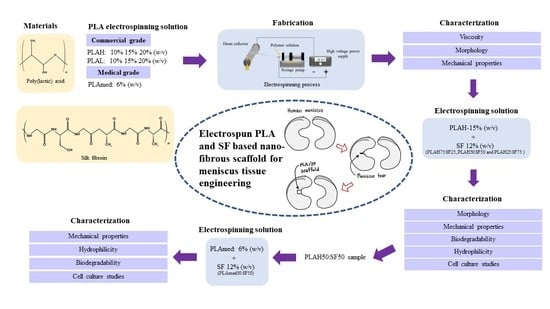
Electrospun Poly(lactic acid) and Silk Fibroin Based Nanofibrous Scaffold for Meniscus Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Silk Fibroin Preparation
2.3. Electrospinning Solution Preparation
2.4. Characterization of PLA Solutions
2.5. Fiber Morphology
2.6. Mechanical Properties
2.7. Thermal Properties
2.8. Fourier-Transform Infrared Spectroscopy (FTIR)
2.9. In Vitro Degradation
2.10. Surface Wettability
2.11. In Vitro Cell Culture Studies
2.12. Quantitative Analysis for Gene Expression
3. Results
3.1. Viscosity of PLA Solutions
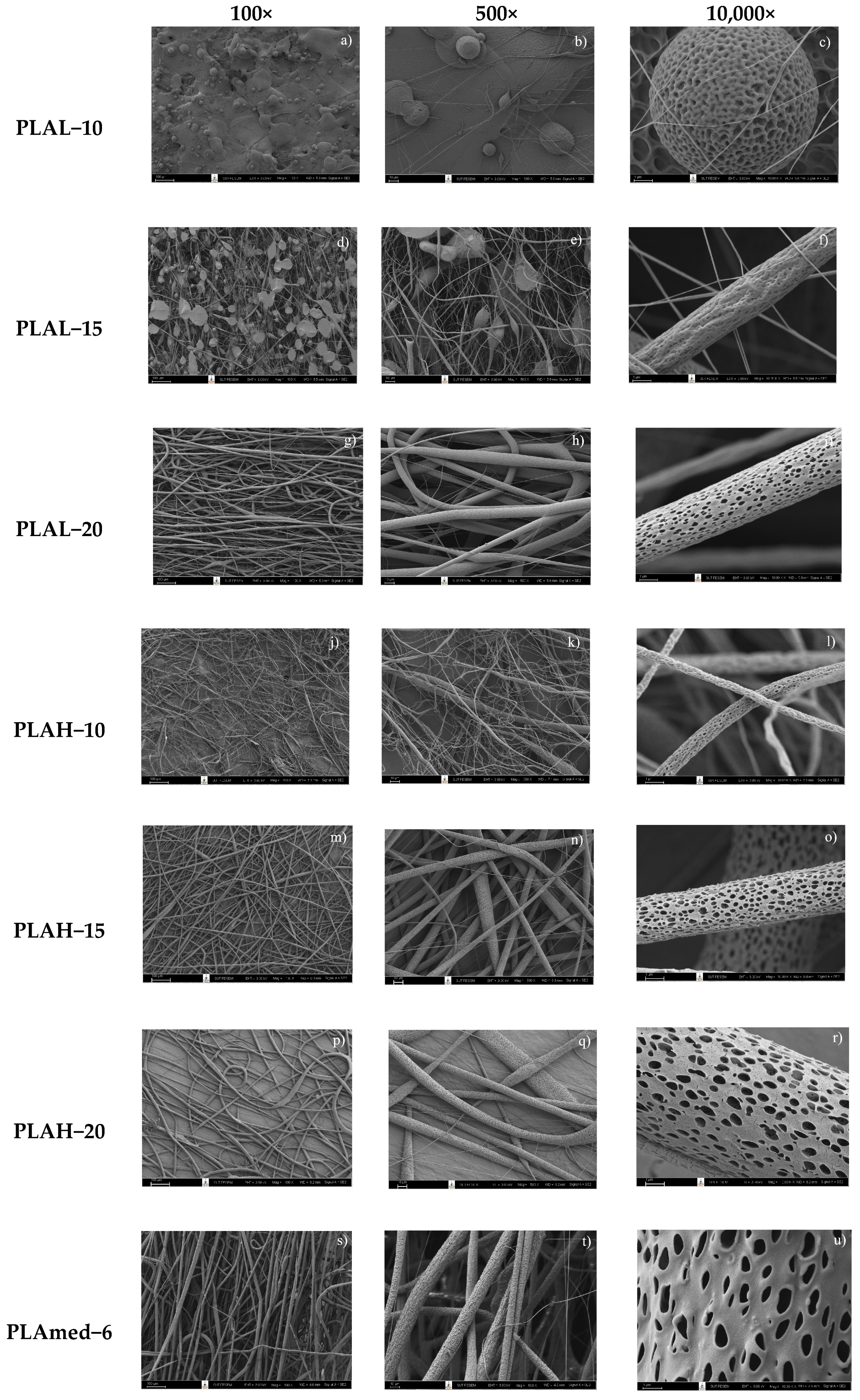
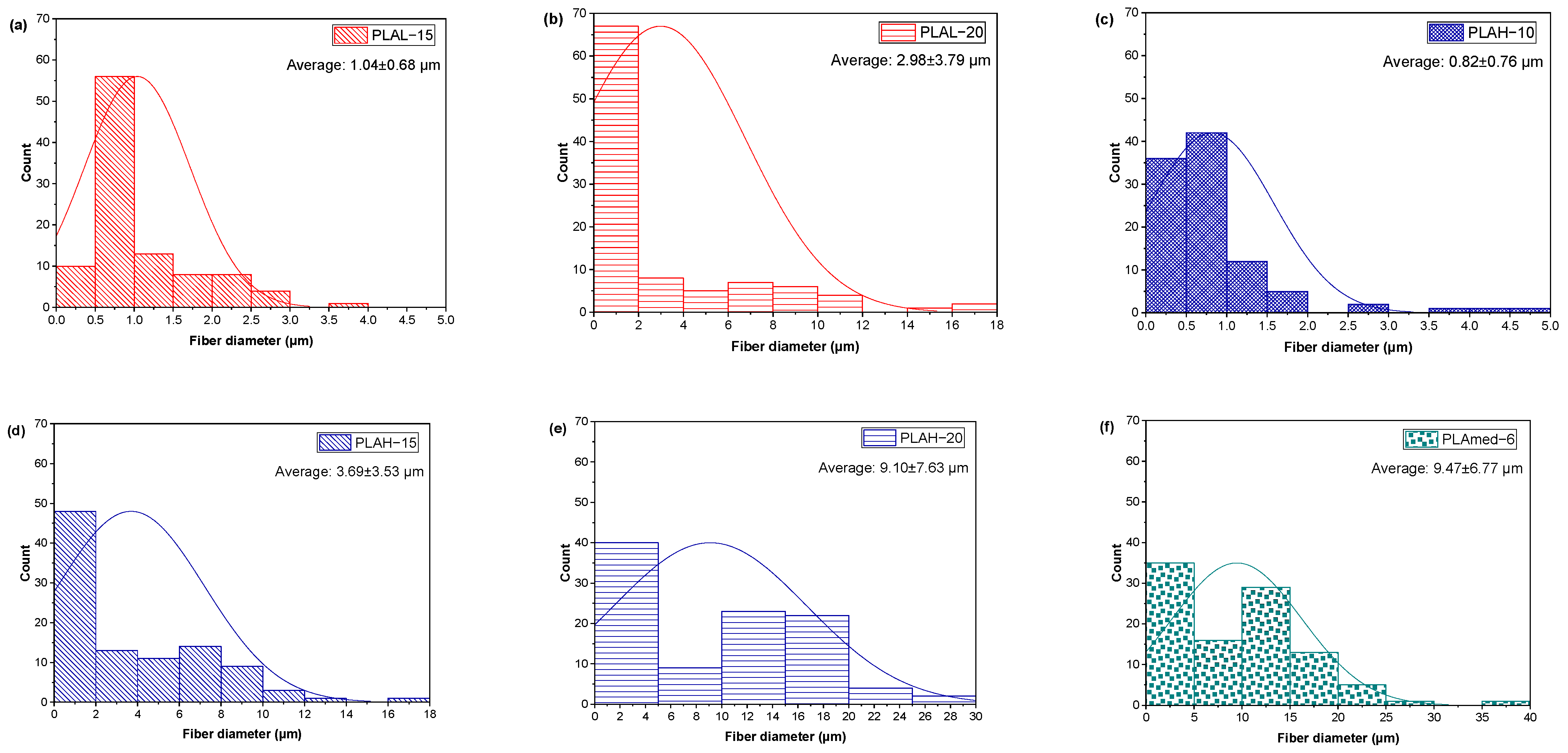
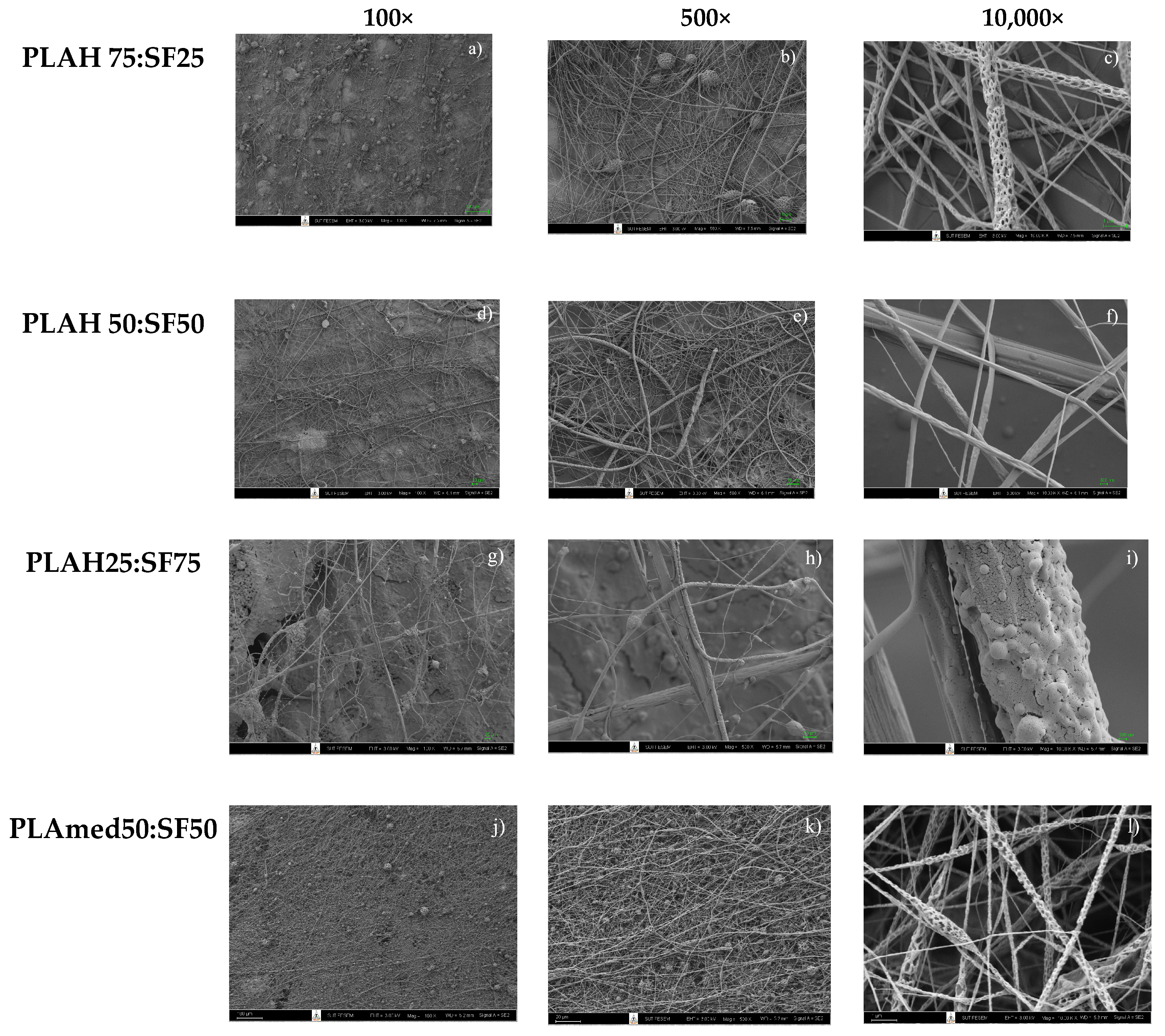
3.2. Fiber Morphology
3.2.1. PLA Fiber Morphology
3.2.2. PLA/SF Fiber Morphology
3.3. FTIR Spectra
3.4. Mechanical Properties
3.5. Thermal Properties
3.6. In Vitro Degradation
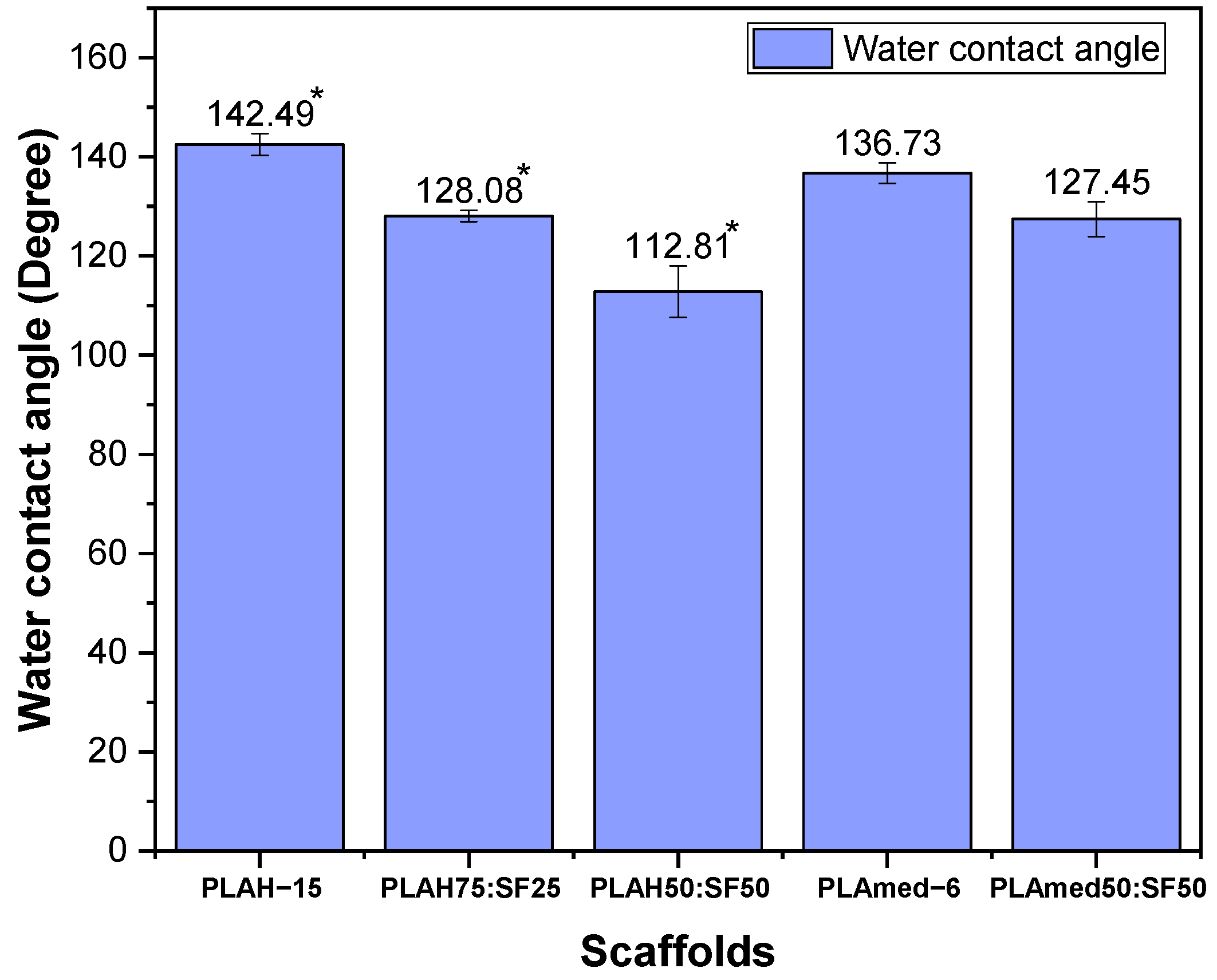
3.7. Surface Wettability
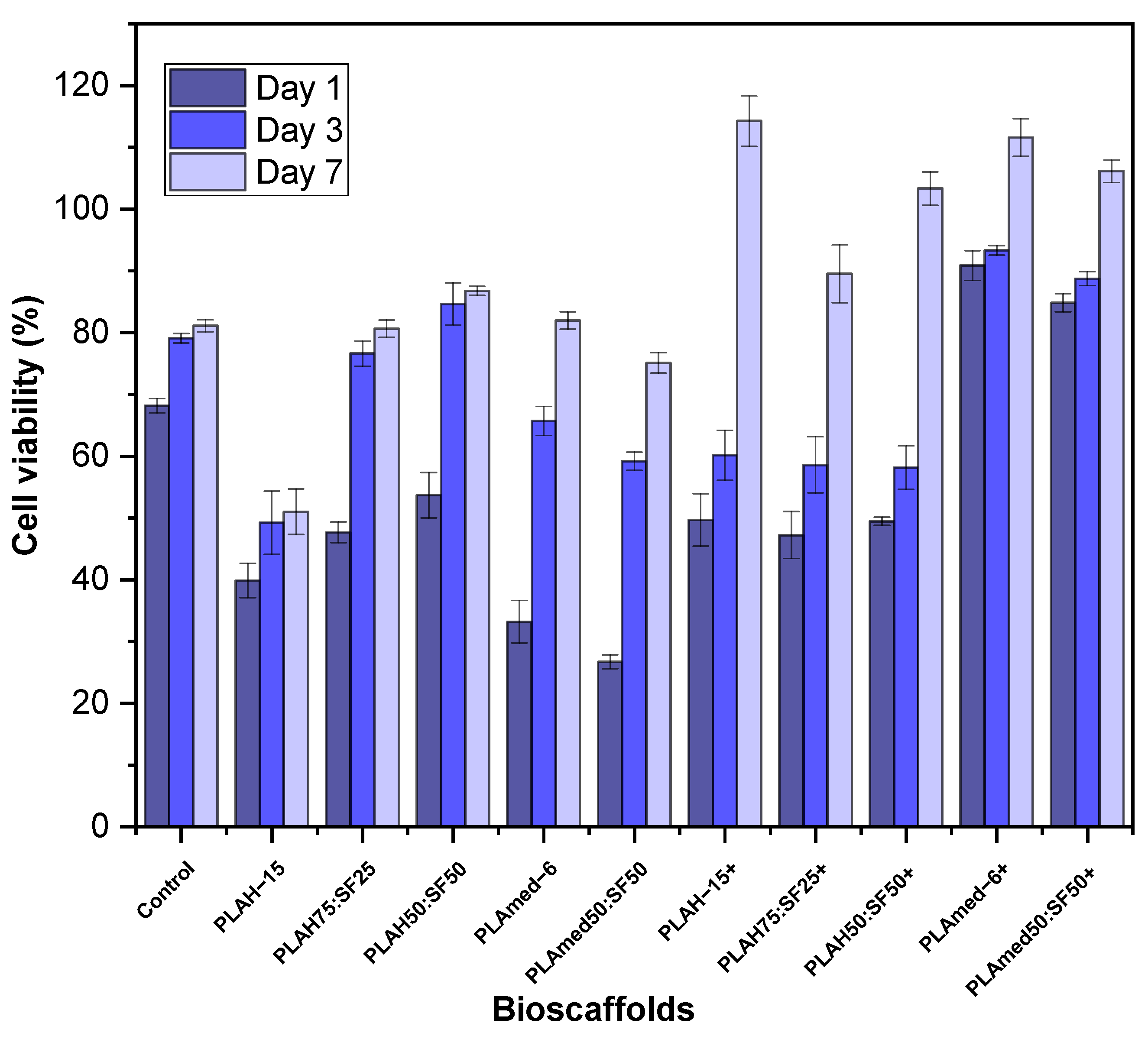
3.8. Cell Viability Test
3.9. Quantitative Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheng, S.-J.; Hu, X.; Wang, F.; Ma, Q.-Y.; Gu, M.-F. Mechanical and thermal property characterization of poly-l-lactide (PLLA) scaffold developed using pressure-controllable green foaming technology. Mater. Sci. Eng. C 2015, 49, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.L.C.; Bahú, J.O.; Xavier, L.F.; Crivellin, S.; de Souza, S.D.A.; Lodi, L.; Jardini, A.L.; Filho, R.M.; Schiavon, M.I.R.B.; Concha, V.O.C.; et al. Lactide: Production Routes, Properties, and Applications. Bioengineering 2022, 9, 164. [Google Scholar] [CrossRef]
- Masutani, K.; Kimura, Y. Chapter 1. PLA Synthesis. From the Monomer to the Polymer. In Poly(lactic acid) Science and Technology: Processing, Properties, Additives and Applications; Royal Society of Chemistry: London, UK, 2014; pp. 1–36. [Google Scholar] [CrossRef]
- Sastri, V.R. 2-Regulations for Medical Devices and Application to Plastics Suppliers: History and Overview. In Plastics in Medical Devices, 2nd ed.; Sastri, V.R., Ed.; William Andrew Publishing: Oxford, UK, 2014; pp. 9–18. [Google Scholar] [CrossRef]
- Liu, W.; Li, Z.; Zheng, L.; Zhang, X.; Liu, P.; Yang, T.; Han, B. Electrospun fibrous silk fibroin/poly(L-lactic acid) scaffold for cartilage tissue engineering. Tissue Eng. Regen. Med. 2016, 13, 516–526. [Google Scholar] [CrossRef]
- Asakura, T.; Suzuki, Y. Silk Fibroin; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–7. [Google Scholar] [CrossRef]
