zainudin, E.S.; Atikah, M.S.N.; Mohd Nurazzi, N.; Atiqah, A.; Ansari, M.N.M.; et al. Effect of sugar palm nanofibrillated celluloseconcentrations on morphological, mechanical andphysical properties of biodegradable films basedon agro-waste sugar palm Arenga pinnata (Wurmb. Merr) starch. J. Mater. Res. Technol. 2019, 8, 4819–4830. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Adeniyi, A.G.; Owolabi, O.O.; Abdulkareem, S.A. Moisture absorption, thermal and microstructural properties of polymer composites developed from rice husk and polystyrene wastes. Int. J. Sustain. Eng. 2021, 14, 1049–1058. [Google Scholar] [CrossRef]
- Hemnath, A.; Anbuchezhiyan, G.; Nanthakumar, P.; Senthilkumar, N. Tensile and flexural behaviour of rice husk and sugarcane bagasse reinforced polyester composites. Mater. Today Proc. 2020, 46, 3451–3454. [Google Scholar] [CrossRef]
- Yeong, M.H.C.; Kristanti, R.A. Potential Use of Rise Husk as Filler in Poly Lactic Acid Bio-composite: Mechanical Properties, Morphology and Biodegradability. Front. Water Environ. 2021, 1, 28–34. [Google Scholar]
- Samad, N.A.; Jon, N.; Lazim, M.A.S.M.; Abdullah, I.; Othaman, R. Preparation of ENR/PVC/RH Composite Membrane for Water Permeation Application. Adv. J. Technol. Vocat. Educ. 2015, 1, 4–11. [Google Scholar]
- Chang, B.P.; Gupta, A.; Muthuraj, R.; Mekonnen, T.H. Bioresourced fillers for rubber composite sustainability: Current development and future opportunities. Green Chem. 2021, 23, 5337–5378. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, H.; Wang, H.; Ji, Z.; Bai, R.; Chen, F.; Li, B.; Chang, C.; Lin, T. Improvement of Air Filtration Performance Using Nanofibrous Membranes with a Periodic Variation in Packing Density. Adv. Mater. Interfaces 2022, 9, 2101848. [Google Scholar] [CrossRef]
- Wang, X.L.; Qu, Z.G.; Lai, T.; Ren, G.F.; Wang, W.K. Enhancing water transport performance of gas diffusion layers through coupling manipulation of pore structure and hydrophobicity. J. Power Sources 2022, 525, 231121. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, W.K.; Qu, Z.G.; Ren, G.F.; Wang, H.C. Surface roughness dominated wettability of carbon fiber in gas diffusion layer materials revealed by molecular dynamics simulations. Int. J. Hydrogen Energy 2021, 46, 26489–26498. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, B.; Qu, Z.G.; Zhang, J.F.; Jin, Z.G. Effects of graphite microstructure evolution on the anisotropic thermal conductivity of expanded graphite/paraffin phase change materials and their thermal energy storage performance. Int. J. Heat Mass Transf. 2020, 155, 119853. [Google Scholar] [CrossRef]
- Wang, X.; Qu, Z.; Ren, G.; Feng, C.; Cheng, F. Prolonged yield platform in bioinspired three dimensional carbon materials derived from crack deflection. Mater. Lett. 2020, 270, 127759. [Google Scholar] [CrossRef]
- Sun, H.; Yang, X.; Zhang, Y.; Cheng, X.; Xu, Y.; Bai, Y.; Shao, L. Segregation-induced in situ hydrophilic modification of poly (vinylidene fluoride) ultrafiltration membranes via sticky poly (ethylene glycol) blending. J. Memb. Sci. 2018, 563, 22–30. [Google Scholar] [CrossRef]
- Saeedi-Jurkuyeh, A.; Jafari, A.J.; Kalantary, R.R.; Esrafili, A. A novel synthetic thin-film nanocomposite forward osmosis membrane modified by graphene oxide and polyethylene glycol for heavy metals removal from aqueous solutions. React. Funct. Polym. 2020, 146, 4397. [Google Scholar] [CrossRef]
- Huang, Q.; Luo, Q.; Chen, Z.; Yao, L.; Fu, P.; Lin, Z. The effect of electrolyte concentration on electrochemical impedance for evaluating polysulfone membranes. Environ. Sci. Water Res. Technol. 2018, 4, 1145–1151. [Google Scholar] [CrossRef]
- Nguyen, H.T.V.; Ngo, T.H.A.; Do, K.D.; Nguyen, M.N.; Dang, N.T.T.; Nguyen, T.T.H.; Vien, V.; Vu, T.A. Preparation and characterization of a hydrophilic polysulfone membrane using graphene oxide. J. Chem. 2019, 2019, 15–20. [Google Scholar] [CrossRef]
- Dong, X.; Al-Jumaily, A.; Escobar, I.C. Investigation of the use of a bio-derived solvent for non-solvent-induced phase separation (NIPS) fabrication of polysulfone membranes. Membranes 2018, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Unno, H. Integrated Production and Separation, 2nd ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 2, ISBN 9780080885049. [Google Scholar]
- Mishra, G.; Mukhopadhyay, M. Enhanced antifouling performance of halloysite nanotubes (HNTs) blended poly(vinyl chloride) (PVC/HNTs) ultrafiltration membranes: For water treatment. J. Ind. Eng. Chem. 2018, 63, 366–379. [Google Scholar] [CrossRef]
- Bhran, A.; Shoaib, A.; Elsadeq, D.; El-gendi, A.; Abdallah, H. Preparation of PVC/PVP composite polymer membranes via phase inversion process for water treatment purposes. Chinese J. Chem. Eng. 2018, 26, 715–722. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Park, M.J.; Tijing, L.; Han, D.S.; Phuntsho, S.; Shon, H.K. Modification of nanofiber support layer for thin film composite forward osmosis membranes via layer-by-layer polyelectrolyte deposition. Membranes 2018, 8, 70. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Ismail, A. Effects of fabrication parameters on the morphology of porous polysulfone hollow fiber membranes. J. Teknol. 2008, 49, 81–89. [Google Scholar]
- Chinpa, W. Preparation and characterization of an asymmetric porous polyvinyl chloride/poly(methyl methacrylate-co-methacrylic acid) membrane. Sci. Asia 2008, 34, 385–389. [Google Scholar] [CrossRef]
- Lin, R.Y.; Chen, B.S.; Chen, G.L.; Wu, J.Y.; Chiu, H.C.; Suen, S.Y. Preparation of porous PMMA/Na+-montmorillonite cation-exchange membranes for cationic dye adsorption. J. Memb. Sci. 2009, 326, 117–129. [Google Scholar] [CrossRef]
- Srivastava, H.P.; Arthanareeswaran, G.; Anantharaman, N.; Starov, V.M. Performance of modified poly(vinylidene fluoride) membrane for textile wastewater ultrafiltration. Desalination 2011, 282, 87–94. [Google Scholar] [CrossRef]
- Zebiri, H.; Van Den Berghe, H.; Sayegh, S.; Chammas, P.E.; Pompée, C.; Chammas, M.; Garric, X. Synthesis of PLA-poly(ether urethane)-PLA copolymers and design of biodegradable anti-adhesive membranes for orthopaedic applications. J. Mater. Chem. B 2021, 9, 832–845. [Google Scholar] [CrossRef] [PubMed]
