Performance and Structure Evaluation of Gln-Lys Isopeptide Bond Crosslinked USYK-SPI Bioplastic Film Derived from Discarded Yak Hair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
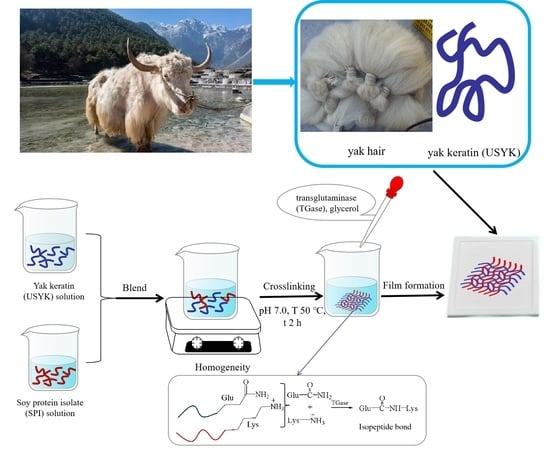
2.2. Extraction of Keratin from Yak Hair (USYK)
2.3. Preparation of the Isopeptide Bond Cross-Linked USYK-SPI Bioplastic Film
2.4. X-ray Diffraction (XRD) Pattern
2.5. Scanning Electron Microscopy (SEM)
2.6. The Thermal Behavior
2.7. The Equilibrium Water Content
2.8. Mechanical Properties
2.9. Water Vapor Permeability (WVP)
2.10. Water Contact Angle (CA)
2.11. Light Transmittance Performance
2.12. Statistical Analysis
3. Results and Discussion
3.1. Crystal Characteristics of the USYK-SPI Bioplastic Film
3.2. Cross-Sectional Micromorphology of the USYK-SPI Bioplastic Film
3.3. Apparent Properties of the USYK-SPI Bioplastic Films
3.4. Mechanical Properties of the USYK—SPI Bioplastic Films
3.5. Water Vapor Permeability of the USYK-SPI Bioplastic Films
3.6. Surface Hydrophobicity of the USYK-SPI Bioplastic Films
3.7. UV Barrier Properties of the USYK-SPI Bioplastic Films
3.8. Thermal Properties of the USYK-SPI Bioplastic Film
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Posati, T.; Giuri, D.; Nocchetti, M.; Sagnella, A.; Gariboldi, M.; Ferroni, C.; Sotgiu, G.; Varchi, G.; Zamboni, R.; Aluigi, A. Keratin-hydrotalcites hybrid films for drug delivery applications. Eur. Polym. J. 2018, 105, 177–185. [Google Scholar] [CrossRef]
- Rani, P.; Yu, X.; Liu, H.; Li, K.; He, Y.; Tian, H.; Kumar, R. Material, Antibacterial and anticancer properties of natural polyphenols incorporated soy protein isolate: A review. Eur. Polym. J. 2021, 152, 110494. [Google Scholar] [CrossRef]
- Song, N.B.; Lee, J.H.; Mijan, M.A.; Song, K.B. Development of a chicken feather protein film containing clove oil and its application in smoked salmon packaging. LWT Food Sci. Technol. 2014, 57, 453–460. [Google Scholar] [CrossRef]
- Reichl, S.; Müller-Goymann, C.C. Keratin film made of human hair as a nail plate model for studying drug permeation. Eur. J. Pharm. Biopharm. 2011, 78, 432–440. [Google Scholar] [CrossRef]
- Gregurec, D.; Wang, G.; Pires, R.H.; Kosutic, M.; Lüdtke, T.; Delcea, M.; Moya, S.E. Bioinspired titanium coatings: Self-assembly of collagen-alginate films for enhanced osseointegration. J. Mater. Chem. B 2016, 4, 1978–1986. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Liu, A.; Wang, W.; Ye, R. Improved mechanical properties and thermal-stability of collagen fiber based film by crosslinking with casein, keratin or SPI: Effect of crosslinking process and concentrations of proteins. Int. J. Biol. Macromol. 2018, 109, 1319–1328. [Google Scholar] [CrossRef]
- Xu, H.; Yang, M.; Hou, X.; Li, W.; Su, X.; Yang, Y. Industrial trial of high-quality all green sizes composed of soy-derived protein and glycerol. J. Cleaner Prod. 2016, 135, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hasanzadeh, M.; Farhadi, K.; Tajik, H. Zein film as a novel natural biopolymer membrane in electrochemical detections. J. Solid State Electrochem. 2021, 25, 1327–1337. [Google Scholar] [CrossRef]
- Chandrasekhar, M.; Prabhakar, M.N.; Song, J. Preparation and Mechanical Properties of Wheat Protein Isolate Films Cross-linked with Resorcinol. Compos. Res. 2015, 28, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Q.; Zhang, G.; Ma, T.; Qian, W.; Wang, J.; Ye, Z.; Cao, C.; Hu, Q.; Kim, J.; Larkin, D.M.; et al. The yak genome and adaptation to life at high altitude. Nat. Genet. 2012, 44, 946–949. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Wei, L.; Xiao, Y.; Bi, H.; Yang, H.; Du, Y. Physicochemical and functional properties of gelatin extracted from Yak skin. Int. J. Biol. Macromol. 2017, 95, 1246–1253. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, J.; Liu, M.; Yan, J.; Zhao, H.; Zheng, L. CO2 Utilization for the dyeing of yak hair: Fracture behaviour in supercritical state. J. CO2 Util. 2017, 18, 117–124. [Google Scholar] [CrossRef]