- Möller, M.; Popescu, C. 10.16-Natural Fibers. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 267–280. [Google Scholar] [CrossRef]
- Lee, H.; Jang, C.H.; Kim, G.H. A polycaprolactone/silk-fibroin nanofibrous composite combined with human umbilical cord serum for subacute tympanic membrane perforation; an in vitro and in vivo study. J. Mater. Chem. B 2014, 2, 2703–2713. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Li, Y.; Li, Y.; Ma, Q.; Zhang, J.; Hu, X. Tunable Biodegradable Polylactide–Silk Fibroin Scaffolds Fabricated by a Solvent-Free Pressure-Controllable Foaming Technology. ACS Appl. Bio Mater. 2020, 3, 8795–8807. [Google Scholar] [CrossRef]
- Yin, G.-B.; Zhang, Y.-Z.; Wang, S.-D.; Shi, D.-B.; Dong, Z.-H.; Fu, W.-G. Study of the electrospun PLA/silk fibroin-gelatin composite nanofibrous scaffold for tissue engineering. J. Biomed. Mater. Res. A 2010, 93, 158–163. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Wang, H.; Yin, G.; Dong, Z. Fabrication and Properties of the Electrospun Polylactide/Silk Fibroin-Gelatin Composite Tubular Scaffold. Biomacromolecules 2009, 10, 2240–2244. [Google Scholar] [CrossRef]
- Wang, F.; Wu, H.; Venkataraman, V.; Hu, X. Silk fibroin-poly(lactic acid) biocomposites: Effect of protein-synthetic polymer interactions and miscibility on material properties and biological responses. Mater. Sci. Eng. C 2019, 104, 109890. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheung, H.-Y.; Lau, K.T.; Xu, C.-L.; Zhao, D.-D.; Li, H.-L. Silkworm silk/poly(lactic acid) biocomposites: Dynamic mechanical, thermal and biodegradable properties. Polym. Degrad. Stab. 2010, 95, 1978–1987. [Google Scholar] [CrossRef]
- Sari, A.; Gunaydin, B.; Dinçel, Y. Meniscus Tears and Review of the Literature; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Vijayavenkataraman, S.; Liu, H. An Overview of Scaffold Design and Fabrication Technology for Engineered Knee Meniscus. Materials 2017, 10, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Caro, F.; Perdisa, F.; Dhollander, A.; Verdonk, R.; Verdonk, P. Meniscus Scaffolds for Partial Meniscus Defects. Clin. Sports Med. 2020, 39, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, D.; Stein, S.; Haffner-Luntzer, M.; de Roy, L.; Skaer, N.; Walker, R.; Kessler, O.; Ignatius, A.; Dürselen, L. Biomechanical, structural and biological characterisation of a new silk fibroin scaffold for meniscal repair. J. Mech. Behav. Biomed. Mater. 2018, 86, 314–324. [Google Scholar] [CrossRef]