- Hermida-Merino, C.; Pardo, F.; Zarca, G.; Araújo, J.M.M.; Urtiaga, A.; Piñeiro, M.M.; Pereiro, A.B. Integration of stable ionic liquid-based nanofluids into polymer membranes. Part i: Membrane synthesis and characterization. Nanomaterials 2021, 11, 607. [Google Scholar] [CrossRef]
- Prihatiningtyas, I.; Hartanto, Y.; Ballesteros, M.S.R.; Van der Bruggen, B. Cellulose triacetate/LUDOX-SiO2 nanocomposite for synthesis of pervaporation desalination membranes. J. Appl. Polym. Sci. 2021, 138, 50000. [Google Scholar] [CrossRef]
- Khan, M.I.; Shanableh, A.; Fernandez, J.; Lashari, M.H.; Shahida, S.; Manzoor, S.; Zafar, S.; Rehman, A.U.; Elboughdiri, N. Synthesis of dmea-grafted anion exchange membrane for adsorptive discharge of methyl orange from wastewaters. Membranes 2021, 11, 166. [Google Scholar] [CrossRef]
- Hong, M.; Dong, Q.; Xie, H.; Wang, X.; Brozena, A.H.; Gao, J.; Wang, C.; Chen, C.; Rao, J.; Luo, J.; et al. Tailoring grain growth and densification toward a high-performance solid-state electrolyte membrane. Mater. Today 2021, 42, 41–48. [Google Scholar] [CrossRef]
- Shi, W.; Yang, C.; Qiu, M.; Chen, X.; Fan, Y. A new method for preparing α-alumina ultrafiltration membrane at low sintering temperature. J. Memb. Sci. 2022, 642, 119992. [Google Scholar] [CrossRef]
- Ji, D.; Xiao, C.; Chen, K.; Zhou, F.; Gao, Y.; Zhang, T.; Ling, H. Solvent-free green fabrication of PVDF hollow fiber MF membranes with controlled pore structure via melt-spinning and stretching. J. Memb. Sci. 2021, 621, 118953. [Google Scholar] [CrossRef]
- Thomas, N.; Kumar, M.; Palmisano, G.; Al-Rub, R.K.A.; Alnuaimi, R.Y.; Alhseinat, E.; Rowshan, R.; Arafat, H.A. Antiscaling 3D printed feed spacers via facile nanoparticle coating for membrane distillation. Water Res. 2021, 189, 116649. [Google Scholar] [CrossRef]
- Abbasi, M.; Sabzehmeidani, M.M.; Ghaedi, M.; Jannesar, R.; Shokrollahi, A. Synthesis of grass-like structured Mn-Fe layered double hydroxides/PES composite adsorptive membrane for removal of malachite green. Appl. Clay Sci. 2021, 203, 105946. [Google Scholar] [CrossRef]
- Ilyas, A.; Yihdego Gebreyohannes, A.; Qian, J.; Reynaerts, D.; Kuhn, S.; Vankelecom, I.F.J. Micro-patterned membranes prepared via modified phase inversion: Effect of modified interface on water fluxes and organic fouling. J. Colloid Interface Sci. 2021, 585, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Li, Y. Novel superhydrophilic antifouling PVDF-BiOCl nanocomposite membranes fabricated via a modified blending-phase inversion method. Sep. Purif. Technol. 2021, 254, 117656. [Google Scholar] [CrossRef]
- Bai, M.; Qiang, L.; Meng, M.; Li, B.; Wang, S.; Wu, Y.; Chen, L.; Dai, J.; Liu, Y.; Pan, J. Upper surface imprinted membrane prepared by magnetic guidance phase inversion method for highly efficient and selective separation of Artemisinin. Chem. Eng. J. 2021, 405, 126899. [Google Scholar] [CrossRef]
- Figoli, A.; Marino, T.; Galiano, F. Polymeric Membranes in Biorefinery. Membr. Technol. Biorefining 2016, 29–59. [Google Scholar]
- Chen, X.; Hu, Y.; Xie, Z.; Wang, H. Materials and Design of Photocatalytic Membranes; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128135495. [Google Scholar]
- Machado, P.S.T.; Habert, A.C.; Borges, C.P. Membrane formation mechanism based on precipitation kinetics and membrane morphology: Flat and hollow fiber polysulfone membranes. J. Memb. Sci. 1999, 155, 171–183. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, X.; Liu, F.; Fan, L.Z. Porous polymer electrolytes for long-cycle stable quasi-solid-state magnesium batteries. J. Energy Chem. 2021, 59, 608–614. [Google Scholar] [CrossRef]
- Ahmad, A.L. Teknologi Membran: Evolusi Proses Pemisahan Kreatif, Inovatif dan Efektif Tanpa Sempadan; Penerbit Universiti Sains Malaysia: Pulau Pinang, Malaysia, 2007. [Google Scholar]
- Fontão, N.C.; Wilhelm, M.; Rezwan, K. Asymmetric polysiloxane-based SiOC membranes produced via phase inversion tape casting process. Mater. Des. 2021, 198, 109328. [Google Scholar] [CrossRef]
- Kamp, J.; Emonds, S.; Borowec, J.; Restrepo Toro, M.A.; Wessling, M. On the organic solvent free preparation of ultrafiltration and nanofiltration membranes using polyelectrolyte complexation in an all aqueous phase inversion process. J. Memb. Sci. 2021, 618, 118632. [Google Scholar] [CrossRef]
- Feng, C.; Shi, B.; Li, G.; Wu, Y. Preparation and properties of microporous membrane from poly(vinylidene fluoride-co-tetrafluoroethylene) (F2.4) for membrane distillation. J. Memb. Sci. 2004, 237, 15–24. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, B.; Ju, J.; Kang, W.; Liu, Y. Development of a novel multi-scale structured superhydrophobic nanofiber membrane with enhanced thermal efficiency and high flux for membrane distillation. Desalination 2021, 501, 114834. [Google Scholar] [CrossRef]
- Polisetti, V.; Ray, P. Nanoparticles modified Polyacrylonitrile/Polyacrylonitrile–Polyvinylidenefluoride blends as substrate of high flux anti-fouling nanofiltration membranes. J. Appl. Polym. Sci. 2021, 138, 50228. [Google Scholar] [CrossRef]
- Yang, G.; Xiong, X.; Zhang, L. Microporous formation of blend membranes from cellulose/konjac glucomannan in NaOH/thiourea aqueous solution. J. Memb. Sci. 2002, 201, 161–173. [Google Scholar] [CrossRef]
- Nazri, A.I.; Ahmad, A.L.; Hussin, M.H. Microcrystalline cellulose-blended polyethersulfone membranes for enhanced water permeability and humic acid removal. Membranes 2021, 11, 660. [Google Scholar] [CrossRef]
- Wardani, A.K.; Ivan, I.; Darmawan, I.R.; Khoiruddin, K.; Wenten, I.G. Fine particle removal using hydrophobic microporous polymeric membranes. J. Teknol. 2019, 81, 99–108. [Google Scholar] [CrossRef]
- Saljoughi, E.; Sadrzadeh, M.; Mohammadi, T. Effect of preparation variables on morphology and pure water permeation flux through asymmetric cellulose acetate membranes. J. Membr. Sci. 2009, 326, 627–634. [Google Scholar] [CrossRef]
- Mahendran, R.; Malaisamy, R.; Mohan, D. Preparation, characterization and effect of annealing on performance of cellulose acetate/sulfonated polysulfone and cellulose acetate/epoxy resin blend ultrafiltration membranes. Eur. Polym. J. 2004, 40, 623–633. [Google Scholar] [CrossRef]
- Francis, L.; Ahmed, F.E.; Hilal, N. Electrospun membranes for membrane distillation: The state of play and recent advances. Desalination 2022, 526, 115511. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Ebadi Amooghin, A.; Shirazi, M.M.A.; Pishnamazi, M.; Shirazian, S. Water desalination and ion removal using mixed matrix electrospun nanofibrous membranes: A critical review. Desalination 2022, 521, 115350. [Google Scholar] [CrossRef]
- Ren, G.; Qu, Z.; Wang, X.; Zhang, J. Liquid water transport and mechanical performance of electrospun gas diffusion layers. Int. J. Green Energy 2022, 19, 210–218. [Google Scholar] [CrossRef]
- Akanda, M.J.H.; Sarker, M.Z.I.; Ferdosh, S.; Manap, M.Y.A.; Rahman, N.N.N.A.; Kadir, M.O.A. Applications of supercritical fluid extraction (SFE) of palm oil and oil from natural sources. Molecules 2012, 17, 1764–1794. [Google Scholar] [CrossRef]
- Hassan, N.; Idris, A.; Akhtar, J. Overview on Bio-refinery Concept in Malaysia: Potential High Value Added Products from Palm Oil Biomass. J. Kejuruter. 2019, 2, 113–124. [Google Scholar]
- Russo, T.; Fucile, P.; Giacometti, R.; Sannino, F. Sustainable removal of contaminants by biopolymers: A novel approach for wastewater treatment. current state and future perspectives. Processes 2021, 9, 719. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Leaper, S.; Skuse, C.; Zaragoza, G.; Gryta, M.; Gorgojo, P. Membrane cleaning and pretreatments in membrane distillation–a review. Chem. Eng. J. 2021, 422, 129696. [Google Scholar] [CrossRef]
- Abu Bakar, S.N.H.; Hasan, H.A.; Mohammad, A.W.; Sheikh Abdullah, S.R.; Muhamad, M.H. Interactions between operating parameters of moving bed biofilm reactors in treating palm oil mill effluent. Process Saf. Environ. Prot. 2022, 158, 567–575. [Google Scholar] [CrossRef]
- Lim, K.S.; Sethu, V.; Selvarajoo, A. Natural plant materials as coagulant and flocculants for the treatment of palm oil mill effluent. Mater. Today Proc. 2021, 48, 871–887. [Google Scholar] [CrossRef]
- Ratnasari, A.; Syafiuddin, A.; Boopathy, R.; Malik, S.; Aamer Mehmood, M.; Amalia, R.; Dwi Prastyo, D.; Syamimi Zaidi, N. Advances in pretreatment technology for handling the palm oil mill effluent: Challenges and prospects. Bioresour. Technol. 2022, 344, 126239. [Google Scholar] [CrossRef]
- Dashti, A.F.; Salman, N.A.S.; Adnan, R.; Zahed, M.A. Palm oil mill effluent treatment using combination of low cost chickpea coagulant and granular activated carbon: Optimization via response surface methodology. Groundw. Sustain. Dev. 2022, 16, 100709. [Google Scholar] [CrossRef]
- Manzoor, K.; Khan, S.J.; Yasmeen, M.; Jamal, Y.; Arshad, M. Assessment of anaerobic membrane distillation bioreactor hybrid system at mesophilic and thermophilic temperatures treating textile wastewater. J. Water Process Eng. 2022, 46, 102603. [Google Scholar] [CrossRef]
- Nnaji, P.C.; Anadebe, V.C.; Onukwuli, O.D.; Okoye, C.C.; Ude, C.J. Multifactor optimization for treatment of textile wastewater using complex salt–Luffa cylindrica seed extract (CS-LCSE) as coagulant: Response surface methodology (RSM) and artificial intelligence algorithm (ANN–ANFIS). Chem. Pap. 2022, 76, 2125–2144. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile Dye Wastewater Characteristics and Constituents of Synthetic Effluents: A Critical Review; Springer: erlin/Heidelberg, Germany, 2019; Volume 16, ISBN 0123456789. [Google Scholar]
- Mohammad, S.; Baidurah, S.; Kobayashi, T.; Ismail, N.; Leh, C.P. Palm oil mill effluent treatment processes—A review. Processes 2021, 9, 739. [Google Scholar] [CrossRef]
- Pal, P. Selection of Water-Treatment Technology. Ind. Water Treat. Process Technol. 2017, 537–544. [Google Scholar]
- Patel, A.; Arkatkar, A.; Singh, S.; Rabbani, A.; Solorza Medina, J.D.; Ong, E.S.; Habashy, M.M.; Jadhav, D.A.; Rene, E.R.; Mungray, A.A.; et al. Physico-chemical and biological treatment strategies for converting municipal wastewater and its residue to resources. Chemosphere 2021, 282, 130881. [Google Scholar] [CrossRef] [PubMed]
- Munyai, S.; Hintsho-Mbita, N.C. Green derived metal sulphides as photocatalysts for waste water treatment. A review. Curr. Res. Green Sustain. Chem. 2021, 4, 100163. [Google Scholar] [CrossRef]
- Andraskar, J.; Yadav, S.; Kapley, A. Challenges and Control Strategies of Odor Emission from Composting Operation. Appl. Biochem. Biotechnol. 2021, 193, 2331–2356. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.H.A.; Natsir, N.S.M.; Rahman, S.N.A.; Daud, F.D.M.; Jamal, N.A.; Ibrahim, N.F.; Nordin, N.H. Development of High Entropy Alloy (HEA) as Catalyst for Azo Dye Degradation in Fenton Process. J. Phys. Conf. Ser. 2021, 012101. [Google Scholar] [CrossRef]
- Yuan, Y.L.; Wen, Y.Z.; Li, X.Y.; Luo, S.Z. Treatment of wastewater from dye manufacturing industry by coagulation. J. Zhejiang Univ. Sci. 2006, 7, 340–344. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Giwa, A.; Ogunribido, A. The Applications of Membrane Operations in the Textile Industry: A Review. Br. J. Appl. Sci. Technol. 2012, 2, 296–310. [Google Scholar] [CrossRef]
- Shinde, P.A.; Ukarde, T.M.; Gogate, P.R.; Pawar, H.S. An integrated approach of adsorption and membrane separation for treatment of sewage water and resource recovery. J. Water Process Eng. 2021, 40, 101795. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Ismail, S.; Bhatia, S. Water recycling from palm oil mill effluent (POME) using membrane technology. Desalination 2003, 157, 87–95. [Google Scholar] [CrossRef]
- Sulaiman, N.M.N.; Ling, C.K. Membrane Ultrafiltration of Treated Palm Oil Mill Effluent (POME). J. Teknol. 2004, 41, 113–120. [Google Scholar] [CrossRef][Green Version]
- Chakraborty, S.; Purkait, M.K.; DasGupta, S.; De, S.; Basu, J.K. Nanofiltration of textile plant effluent for color removal and reduction in COD. Sep. Purif. Technol. 2003, 31, 141–151. [Google Scholar] [CrossRef]
- Lau, W.J.; Ismail, A.F. Polymeric nanofiltration membranes for textile dye wastewater treatment: Preparation, performance evaluation, transport modelling, and fouling control-a review. Desalination 2009, 245, 321–348. [Google Scholar] [CrossRef]
- Townsend, R.B.; Neytzell-de Wilde, F.G.; Buckley, C.A.; Turpie, D.W.F.; Steenkamp, C. Use of dynamic membranes for the treatment of effluents arising from wool scouring and textile dyeing effluents. Water SA 1992, 18, 81–86. [Google Scholar]