- Kalva, S.N.; Augustine, R.; Al Mamun, A.; Dalvi, Y.B.; Vijay, N.; Hasan, A. Active agents loaded extracellular matrix mimetic electrospun membranes for wound healing applications. J. Drug Deliv. Sci. Technol. 2021, 63, 102500. [Google Scholar] [CrossRef]
- Friné, V.-C.; Hector, A.-P.; Manuel, N.-D.S.; Estrella, N.-D.; Antonio, G.J. Development and Characterization of a Biodegradable PLA Food Packaging Hold Monoterpene-Cyclodextrin Complexes against Alternaria alternata. Polymers 2019, 11, 1720. [Google Scholar] [CrossRef] [Green Version]
- Gil-Castell, O.; Badia, J.D.; Ingles-Mascaros, S.; Teruel-Juanes, R.; Serra, A.; Ribes-Greus, A. Polylactide-based self-reinforced composites biodegradation: Individual and combined influence of temperature, water and compost. Polym. Degrad. Stab. 2018, 158, 40–51. [Google Scholar] [CrossRef]
- Jazrawi, B.; Noroozi, N.; Ansari, M.; Hatzikiriakos, S. Processing aids for biodegradable polymers. J. Appl. Polym. Sci. 2013, 128, 3592–3600. [Google Scholar] [CrossRef]
- Ortenzi, M.A.; Gazzotti, S.; Marcos, B.; Antenucci, S.; Camazzola, S.; Piergiovanni, L.; Farina, H.; Di Silvestro, G.; Verotta, L. Synthesis of Polylactic Acid Initiated through Biobased Antioxidants: Towards Intrinsically Active Food Packaging. Polymers 2020, 12, 1183. [Google Scholar] [CrossRef]
- Meng, X.; Nguyen, N.A.; Tekinalp, H.; Lara-Curzio, E.; Ozcan, S. Supertough PLA-Silane Nanohybrids by in Situ Condensation and Grafting. ACS Sustain. Chem. Eng. 2018, 6, 1289–1298. [Google Scholar] [CrossRef]
- Torino, E.; Aruta, R.; Sibillano, T.; Giannini, C.; Netti, P.A. Synthesis of semicrystalline nanocapsular structures obtained by Thermally Induced Phase Separation in nanoconfinement. Sci. Rep. 2016, 6, 32727. [Google Scholar] [CrossRef] [PubMed]
- Baydemir, T. Investigations on the Properties and Drug Releases of Biodegradable Polymer Coatings on Metal Substrates as Drug Carriers. Ph.D. Thesis, Middle East Technical University, Ankara, Turkey, 2009. [Google Scholar]
- Nguyen, T.C.; Ruksakulpiwat, C.; Ruksakulpiwat, Y. The Study on the Grafting of Glycidyl Methacrylate onto Poly(lactic acid) in an Internal Mixer. Walailak J. Sci. Technol. (WJST) 2016, 13, 1037–1046. [Google Scholar]
- Nagarajan, V.; Zhang, K.; Misra, M.; Mohanty, A.K. Overcoming the Fundamental Challenges in Improving the Impact Strength and Crystallinity of PLA Biocomposites: Influence of Nucleating Agent and Mold Temperature. ACS Appl. Mater. Interfaces 2015, 7, 11203–11214. [Google Scholar] [CrossRef] [PubMed]