- Fersi, C.; Dhahbi, M. Treatment of textile plant effluent by ultrafiltration and/or nanofiltration for water reuse. Desalination 2008, 222, 263–271. [Google Scholar] [CrossRef]
- Laqbaqbi, M.; García-Payo, M.C.; Khayet, M.; El Kharraz, J.; Chaouch, M. Application of direct contact membrane distillation for textile wastewater treatment and fouling study. Sep. Purif. Technol. 2019, 209, 815–825. [Google Scholar] [CrossRef]
- Karim, Z.; Mathew, A.P.; Grahn, M.; Mouzon, J.; Oksman, K. Nanoporous membranes with cellulose nanocrystals as functional entity in chitosan: Removal of dyes from water. Carbohydr. Polym. 2014, 112, 668–676. [Google Scholar] [CrossRef]
- Fersi, C.; Gzara, L.; Dhahbi, M. Treatment of textile effluents by membrane technologies. Desalination 2005, 185, 399–409. [Google Scholar] [CrossRef]
- Suksaroj, C.; Héran, M.; Allègre, C.; Persin, F. Treatment of textile plant effluent by nanofiltration and/or reverse osmosis for water reuse. Desalination 2005, 178, 333–341. [Google Scholar] [CrossRef]
- Karkooti, A.; Yazdi, A.Z.; Chen, P.; McGregor, M.; Nazemifard, N.; Sadrzadeh, M. Development of advanced nanocomposite membranes using graphene nanoribbons and nanosheets for water treatment. J. Memb. Sci. 2018, 560, 97–107. [Google Scholar] [CrossRef]
- Abouzeid, R.E.; Khiari, R.; El-Wakil, N.; Dufresne, A. Current State and New Trends in the Use of Cellulose Nanomaterials for Wastewater Treatment. Biomacromolecules 2019, 20, 573–597. [Google Scholar] [CrossRef] [PubMed]
- Bicy, K.; Rouxel, D.; Poncot, M.; Royaud, I.; Bourson, P.; Chapron, D.; Kalarikkal, N.; Thomas, S. Interfacial tuning and designer morphologies of microporous membranes based on polypropylene/natural rubber nanocomposites. J. Appl. Polym. Sci. 2021, 138, 1–16. [Google Scholar] [CrossRef]
- Alquraish, M.; Jeng, Y.T.; Kchaou, M.; Munusamy, Y.; Abuhasel, K. Development of environment-friendly membrane for oily industrial wastewater filtration. Membranes 2021, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, C.; Wei, Y.; Zhou, Y.; Liao, S. Characterization of the trans-structure in the molecular chain structure of natural rubber. J. Mol. Struct. 2021, 1246, 131209. [Google Scholar] [CrossRef]
- Gelling, I.R. Modification of Natural Rubber Latex With Peracetic Acid. Rubber Chem. Technol. 1985, 58, 86–96. [Google Scholar] [CrossRef]
- Gelling, I. Epoxidised natural rubber. J. Nat. Rubber Res. 1991, 6, 184–205. [Google Scholar]
- Tanrattanakul, V.; Wattanathai, B.; Tiangjunya, A.; Muhamud, P. In situ epoxidized natural rubber: Improved oil resistance of natural rubber. J. Appl. Polym. Sci. 2003, 90, 261–269. [Google Scholar] [CrossRef]
- Ismail, H. Bin Pengisi dan Penguatan Getah; Penerbit Universiti Sains Malaysia: Pulau Pinang, Malaysia, 2000; ISBN 9838611964. [Google Scholar]
- Surya, I.; Maulina, S.; Ismail, H. Effects of alkanolamide and epoxidation in natural rubber and epoxidized natural rubbers compounds. IOP Conf. Ser. Mater. Sci. Eng. 2018, 299, 1–7. [Google Scholar] [CrossRef]
- Haxo, H.E.; Mehta, P.K. Ground Rice-Hull Ash As a Filler for Rubber. Rubber Chem. Technol. 1975, 48, 271–288. [Google Scholar] [CrossRef]
- Fuad, M.Y.A.; Yaakob, I.; Rusli, O.; Ishak, Z.A.M.; Omar, A.K.M. Determination of filler content in thermoplastic composites by FTIR analysis. J. Appl. Polym. Sci. 1995, 56, 1557–1560. [Google Scholar] [CrossRef]
- Yoksan, R. Epoxidized Natural Rubber for Adhesive Applications. Nat. Sci. 2008, 42, 325–332. [Google Scholar]
- Jiang, Y.; Wang, J.; Wu, J.; Zhang, Y. Preparation of high-performance natural rubber/carbon black/molybdenum disulfide composite by using the premixture of epoxidized natural rubber and cysteine-modified molybdenum disulfide. Polym. Bull. 2021, 78, 1213–1230. [Google Scholar] [CrossRef]
- Raju, G.; Khalid, M.; Shaban, M.M.; Azahari, B. Preparation and characterization of eco-friendly spent coffee/enr50 biocomposite in comparison to carbon black. Polymers 2021, 13, 2796. [Google Scholar] [CrossRef]
- Songtipya, L.; Songtipya, P.; Sengsuk, T.; Kalkornsurapranee, E.; Nakaramontri, Y.; Johns, J. Improved adhesion properties of natural rubber-based pressure-sensitive adhesives by incorporating particulate fillers. Compos. Commun. 2021, 27, 100880. [Google Scholar] [CrossRef]
- de Maria, V.P.K.; de Paiva, F.F.G.; Cabrera, F.C.; Hiranobe, C.T.; Ribeiro, G.D.; Paim, L.L.; Job, A.E.; dos Santos, R.J. Mechanical and rheological properties of partial replacement of carbon black by treated ultrafine calcium carbonate in natural rubber compounds. Polym. Bull. 2021. [Google Scholar] [CrossRef]
- Ismail, H.; Freakley, P.K.; Sutherland, I.; Sheng, E. Effects of multifunctional additive on mechanical properties of silica filled natural rubber compound. Eur. Polym. J. 1995, 31, 1109–1117. [Google Scholar] [CrossRef]
- Ismail, H.; Nizam, J.M.; Abdul Khalil, H.P.S. Effect of a compatibilizer on the mechanical properties and mass swell of white rice husk ash filled natural rubber/linear low density polyethylene blends. Polym. Test. 2001, 20, 125–133. [Google Scholar] [CrossRef]
- Ryu, G.; Kim, D.; Song, S.; Hwang, K.; Ahn, B.; Kim, W. Effect of epoxide content on the vulcanizate structure of silica-filled epoxidized natural rubber (Enr) compounds. Polymers 2021, 13, 1862. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, R.; Mascia, L.; Clarke, J.; Malucelli, G. Effect of SiO2 particles on the relaxation dynamics of epoxidized natural rubber (ENR) in the melt state by time-resolved mechanical spectroscopy. Polymers 2021, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Eom, Y.-G. Thermogravimetric analysis of rice husk flour for a new raw material of lignocellulosic fiber-thermoplastic polymer composites. J. Korean Wood Sci. Technol. 2001, 29, 59–67. [Google Scholar]
- Ahmad, A.; Mohd, D.H.; Abdullah, I. Mechanical properties of filled NR/LLDPE blends. Iran. Polym. J. Engl. Ed. 2004, 13, 173–178. [Google Scholar]
- Pham, L.Q.; Uspenskaya, M.V.; Olekhnovich, R.O.; Bernal, R.A.O. A review on electrospun pvc nanofibers: Fabrication, properties, and application. Fibers 2021, 9, 12. [Google Scholar] [CrossRef]
- Turner, A.; Filella, M. Polyvinyl chloride in consumer and environmental plastics, with a particular focus on metal-based additives. Environ. Sci. Process. Impacts 2021, 23, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.G.; Wei, H.J.; Luo, J.; Chen, Y.; Cao, P.F. Highly Stretchable, Ultratough, and Multifunctional Poly(vinyl chloride)-Based Plastics via a Green, Star-Shaped Macromolecular Additive. Macromolecules 2021, 54, 3169–3180. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Kodsangma, A.; Homsaard, N.; Nadon, S.; Jantrawut, P.; Ruksiriwanich, W.; Seesuriyachan, P.; Leksawasdi, N.; Phimolsiripol, Y.; Chaiyaso, T.; et al. Thermoplastic mung bean starch/natural rubber/sericin blends for improved oil resistance. Int. J. Biol. Macromol. 2021, 188, 283–289. [Google Scholar] [CrossRef]
- Ismail, H.; Mohamad, Z.; Abu Bakar, A. The effect of dynamic vulcanization on properties of rice husk powder filled polystyrene/styrene butadiene rubber blends. Iran. Polym. J. Engl. Ed. 2004, 13, 11–19. [Google Scholar]
- Toh, H.W.; Toong, D.W.Y.; Ng, J.C.K.; Ow, V.; Lu, S.; Tan, L.P.; Wong, P.E.H.; Venkatraman, S.; Huang, Y.; Ang, H.Y. Polymer blends and polymer composites for cardiovascular implants. Eur. Polym. J. 2021, 146, 110249. [Google Scholar] [CrossRef]
- Steube, M.; Johann, T.; Barent, R.D.; Müller, A.H.E.; Frey, H. Rational design of tapered multiblock copolymers for thermoplastic elastomers. Prog. Polym. Sci. 2022, 124, 101488. [Google Scholar] [CrossRef]
- Abdullah, I. Strategi Penyelidikan Kimia Getah Asli; Universiti Kebangsaan Malaysia: Bangi, Malaysia, 2002. [Google Scholar]
- Bhowmick, S.K.D. and A.K. Thermoplastic Elastomers from Rubber-Plastic Blends; Ellis Horwood: Chichester, UK, 1990. [Google Scholar]
- Bui, Q.B.; Colin, J.; Nguyen, T.D.; Mao, N.D.; Nguyen, T.M.L.; Perré, P. Mechanical and thermal properties of a biocomposite based on polyvinylchloride/epoxidized natural rubber blend reinforced with rice husk microfiller. J. Thermoplast. Compos. Mater. 2019. [Google Scholar] [CrossRef]
- Kanthiya, T.; Kiattipornpithak, K.; Thajai, N.; Phimolsiripol, Y. Modified Poly ( Lactic Acid ) Epoxy Resin Using Chitosan for Reactive Blending with Epoxidized Natural Rubber: Analysis of Annealing Time. Polymers 2022, 14, 1085. [Google Scholar] [CrossRef]
- Li, L.; Schneider, Y.; Hoeglund, A.B.; Braslau, R. Advances in Internal Plasticization of PVC: Copper-Mediated Atom-Transfer Radical Polymerization from PVC Defect Sites to Form Acrylate Graft Copolymers. Synlett 2021, 32, 497–501. [Google Scholar] [CrossRef]
- Ka Wei, K.; Leng, T.P.; Keat, Y.C.; Osman, H.; Ying, L.B. Enhancing compatibility in epoxy/vulcanized natural rubber (VNR)/Graphene nano-platelets (GNP) system using epoxidized natural rubber (ENR-50). Compos. Part B Eng. 2019, 174, 107058. [Google Scholar] [CrossRef]
- Ibrahim, A.; Dahlan, M. Thermoplastic Natural Rubber Blends. Prog. Polym. Sci. 1998, 23, 665–706. [Google Scholar] [CrossRef]
- Ramesh, P.; De, S.K. Self-crosslinkable plastic-rubber blend system based on poly (vinyl chloride) and epoxidiezed natural rubber. J. Mater. Polym. Sci. 1991, 26, 2846–2850. [Google Scholar]
- Varughese, K.T.; Nando, G.B.; De, P.P.; De, S.K. Miscible blends from rigid poly(vinyl chloride) and epoxidized natural rubber-Part 1 Phase morphology. J. Mater. Sci. 1988, 23, 3894–3902. [Google Scholar] [CrossRef]
- Ratnam, C.T.; Zaman, K. Enhancement of polyvinyl chloride (PVC)/epoxidised natural rubber (ENR) blend properties by electron beam irradiation: Effect of antioxidants. Polym. Degrad. Stab. 1999, 65, 481–490. [Google Scholar] [CrossRef]
- Manoj, N.R.; De, P.P.; De, S.K. Self-crosslinkable plastic-rubber blend system based on poly (vinyl chloride) and acrylonitrile-co-butadiene rubber. J. Appl. Polym. Sci. 1993, 49, 133–142. [Google Scholar] [CrossRef]
- Nasir, Z.A.; Ishiaku, U.S.; Ishak, Z.A.M. Determination of optimum blending conditions for poly(vinyl chloride)/epoxidized natural rubber blends. J. Appl. Polym. Sci. 1993, 47, 951–959. [Google Scholar] [CrossRef]
- Samad, N.A.; Othaman, R.; Abdullah, I. Preparation and characterisation of epoxidised natural rubber/polyvinyl chloride/rice husk (ENR/PVC/RH) thin film composite by solution casting technique. Int. J. Mater. Eng. Innov. 2014, 5, 61–69. [Google Scholar] [CrossRef]
- Jon, N.; Samad, N.A.; Abdullah, N.A.; Abdullah, I.; Othaman, R. Influence of silica addition on the properties of epoxidised natural rubber/polyvinyl chloride composite membrane. J. Appl. Polym. Sci. 2013, 129, 2789–2795. [Google Scholar] [CrossRef]
- Premalal, H.G.B.; Ismail, H.; Baharin, A. Comparison of the mechanical properties of rice husk powder filled polypropylene composites with talc filled polypropylene composites. Polym. Test. 2002, 21, 833–839. [Google Scholar] [CrossRef]
- Ray, S.; Ray, S.K. Separation of organic mixtures by pervaporation using crosslinked and filled rubber membranes. J. Memb. Sci. 2006, 285, 108–119. [Google Scholar] [CrossRef]
- Sapuan, S.M.; Bachtiar, D. Mechanical Properties of Sugar Palm Fibre Reinforced High Impact Polystyrene Composites. Procedia Chem. 2012, 4, 101–106. [Google Scholar] [CrossRef]
- Bachtiar, D.; Sapuan, S.M.; Zainudin, E.S.; Khalina, A.; Dahlan, K.Z.M. The tensile properties of single sugar palm (Arenga pinnata) fibre. IOP Conf. Ser. Mater. Sci. Eng. 2010, 11, 012012. [Google Scholar] [CrossRef]
- Ratnam, C.T.; Fazlina, R.S.; Shamsuddin, S. Mechanical Properties of Rubber-Wood Fiber Filled PVC / ENR Blend. Malays. Polym. J. 2010, 5, 17–25. [Google Scholar]
- Ratnam, C.T.; Raju, G.; Yunus, W.M.Z.W. Oil palm empty fruit bunch (OPEFB) fiber reinforced PVC/ENR blend-electron beam irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2007, 265, 510–514. [Google Scholar] [CrossRef]
- Raju, G.; Ratnam, C.T.; Ibrahim, N.A.; Zaki, M.; Rahman, A.; Zin, W.; Yunus, W. Enhancement of PVC/ENR Blend Properties by Poly (methyl acrylate) Grafted Oil Palm Empty Fruit Bunch Fiber. J. Appl. Polym. Sci. 2008. [Google Scholar] [CrossRef]
- Ramlee, N.A.; Ratnam, C.T.; Rahman, S.A.; Azua, N.; Samat, A. Incorporation of TiO 2 nanoparticles in PVC/ENR blends. 2013, 557–560. In Proceedings of the 2013 IEEE Business Engineering and Industrial Applications Colloquium (BEIAC), Langkawi, Malaysia, 7–9 April 2013; pp. 557–560. [Google Scholar]
- Ab, N.; Jon, N.; Azwan, M.; Mat, S.; Abdullah, I. Preparation of ENR / PVC / RH Composite Membrane for Water Permeation Application. Adv. J. Technol. Vocat. Educ. 2017, 1, 20–30. [Google Scholar]