- Wongnarat, C.; Srihanam, P. Degradation Behaviors of Thai Bombyx mori Silk Fibroins Exposure to Protease Enzymes. Engineering 2013, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Promnil, S.; Ruksakulpiwat, Y.; Ruksakulpiwat, C.; Numpaisal, P.-O. Effect of Silk Fibroin Content on Physical and Mechanical Properties of Electrospun Poly(lactic acid)/Silk Fibroin Nanofibers for Meniscus Tissue Engineering Scaffold. J. Phys. Conf. Ser. 2022, 2175, 012016. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Qian, Y.; Li, X.; Singh, G.K.; Zhong, L.; Liu, W.; Lv, Y.; Cai, K.; Yang, L. Electrospun poly (ε-caprolactone)/silk fibroin core-sheath nanofibers and their potential applications in tissue engineering and drug release. Int. J. Biol. Macromol. 2011, 49, 223–232. [Google Scholar] [CrossRef]
- Kim, G.-T.; Lee, J.-S.; Shin, J.-H.; Ahn, Y.-C.; Hwang, Y.-J.; Shin, H.-S.; Lee, J.-K.; Sung, C.-M. Investigation of pore formation for polystyrene electrospun fiber: Effect of relative humidity. Korean J. Chem. Eng. 2005, 22, 783–788. [Google Scholar] [CrossRef]
- Udayakumar, M.; Kollár, M.; Kristály, F.; Leskó, M.; Szabó, T.; Marossy, K.; Tasnádi, I.; Németh, Z. Temperature and Time Dependence of the Solvent-Induced Crystallization of Poly(l-lactide). Polymers 2020, 12, 1065. [Google Scholar] [CrossRef]
- Rissanen, M.; Puolakka, A.; Nousiainen, P.; Kellomäki, M.; Ellä, V. Solubility and Phase Separation of Poly(l,d-Lactide) Copolymers. J. Appl. Polym. Sci. 2008, 110, 2399–2404. [Google Scholar] [CrossRef]
- Burg, K. Chapter 6-Poly(α-ester)s. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Oxford, UK, 2014; pp. 115–121. [Google Scholar] [CrossRef]
- Puchalski, M.; Kwolek, S.; Szparaga, G.; Chrzanowski, M.; Krucińska, I. Investigation of the Influence of PLA Molecular Structure on the Crystalline Forms (α’ and α) and Mechanical Properties of Wet Spinning Fibres. Polymers 2017, 9, 18. [Google Scholar] [CrossRef]
- Nezarati, R.M.; Eifert, M.B.; Cosgriff-Hernandez, E. Effects of humidity and solution viscosity on electrospun fiber morphology. Tissue Eng. Part C Methods 2013, 19, 810–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, G.R.; Raimi-Abraham, B.T.; Luo, C.J. Electrospinning fundamentals. In Nanofibres in Drug Delivery; UCL Press: London, UK, 2018; pp. 24–59. [Google Scholar] [CrossRef]
- Promnil, S.; Numpaisal, P.-O.; Ruksakulpiwat, Y. Effect of molecular weight on mechanical properties of electrospun poly (lactic acid) fibers for meniscus tissue engineering scaffold. Mater. Today Proc. 2021, 47, 3496–3499. [Google Scholar] [CrossRef]