- Ismail, N.F.H.; Chai, T.M.; Daik, R.; Othaman, R. Epoxidised natural rubber (ENR)/polyvinyl chloride (PVC)/silica (SiO2) membrane for treating palm oil mill effluents (POME). Plast. Rubber Compos. 2020, 49, 134–140. [Google Scholar] [CrossRef]
- Mohd Nor, F.; Othaman, R. Effects of MgO particle loading on gas permeation properties of epoxidized natural rubber (ENR)/polyvinyl chloride (PVC) membrane. Sains Malays. 2015, 44, 875–881. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Fatimah Athiyah, S.; Shazleen, S.S.; Rafiqah, S.A.; Harussani, M.M.; Kamarudin, S.H.; Razman, M.R.; Rahmah, M.; Zainudin, E.S.; et al. A Review on Mechanical Performance of Hybrid Natural Fiber Polymer Composites for Structural Applications. Polymers 2021, 13, 2170. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Asyraf, M.R.M.; Dayana, D.A.Z.N.; Amelia, J.J.N.; Rani, M.S.A.; Norrrahim, M.N.F.; Nurazzi, N.M.; Aisyah, H.A.; Sharma, S.; et al. Polymer Composites Filled with Metal Derivatives: A Review of Flame Retardants. Polymers 2021, 13, 1701. [Google Scholar] [CrossRef]
- Thyavihalli Girijappa, Y.G.; Mavinkere Rangappa, S.; Parameswaranpillai, J.; Siengchin, S. Natural Fibers as Sustainable and Renewable Resource for Development of Eco-Friendly Composites: A Comprehensive Review. Front. Mater. 2019, 6, 1–14. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Aisyah, H.A.; Rafiqah, S.A.; Sabaruddin, F.A.; Kamarudin, S.H.; Norrrahim, M.N.F.; Ilyas, R.A.; et al. A Review on Natural Fiber Reinforced Polymer Composite for Bullet Proof and Ballistic Applications. Polymers 2021, 13, 646. [Google Scholar] [CrossRef]
- Sabaruddin, F.A.; Paridah, M.T.; Sapuan, S.M.; Ilyas, R.A.; Lee, S.H.; Abdan, K.; Mazlan, N.; Roseley, A.S.M.; Abdul Khalil, H.P.S. The effects of unbleached and bleached nanocellulose on the thermal and flammability of polypropylene-reinforced kenaf core hybrid polymer bionanocomposites. Polymers 2020, 13, 116. [Google Scholar] [CrossRef]
- Rozilah, A.; Jaafar, C.N.A.; Sapuan, S.M.; Zainol, I.; Ilyas, R.A. The Effects of Silver Nanoparticles Compositions on the Mechanical, Physiochemical, Antibacterial, and Morphology Properties of Sugar Palm Starch Biocomposites for Antibacterial Coating. Polymers 2020, 12, 2605. [Google Scholar] [CrossRef]
- Mark, U.C.; Madufor, I.C.; Obasi, H.C.; Mark, U. Influence of filler loading on the mechanical and morphological properties of carbonized coconut shell particles reinforced polypropylene composites. J. Compos. Mater. 2020, 54, 397–407. [Google Scholar] [CrossRef]
- Alsubari, S.; Zuhri, M.Y.M.; Sapuan, S.M.; Ishak, M.R.; Ilyas, R.A.; Asyraf, M.R.M. Potential of natural fiber reinforced polymer composites in sandwich structures: A review on its mechanical properties. Polymers 2021, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, I.; Leman, Z.; Ishak, M.R.; Zainudin, E.S. Sugar Palm Fibre and its Composites: A Review of Recent Developments. Bioresource 2016, 11, 10756–10782. [Google Scholar]
- Suriani, M.J.; Ilyas, R.A.; Zuhri, M.Y.M.; Khalina, A.; Sultan, M.T.H.; Sapuan, S.M.; Ruzaidi, C.M.; Wan, F.N.; Zulkifli, F.; Harussani, M.M.; et al. Critical Review of Natural Fiber Reinforced Hybrid Composites: Processing, Properties, Applications and Cost. Polymers 2021, 13, 3514. [Google Scholar] [CrossRef]
- Suriani, M.J.; Rapi, H.Z.; Ilyas, R.A.; Petrů, M.; Sapuan, S.M. Delamination and Manufacturing Defects in Natural Fiber-Reinforced Hybrid Composite: A Review. Polymers 2021, 13, 1323. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Zuhri, M.Y.M.; Norrrahim, M.N.F.; Misenan, M.S.M.; Jenol, M.A.; Samsudin, S.A.; Nurazzi, N.M.; Asyraf, M.R.M.; Supian, A.B.M.; Bangar, S.P.; et al. Natural Fiber-Reinforced Polycaprolactone Green and Hybrid Biocomposites for Various Advanced Applications. Polymers 2022, 14, 182. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Zuhri, M.Y.M.; Aisyah, H.A.; Asyraf, M.R.M.; Hassan, S.A.; Zainudin, E.S.; Sapuan, S.M.; Sharma, S.; Bangar, S.P.; Jumaidin, R.; et al. Natural Fiber-Reinforced Polylactic Acid, Polylactic Acid Blends and Their Composites for Advanced Applications. Polymers 2022, 14, 202. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Aisyah, H.A.; Nordin, A.H.; Ngadi, N.; Zuhri, M.Y.M.; Asyraf, M.R.M.; Sapuan, S.M.; Zainudin, E.S.; Sharma, S.; Abral, H.; et al. Natural-Fiber-Reinforced Chitosan, Chitosan Blends and Their Nanocomposites for Various Advanced Applications. Polymers 2022, 14, 874. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A.; Sherwani, S.F.K.; Yusuf, J.; Ilyas, R.A. Recent developments in sustainable arrowroot (Maranta arundinacea Linn) starch biopolymers, fibres, biopolymer composites and their potential industrial applications: A review. J. Mater. Res. Technol. 2021, 13, 1191–1219. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Rayung, M.; Norrrahim, M.N.F.; Shazleen, S.S.; Rani, M.S.A.; Shafi, A.R.; Aisyah, H.A.; Radzi, M.H.M.; Sabaruddin, F.A.; et al. Thermogravimetric Analysis Properties of Cellulosic Natural Fiber Polymer Composites: A Review on Influence of Chemical Treatments. Polymers 2021, 13, 2710. [Google Scholar] [CrossRef]
- Nabilah Haris, N.I.; Hassan, M.Z.; Ilyas, R.A.; Suhot, M.A.; Sapuan, S.M.; Dolah, R.; Mohammad, R.; Asyraf, M.R.M. Dynamic mechanical properties of natural fiber reinforced hybrid polymer composites: A review. J. Mater. Res. Technol. 2022. [Google Scholar] [CrossRef]
- Norfarhana, A.S.; Ilyas, R.A.; Ngadi, N. A review of nanocellulose adsorptive membrane as multifunctional wastewater treatment. Carbohydr. Polym. 2022, 291, 119563. [Google Scholar] [CrossRef]
- Thiruganasambanthan, T.; Ilyas, R.A.; Norrrahim, M.N.F.; Kumar, T.S.M.; Siengchin, S.; Misenan, M.S.M.; Farid, M.A.A.; Nurazzi, N.M.; Asyraf, M.R.M.; Zakaria, S.Z.S.; et al. Emerging Developments on Nanocellulose as Liquid Crystals: A Biomimetic Approach. Polymers 2022, 14, 1546. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Harussani, M.M.; Ilyas, R.A.; Ashogbon, A.O.; Singh, A.; Trif, M.; Jafari, S.M. Surface modifications of cellulose nanocrystals: Processes, properties, and applications. Food Hydrocoll. 2022, 130, 107689. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, M.Q.; Rong, M.Z.; Friedrich, K. Tensile performance improvement of low nanoparticles filled-polypropylene composites. Compos. Sci. Technol. 2002, 62, 1327–1340. [Google Scholar] [CrossRef]