- Mehta, P.P.; Pawar, V.S. 22-Electrospun nanofiber scaffolds: Technology and applications. In Applications of Nanocomposite Materials in Drug Delivery; Inamuddin, Asiri, A.M., Mohammad, A., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 509–573. [Google Scholar] [CrossRef]
- Roy, T.; Maity, P.P.; Rameshbabu, A.P.; Das, B.; John, A.; Dutta, A.; Ghorai, S.K.; Chattopadhyay, S.; Dhara, S. Core-Shell Nanofibrous Scaffold Based on Polycaprolactone-Silk Fibroin Emulsion Electrospinning for Tissue Engineering Applications. Bioengineering 2018, 5, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taddei, P.; Tozzi, S.; Zuccheri, G.; Martinotti, S.; Ranzato, E.; Chiono, V.; Carmagnola, I.; Tsukada, M. Intermolecular interactions between B. mori silk fibroin and poly(l-lactic acid) in electrospun composite nanofibrous scaffolds. Mater. Sci. Eng. C 2017, 70, 777–787. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qin, Y.; Cui, S.; Gao, Y.; Wang, S. Structure and properties of novel electrospun tussah silk fibroin/poly(lactic acid) composite nanofibers. J. Mater. Sci. 2011, 46, 2938–2946. [Google Scholar] [CrossRef]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The basic science of human knee menisci: Structure, composition, and function. Sports Health 2012, 4, 340–351. [Google Scholar] [CrossRef] [Green Version]
- Thanh Tam, T.; Abdul Hamid, Z.; Cheong, K.Y. A Review of Mechanical Properties of Scaffold in Tissue Engineering: Aloe Vera Composites. J. Phys. Conf. Ser. 2018, 1082, 012080. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.; Karpova, S.; Moskovskiy, M.; Dorokhov, A. Electrospun Polylactide/Natural Rubber Fibers: Effect Natural Rubber Content on Fiber Morphology and Properties. Polymers 2021, 13, 2232. [Google Scholar] [CrossRef]
- Wu, J.; Hong, Y. Enhancing cell infiltration of electrospun fibrous scaffolds in tissue regeneration. Bioact. Mater. 2016, 1, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Maharana, T.; Mohanty, B.; Negi, Y.S. Melt–solid polycondensation of lactic acid and its biodegradability. Prog. Polym. Sci. 2009, 34, 99–124. [Google Scholar] [CrossRef]
- Pölöskei, K.; Csézi, G.; Hajba, S.; Tábi, T. Investigation of the thermoformability of various d-Lactide content poly(lactic acid) films by ball burst test. Polym. Eng. Sci. 2020, 60, 1266–1277. [Google Scholar] [CrossRef] [Green Version]
- Oksiuta, Z.; Jalbrzykowski, M.; Mystkowska, J.; Romanczuk, E.; Osiecki, T. Mechanical and Thermal Properties of Polylactide (PLA) Composites Modified with Mg, Fe, and Polyethylene (PE) Additives. Polymers 2020, 12, 2939. [Google Scholar] [CrossRef] [PubMed]
- Rothrauff, B.B.; Numpaisal, P.-O.; Lauro, B.B.; Alexander, P.G.; Debski, R.E.; Musahl, V.; Tuan, R.S. Augmented repair of radial meniscus tear with biomimetic electrospun scaffold: An in vitro mechanical analysis. J. Exp. Orthop. 2016, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Shimomura, K.; Bean, A.C.; Lin, H.; Nakamura, N.; Tuan, R.S. In Vitro Repair of Meniscal Radial Tear Using Aligned Electrospun Nanofibrous Scaffold. Tissue Eng. Part A 2015, 21, 2066–2075. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Ou-Yang, H.-K.; Shan, Y.-L.; Luo, D.-Z.; Wang, H.; Zhang, K. Tensile biomechanical characteristics of human meniscus. Emerg. Mater. Res. 2016, 5, 44–49. [Google Scholar] [CrossRef]
- McCarron, J.A.; Milks, R.A.; Chen, X.; Iannotti, J.P.; Derwin, K.A. Improved time-zero biomechanical properties using poly-L-lactic acid graft augmentation in a cadaveric rotator cuff repair model. J. Shoulder Elbow Surg. 2010, 19, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Y.; Gough, C.R.; Liu, Q.; Hu, X. Dual-Crystallizable Silk Fibroin/Poly(l-lactic Acid) Biocomposite Films: Effect of Polymer Phases on Protein Structures in Protein-Polymer Blends. Int. J. Mol. Sci. 2021, 22, 1871. [Google Scholar] [CrossRef]
- Holjevac Grgurić, T.; Mijović, B.; Zimić, I.; Dolenec, T.; Kuzmić, S.; Mrkonjić, N.; Tominac-Trcin, M.; Slivac, I.; Zdraveva, E.; Govorcin Bajsic, E. Preparation and Characterization of Electrospun PCL/Silk Fibroin Scaffolds. Chem. Biochem. Eng. Q. 2021, 35, 31–42. [Google Scholar] [CrossRef]
- Yu, W.; Wang, X.; Ferraris, E.; Zhang, J. Melt crystallization of PLA/Talc in fused filament fabrication. Mater. Des. 2019, 182, 108013. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [Green Version]
- Bitar, K.N.; Zakhem, E. Design strategies of biodegradable scaffolds for tissue regeneration. Biomed. Eng. Comput. Biol. 2014, 6, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Leonés, A.; Peponi, L.; Lieblich, M.; Benavente, R.; Fiori, S. In Vitro Degradation of Plasticized PLA Electrospun Fiber Mats: Morphological, Thermal and Crystalline Evolution. Polymers 2020, 12, 2975. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, B.; Li, M.; Zuo, B.; Kaplan, D.L.; Huang, Y.; Zhu, H. Degradation mechanism and control of silk fibroin. Biomacromolecules 2011, 12, 1080–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholipourmalekabadi, M.; Mozafari, M.; Bandehpour, M.; Sameni, M.; Ghanbarian, H. How Ethanol Treatment Affects The Physico-chemical And Biological Characteristics Of Silk Fibroin Nanofibrous Scaffolds. Adv. Mater. Lett. 2015, 6, 391–394. [Google Scholar] [CrossRef]