- Syafri, E.; Jamaluddin; Sari, N. H.; Mahardika, M.; Amanda, P.; Ilyas, R.A. Isolation and characterization of cellulose nanofibers from Agave gigantea by chemical-mechanical treatment. Int. J. Biol. Macromol. 2022, 200, 25–33. [Google Scholar] [CrossRef]
- Mohd Nurazzi, N.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Sabaruddin, F.A.; Kamarudin, S.H.; Ahmad, S.; Mahat, A.M.; Lee, C.L.; Aisyah, H.A.; et al. Fabrication, Functionalization, and Application of Carbon Nanotube-Reinforced Polymer Composite: An Overview. Polymers 2021, 13, 1047. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Tawakkal, I.S.M.A.; Ilyas, R.A. Water barrier and mechanical properties of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch (TPS)/poly(lactic acid) (PLA) blend bionanocomposites. Nanotechnol. Rev. 2021, 10, 431–442. [Google Scholar] [CrossRef]
- Syafiq, R.M.O.; Sapuan, S.M.; Zuhri, M.Y.M.; Othman, S.H.; Ilyas, R.A. Effect of plasticizers on the properties of sugar palm nanocellulose/cinnamon essential oil reinforced starch bionanocomposite films. Nanotechnol. Rev. 2022, 11, 423–437. [Google Scholar] [CrossRef]
- Nazrin, A.; Sapuan, S.M.; Zuhri, M.Y.M.; Tawakkal, I.S.M.A.; Ilyas, R.A. Flammability and physical stability of sugar palm crystalline nanocellulose reinforced thermoplastic sugar palm starch / poly ( lactic acid ) blend bionanocomposites. Nanotechnol. Rev. 2022, 11, 86–95. [Google Scholar] [CrossRef]
- Chan, J.X.; Wong, J.F.; Petrů, M.; Hassan, A.; Nirmal, U.; Othman, N.; Ilyas, R.A. Effect of Nanofillers on Tribological Properties of Polymer Nanocomposites: A Review on Recent Development. Polymers 2021, 13, 2867. [Google Scholar] [CrossRef]
- Nawaz, H.; Umar, M.; Ullah, A.; Razzaq, H.; Zia, K.M.; Liu, X. Polyvinylidene fluoride nanocomposite super hydrophilic membrane integrated with Polyaniline-Graphene oxide nano fillers for treatment of textile effluents. J. Hazard. Mater. 2021, 403, 123587. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mandal, T.; Goswami, S. Fabrication of cellulose acetate nanocomposite films with lignocelluosic nanofiber filler for superior effect on thermal, mechanical and optical properties. Nano-Struct. Nano-Objects 2021, 25, 100642. [Google Scholar] [CrossRef]
- Sarkawi, S.S.; Aziz, Y. Ground Rice Husk as Filler in Rubber Compounding. J. Teknol. 2003, 39, 135–148. [Google Scholar] [CrossRef]
- Kuan, H.T.N.; Tan, M.Y.; Shen, Y.; Yahya, M.Y. Mechanical properties of particulate organic natural filler-reinforced polymer composite: A review. Compos. Adv. Mater. 2021, 30, 1–17. [Google Scholar] [CrossRef]
- Cazan, C.; Enesca, A.; Andronic, L. Synergic effect of tio2 filler on the mechanical properties of polymer nanocomposites. Polymers 2021, 13, 2017. [Google Scholar] [CrossRef] [PubMed]
- Jusoh, M.J. bin Studies on the Properties of Woven Natural Fibers Reinforced Unsaturated Polyester Composites. Master’s Thesis, Universiti Sains Malaysia, George Town, Malaysia, September 2008. [Google Scholar]
- Matykiewicz, D.; Barczewski, M.; Mousa, M.S.; Sanjay, M.R.; Siengchin, S. Impact strength of hybrid epoxy–basalt composites modified with mineral and natural fillers. ChemEngineering 2021, 5, 56. [Google Scholar] [CrossRef]
- Torres, F.G.; Cubillas, M.L. Study of the interfacial properties of natural fibre reinforced polyethylene. Polym. Test. 2005, 24, 694–698. [Google Scholar] [CrossRef]
- Saba, N.; Tahir, P.M.; Jawaid, M. A review on potentiality of nano filler/natural fiber filled polymer hybrid composites. Polymers 2014, 6, 2247–2273. [Google Scholar] [CrossRef]
- Zurina, M.; Ismail, H.; Bakar, A.A. Rice husk powder-filled polystyrene/styrene butadiene rubber blends. J. Appl. Polym. Sci. 2004, 92, 3320–3332. [Google Scholar] [CrossRef]
- Yang, H.S.; Kim, H.J.; Son, J.; Park, H.J.; Lee, B.J.; Hwang, T.S. Rice-husk flour filled polypropylene composites; mechanical and morphological study. Compos. Struct. 2004, 63, 305–312. [Google Scholar] [CrossRef]
- Reis, P.N.B.; Ferreira, J.A.M.; Silva, P.A.A. Mechanical behaviour of composites filled by agro-waste materials. Fibers Polym. 2011, 12, 240–246. [Google Scholar] [CrossRef]
- Syafri, R.; Ahmad, I.; Abdullah, I. Effect of rice husk surface modification by LENR the on mechanical properties of NR/HDPE reinforced rice husk composite. Sains Malays. 2011, 40, 749–756. [Google Scholar]
- Handayani, E. Sintesa Membran Nanokomposit Berbasis Nanopartikel Biosilika Dari Sekam Padi Dan Kitosan Sebagai Matriks Biopolimer; IPB University: West Java, Indonesia, 2009. [Google Scholar]
- Ahmad, I.; Jamil, M.S.; Abdullah, I. Pengisian sekam padi & tanah liat ke dalam matriks polietilena berketumpatan tinggi-getah asli getah asli cecair. Sains Malays. 2009, 38, 381–386. [Google Scholar]
- Hardinnawirda, K.; Aisha, I.S. Effect of Rice Husks As Filler in Polymer Matrix Composites. J. Mech. Eng. Sci. 2012, 2, 181–186. [Google Scholar] [CrossRef]
- Jamil, M.S.; Ahmad, I.; Abdullah, I. Effects of rice husk filler on the mechanical and thermal properties of liquid natural rubber compatibilized high-density polyethylene/natural rubber blends. J. Polym. Res. 2006, 13, 315–321. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I. Morphological, thermal, and mechanical properties of starch biocomposite films reinforced by cellulose nanocrystals from rice husks. BioResources 2012, 7, 5469–5477. [Google Scholar] [CrossRef]
- Pornwannachai, W.; Richard Horrocks, A.; Kandola, B.K. Surface Modification of Commingled Flax/PP and Flax/PLA Fibres by Silane or Atmospheric Argon Plasma Exposure to Improve Fibre–Matrix Adhesion in Composites. Fibers 2022, 10, 2. [Google Scholar] [CrossRef]
- George, J.; Sreekala, M.S.; Thomas, S. A review on interface modification and characterization of natural fiber reinforced plastic composites. Polym. Eng. Sci. 2001, 41, 1471–1485. [Google Scholar] [CrossRef]
- Ndazi, B.S.; Karlsson, S.; Tesha, J.V.; Nyahumwa, C.W. Chemical and physical modifications of rice husks for use as composite panels. Compos. Part A Appl. Sci. Manuf. 2007, 38, 925–935. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chowdhury, S.; Das Saha, P. Adsorption of Crystal Violet from aqueous solution onto NaOH-modified rice husk. Carbohydr. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Hanafiah, M.A.K.M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar] [CrossRef] [PubMed]