- Numpaisal, P.-O.; Jiang, C.-C.; Hsieh, C.-H.; Chiang, H.; Chien, C.-L. Prospective Application of Partially Digested Autologous Chondrocyte for Meniscus Tissue Engineering. Pharmaceutics 2022, 14, 605. [Google Scholar] [CrossRef]
- Hirano, N.; Kusuhara, H.; Sueyoshi, Y.; Teramura, T.; Murthy, A.; Asamura, S.; Isogai, N.; Jacquet, R.D.; Landis, W.J. Ethanol treatment of nanoPGA/PCL composite scaffolds enhances human chondrocyte development in the cellular microenvironment of tissue-engineered auricle constructs. PLoS ONE 2021, 16, e0253149. [Google Scholar] [CrossRef]
- D’Amato, A.; Schaub, N.; Cardenas, J.; Franz, E.; Rende, D.; Ziemba, A.; Gilbert, R. Evaluation of procedures to quantify solvent retention in electrospun fibers and facilitate solvent removal. Fibers Polym. 2017, 18, 483–492. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Salvage, R.; Hull, C.M.; Kelly, D.E.; Kelly, S.L. Use of 70% alcohol for the routine removal of microbial hard surface bioburden in life science cleanrooms. Future Microbiol. 2014, 9, 1123–1130. [Google Scholar] [CrossRef]

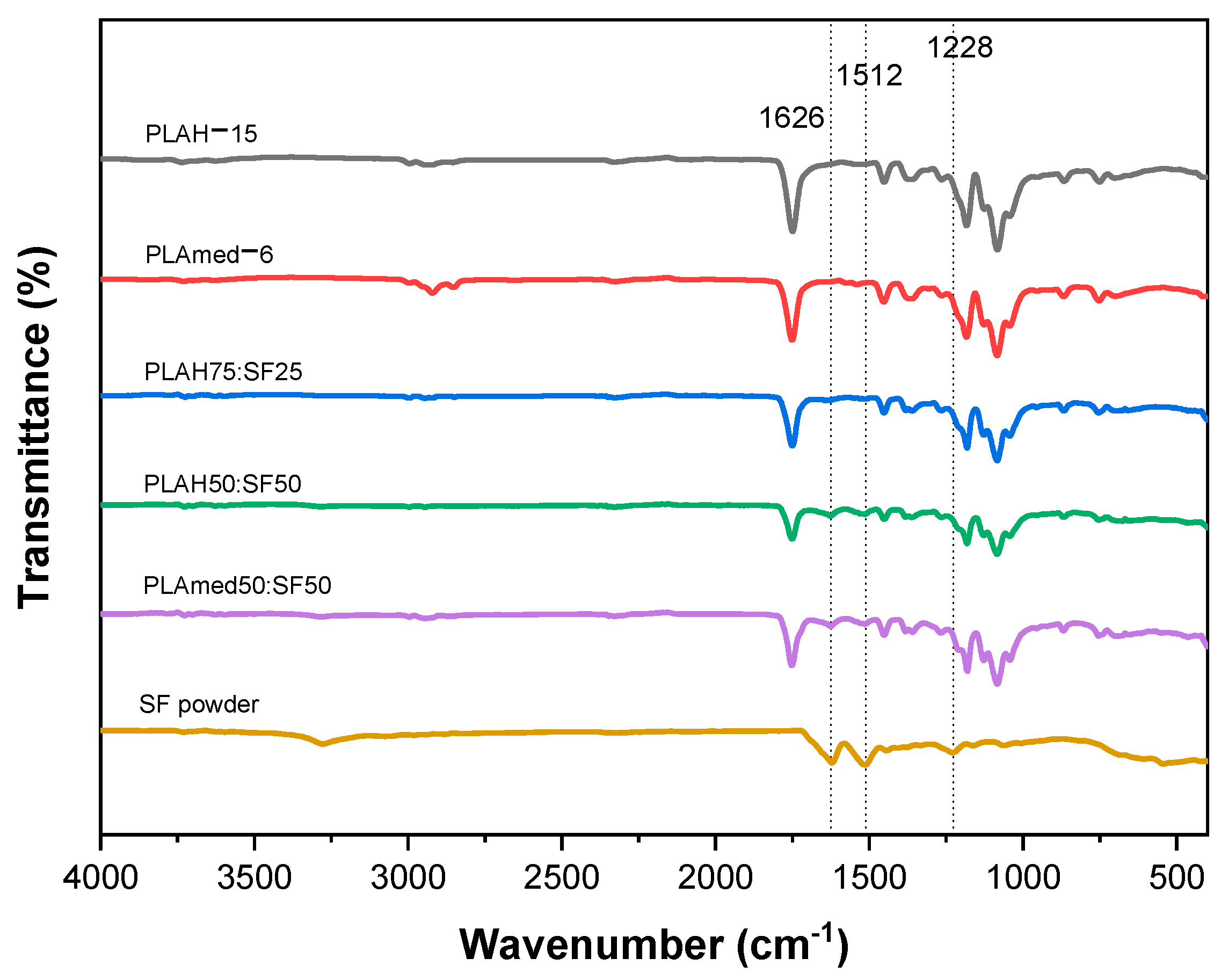
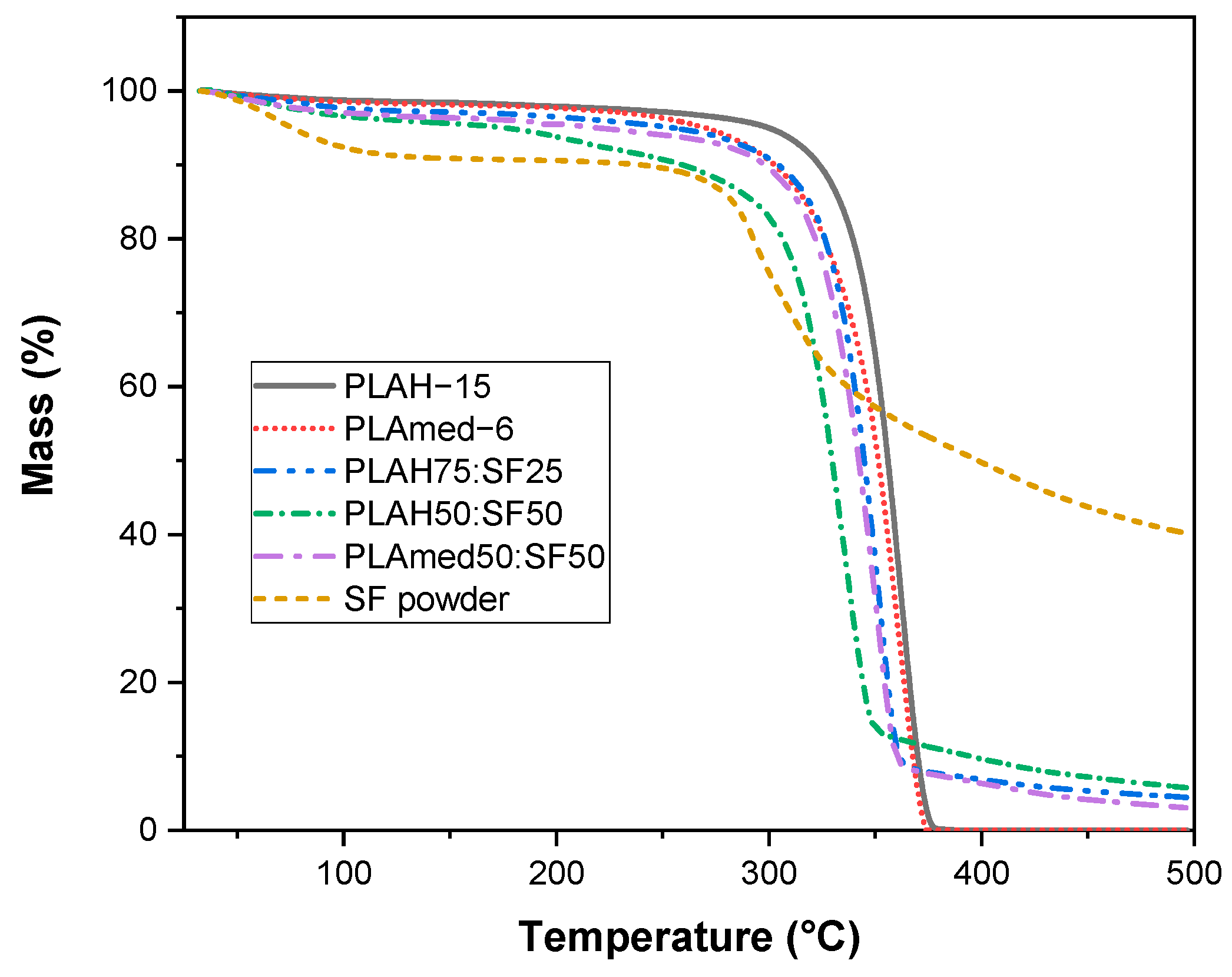
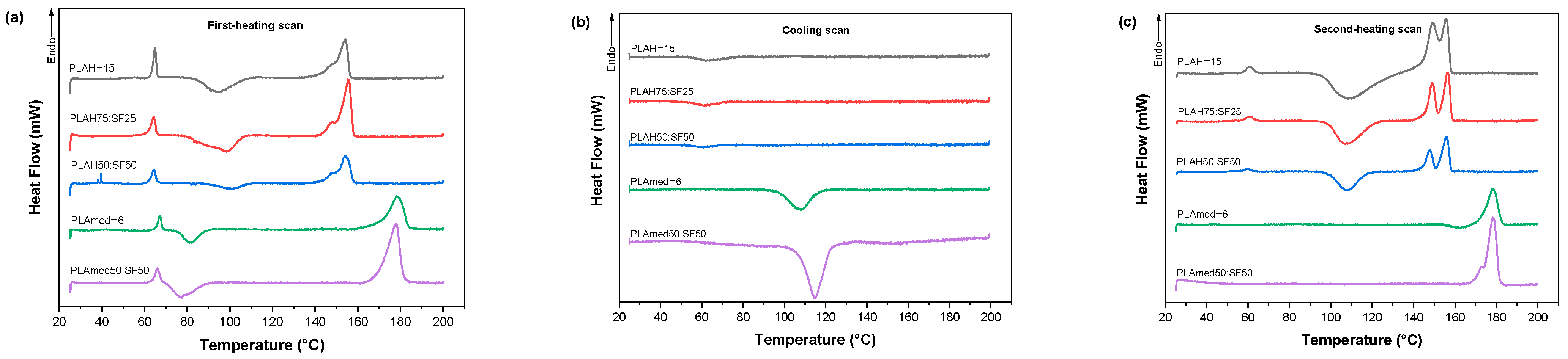
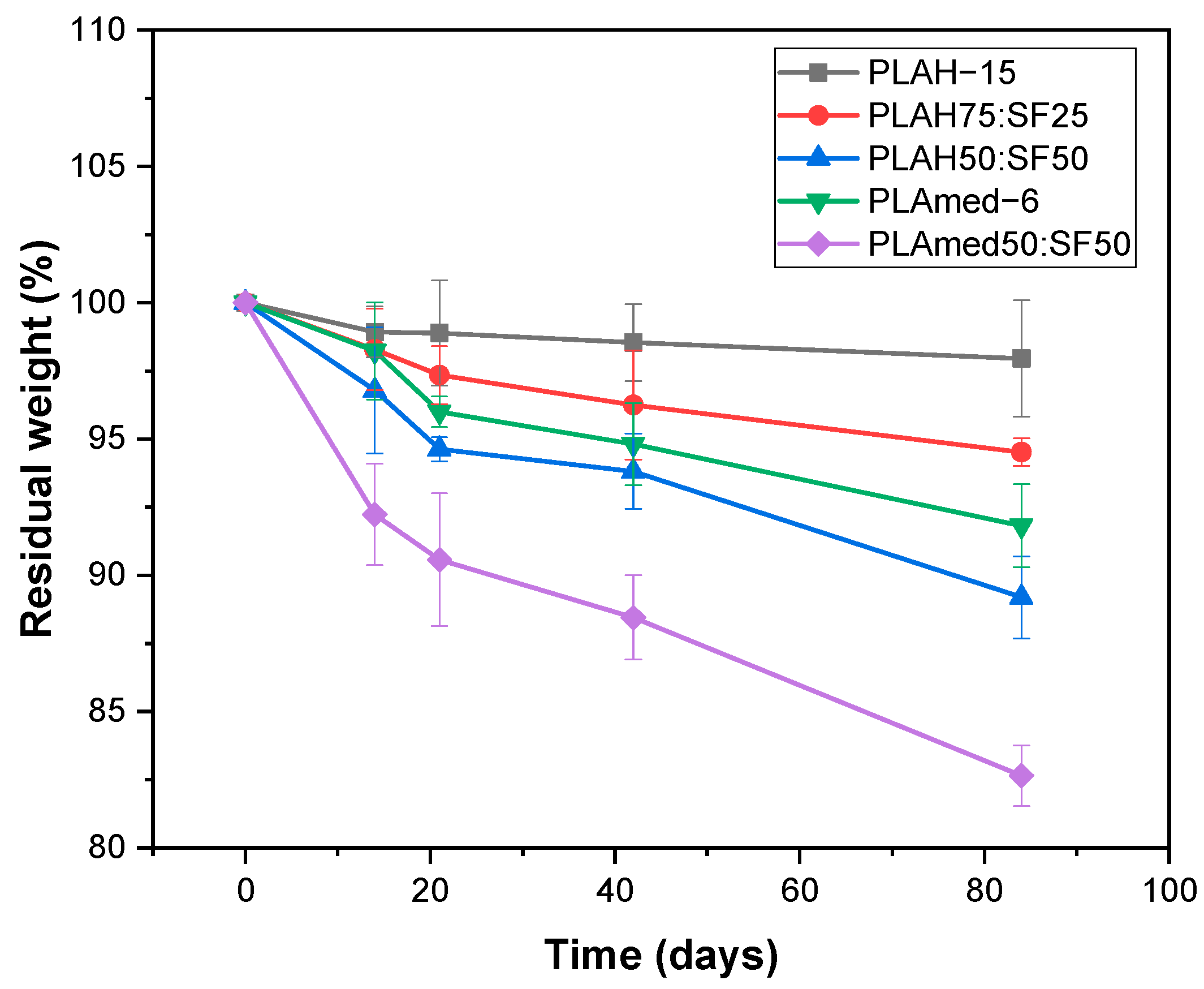

| Sample | Grade | Manufacturer | Molecular Weight (g/mol) | Concentration (% w/v) |
|---|---|---|---|---|
| PLAL−10 | 3251D | NatureWorks LLC | 55,400 [21,23] | 10 |
| PLAL−15 | 3251D | NatureWorks LLC | 55,400 [21,23] | 15 |
| PLAL−20 | 3251D | NatureWorks LLC | 55,400 [21,23] | 20 |
| PLAH−10 | 4043D | NatureWorks LLC | 127,300–147,400 [24,25] | 10 |
| PLAH−15 | 4043D | NatureWorks LLC | 127,300–147,400 [24,25] | 15 |
| PLAH−20 | 4043D | NatureWorks LLC | 127,300–147,400 [24,25] | 20 |
| PLAmed−6 | L209S | Sigma-Aldrich LLC | 177,000 [26,27] | 6 |
| Samples | PLA Concentration (% w/v) | SF Concentration (% w/v) | PLA Ratio (Volume) | SF Ratio (Volume) |
|---|---|---|---|---|
| PLAH75:SF25 | 15 | 12 | 75 | 25 |
| PLAH50:SF50 | 15 | 12 | 50 | 50 |
| PLAH25:SF75 | 15 | 12 | 25 | 75 |
| PLAmed50:SF50 | 15 | 12 | 50 | 50 |
| Genes | Primer Sequence (5′ to 3′) | |
|---|---|---|
| Type I collagen | Sense | GGAGGAGAGTCAGGAAGG |
| (COL1A1) | Antisense | GCAACACAGTTACACAAGG |
| Type II collagen | Sense | GGCAGAGGTATAATGATAAG |
| (COL2A1) | Antisense | ATGTCGTCGCAGAGG |
| 18S rRNA | Sense | ATACCGTCGTAGTTCC |
| Antisense | GTCTCGTTCGTTATCG |
| PLA Solution (w/v) | Viscosity (cP) |
|---|---|
| PLAL−10 | 58.02 ± 0.80 |
| PLAL−15 | 278.68 ± 5.47 |
| PLAL−20 | 690.54 ± 30.18 |