- Sreekala, M.S.; Thomas, S. Effect of fibre surface modification on water-sorption characteristics of oil palm fibres. Compos. Sci. Technol. 2003, 63, 861–869. [Google Scholar] [CrossRef]
- Priya, A.K.; Yogeshwaran, V.; Rajendran, S.; Hoang, T.K.A.; Soto-Moscoso, M.; Ghfar, A.A.; Bathula, C. Investigation of mechanism of heavy metals (Cr6+, Pb2+& Zn2+) adsorption from aqueous medium using rice husk ash: Kinetic and thermodynamic approach. Chemosphere 2022, 286, 131796. [Google Scholar] [CrossRef] [PubMed]
- Ponce, J.; da Silva Andrade, J.G.; dos Santos, L.N.; Bulla, M.K.; Barros, B.C.B.; Favaro, S.L.; Hioka, N.; Caetano, W.; Batistela, V.R. Alkali pretreated sugarcane bagasse, rice husk and corn husk wastes as lignocellulosic biosorbents for dyes. Carbohydr. Polym. Technol. Appl. 2021, 2, 100061. [Google Scholar] [CrossRef]
- Akhtar, M.; Bhanger, M.I.; Iqbal, S.; Hasany, S.M. Sorption potential of rice husk for the removal of 2,4-dichlorophenol from aqueous solutions: Kinetic and thermodynamic investigations. J. Hazard. Mater. 2006, 128, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, M.; Ali Khan Rao, R.; Anwar, S.; Ahmad, J.; Ahmad, R. Adsorption studies on rice husk: Removal and recovery of Cd(II) from wastewater. Bioresour. Technol. 2003, 86, 147–149. [Google Scholar] [CrossRef]
- Katal, R.; Baei, M.S.; Rahmati, H.T.; Esfandian, H. Kinetic, isotherm and thermodynamic study of nitrate adsorption from aqueous solution using modified rice husk. J. Ind. Eng. Chem. 2012, 18, 295–302. [Google Scholar] [CrossRef]

| Membrane Separation Technique | Pore Size |
|---|---|
| Mikrofiltration (MF) | 0.04–100 µm |
| Ultrafiltration (UF) | 0.1–1 µm |
| Nanofiltration (NF) | 100 Å–0.001 Å |
| Reverse Osmosis (RO) | 1 Å–10 Å |
| Technique | Principle |
|---|---|
| Thermally-induced phase separation (TIPS) |
|
| Air-casting of a polymer solution |
|
| Precipitation from the vapor phase |
|
| Immersion precipitation |
|
| ENR-10 | ENR-25 | ENR-50 | |
|---|---|---|---|
| Glass Transition Temperature, Tg (°C) | −60 | −45 | −20 |
| Specific Gravity | 0.94 | 0.97 | 1.03 |
| Mooney Viscosity, ML, 1 + 4 (100 °C) | 90 | 110 | 140 |
| Filler | Fabrication Techniques | Properties | Applications | Ref. |
|---|---|---|---|---|
| Oil palm empty fruit bunch (OPEFB) | Electron-beam irradation | Tensile strength, Young’s modulus, and gel content increase with a concurrent reduction in the elongation at break (Eb) of the composites. | Composite material | [195] |
| Oil palm empty fruit bunch (OPEFB) | Melt blending | Young’s modulus, hardness, and flexural modulus of the PVC/ ENR blend increase with the increase in OPEFB loading | Composite material | [196] |
| Rubber-wood | Melt blending | Flexural modulus, Young’s modulus and hardness increased with the RW loading. The impact strength, Ts and Eb decrease with the increase in RW loading | Composite material | [194] |
| Titnium dioxide (TiO2) | Melt blending, radiation | Good distribution of TiO2 in the PVC/ENR blends matrix | Composite material | [197] |
| Pineapple leaves fiber cellulose | Solution blending, casting technique, phase inversion method | Number of pores increased with the addition of cellulose. Decoloration of palm oil mill effluent after treated by ENR/PVC/Cell-20% and ENR/PVC/Cell-g-PMMA-10% membranes. | Composite material | [36] |
| Rice husk powder | Solution blending, casting technique, phase inversion method | Relative humidity (RH) reduces tensile strength and increases the tensile modulus. The number of pores increased with the increasing wt% of RH. | Water permeation | [198] |
| Silica | Solution blending, casting technique, phase inversion method | Thermal and mechanical stability of the membranes improved with the incorporation of silica.CO2 and N2 gas permeation of silica-filled membranes increased with increasing silica content | Gas permeation | [189] |
| Silica | Solution blending, casting technique, phase inversion method | Silica as pore former. Mechanical properties of the membrane improved by the addition of silica. COD and BOD showed a reduction of 44% and 38.3%, respectively, after POME | POME treatment | [199] |
| Magnesium Oxide, MgO | Solution blending, casting technique, phase inversion method | Pores developed as fillers were introduced to the membrane.Permeability values of CO2 and N2 increased with the addition of MgO. | Gas permeation | [200] |
| Microcrystalline Cellulose, MCC | Solution blending technique | Chemical oxygen demand (COD), biochemical oxygen demand (BOD) and total suspended solid (TSS) were reduced to 99.9%, 70.3%, and 16.9%, respectively. | POME treatment | [38] |
| Element | Percent (%) |
|---|---|
| Cellulose | 25–35 |
| Hemicellulose | 18–21 |
| Lignin | 26–31 |
| Silica | 15–17 |
| Solute | 2–5 |
| Humidity | 7.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norfarhana, A.S.; Ilyas, R.A.; Ngadi, N.; Sharma, S.; Sayed, M.M.; El-Shafay, A.S.; Nordin, A.H. Natural Fiber-Reinforced Thermoplastic ENR/PVC Composites as Potential Membrane Technology in Industrial Wastewater Treatment: A Review. Polymers 2022, 14, 2432. https://doi.org/10.3390/polym14122432
Norfarhana AS, Ilyas RA, Ngadi N, Sharma S, Sayed MM, El-Shafay AS, Nordin AH. Natural Fiber-Reinforced Thermoplastic ENR/PVC Composites as Potential Membrane Technology in Industrial Wastewater Treatment: A Review. Polymers. 2022; 14(12):2432. https://doi.org/10.3390/polym14122432
Chicago/Turabian StyleNorfarhana, A.S., R.A. Ilyas, N. Ngadi, Shubham Sharma, Mohamed Mahmoud Sayed, A.S. El-Shafay, and A.H. Nordin. 2022. "Natural Fiber-Reinforced Thermoplastic ENR/PVC Composites as Potential Membrane Technology in Industrial Wastewater Treatment: A Review" Polymers 14, no. 12: 2432. https://doi.org/10.3390/polym14122432
APA StyleNorfarhana, A. S., Ilyas, R. A., Ngadi, N., Sharma, S., Sayed, M. M., El-Shafay, A. S., & Nordin, A. H. (2022). Natural Fiber-Reinforced Thermoplastic ENR/PVC Composites as Potential Membrane Technology in Industrial Wastewater Treatment: A Review. Polymers, 14(12), 2432. https://doi.org/10.3390/polym14122432