| PLAH−10 | 652.98 ± 36.15 |
| PLAH−15 | 2304.73 ± 74.49 |
| PLAH−20 | 9319.67 ± 91.40 |
| PLAmed−6 | 1024.81 ± 83.15 |
| Samples | Ultimate Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| PLAH−15 | 1.14 ± 0.09 * | 15.97 ± 1.80 * | 70.21 ± 4.99 * |
| PLAH75:SF25 | 0.47 ± 0.07 * | 26.22 ± 10.64 * | 20.51 ± 3.97 * |
| PLAH50:SF50 | 1.05 ± 0.43 * | 17.51 ± 3.89 * | 16.49 ± 8.71 * |
| PLAmed−6 | 2.06 ± 0.28 | 14.34 ± 1.00 | 109.38 ± 12.21 |
| PLAmed50:SF50 | 0.85 ± 0.11 | 12.14 ± 2.20 | 22.59 ± 6.59 |
| Samples | First-Heating Scan | Cooling Scan | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PLAH−15 | 64.90 | 94.22 | 14.81 | 148.05 | 154.07 | 20.53 | 21.94 | 63.68 | - | - |
| PLAH75:SF25 | 64.38 | 98.44 | 13.35 | 147.81 | 155.49 | 25.86 | 21.83 | 62.66 | - | - |
| PLAH50:SF50 | 64.32 | 100.55 | 9.09 | 148.12 | 154.19 | 21.41 | 12.81 | 61.55 | - | - |
| PLAmed−6 | 67.19 | 82.01 | 12.00 | - | 178.61 | 42.61 | 45.52 | - | 107.91 | 19.01 |
| PLAmed50:SF50 | 66.03 | 77.42 | 8.56 | - | 177.82 | 42.71 | 25.55 | - | 114.84 | 30.52 |
| Samples | Second-Heating Scan | ||||||
|---|---|---|---|---|---|---|---|
| Tg (°C) | Tcc (°C) | ||||||
| PLAH−15% | 59.28 | 108.78 | 14.48 | 149.42 | 155.76 | 21.53 | 23.00 |
| PLAH75:SF25 | 58.55 | 107.67 | 24.21 | 149.03 | 156.40 | 25.71 | 21.70 |
| PLAH50:SF50 | 57.41 | 107.79 | 21.58 | 147.85 | 155.92 | 23.71 | 14.19 |
| PLAmed−6% | 67.57 | - | - | - | 178.33 | 39.97 | 42.71 |
| PLAmed50:SF50 | 63.94 | - | - | 172.50 | 178.41 | 41.04 | 24.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Promnil, S.; Ruksakulpiwat, C.; Numpaisal, P.-o.; Ruksakulpiwat, Y. Electrospun Poly(lactic acid) and Silk Fibroin Based Nanofibrous Scaffold for Meniscus Tissue Engineering. Polymers 2022, 14, 2435. https://doi.org/10.3390/polym14122435
Promnil S, Ruksakulpiwat C, Numpaisal P-o, Ruksakulpiwat Y. Electrospun Poly(lactic acid) and Silk Fibroin Based Nanofibrous Scaffold for Meniscus Tissue Engineering. Polymers. 2022; 14(12):2435. https://doi.org/10.3390/polym14122435
Chicago/Turabian StylePromnil, Siripanyo, Chaiwat Ruksakulpiwat, Piya-on Numpaisal, and Yupaporn Ruksakulpiwat. 2022. "Electrospun Poly(lactic acid) and Silk Fibroin Based Nanofibrous Scaffold for Meniscus Tissue Engineering" Polymers 14, no. 12: 2435. https://doi.org/10.3390/polym14122435
APA StylePromnil, S., Ruksakulpiwat, C., Numpaisal, P.-o., & Ruksakulpiwat, Y. (2022). Electrospun Poly(lactic acid) and Silk Fibroin Based Nanofibrous Scaffold for Meniscus Tissue Engineering. Polymers, 14(12), 2435. https://doi.org/10.3390/polym14122435