- Liu, H.L.; Zhao, B.Y.; Yu, W.D. Structural changes in slenderized yak hair induced by heat–humidity conditions using Raman spectroscopy. J. Mol. Struct. 2013, 1037, 57–62. [Google Scholar] [CrossRef]
- Fortunati, E.; Aluigi, A.; Armentano, I.; Morena, F.; Emiliani, C.; Martino, S.; Santulli, C.; Torre, L.; Kenny, J.M.; Puglia, D. Keratins extracted from Merino wool and Brown Alpaca fibres: Thermal, mechanical and biological properties of PLLA based biocomposites. Mat. Sci. Eng. C-Mater. 2015, 47, 394–406. [Google Scholar] [CrossRef]
- Sadeghi, S.; Nourmohammadi, J.; Ghaee, A.; Soleimani, N. Carboxymethyl cellulose-human hair keratin hydrogel with controlled clindamycin release as antibacterial wound dressing. Int. J. Biol. Macromol. 2020, 147, 1239–1247. [Google Scholar] [CrossRef]
- Lazarus, B.S.; Chadha, C.; Velasco-Hogan, A.; Barbosa, J.D.V.; Jasiuk, I.; Meyers, M.A. Engineering with keratin: A functional material and a source of bioinspiration. iScience 2021, 24, 102798–102846. [Google Scholar] [CrossRef]
- Rajabi, M.; Ali, A.; Mcconnell, M.; Cabral, J. Keratinous materials: Structures and functions in biomedical applications. Mat. Sci. Eng. C 2020, 110, 110612–110634. [Google Scholar] [CrossRef]
- Gaidau, C.; Epure, D.G.; Enascuta, C.E.; Carsote, C.; Sendrea, C.; Proietti, N.; Chen, W.; Gu, H. Wool keratin total solubilisation for recovery and reintegration-an ecological approach. J. Clean. Prod. 2019, 236, 117586–117598. [Google Scholar] [CrossRef]
- Bertini, F.; Canetti, M.; Patroccu, A.; Zoccola, M. Wool keratin-polypropylene composites: Properties and thermal degradation. Polym. Degrad. Stab. 2013, 98, 980–987. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Li, J.; Zhao, Z.; Liu, X.; Li, Z.; Han, Y.; Hu, J.; Chen, A. Isolation and characterization of biofunctional keratin particles extracted from wool wastes. Powder Technol. 2013, 246, 356–362. [Google Scholar] [CrossRef]
- Bragulla, H.H.; Homberger, D.G. Structure and functions of keratin proteins in simple, stratifified, keratinized and cornifified epithelia. J. Anat. 2009, 214, 516–559. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Ham, T.R.; Haque, S.; Sparks, J.L.; Saul, J.M. Alkylation of human hair keratin for tunable hydrogel erosion and drug delivery in tissue engineering applications. Acta Biomater. 2015, 23, 201–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shavandi, A.; Silva, T.H.; Bekhit, A.A.; Bekhit, A.E.D.A. Keratin: Dissolution, extraction and biomedical application. Biomater. Sci. 2017, 5, 1699–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramya, K.R.; Thangam, R.; Madhan, B. Comparative analysis of the chemical treatments used in keratin extraction from red sheep’s hair and the cell viability evaluations of this keratin for tissue engineering applications. Process Biochem. 2020, 90, 223–232. [Google Scholar] [CrossRef]
- Fagbemi, O.D.; Sithole, B.; Tesfaye, T. Optimization of keratin protein extraction from waste chicken feathers using hybrid pre-treatment techniques. Sustain. Chem. Pharm. 2020, 17, 100267–100279. [Google Scholar] [CrossRef]
- Ranjit, E.; Hamlet, S.; George, R.; Sharma, A.; Love, R.M. Biofunctional approaches of wool-based keratin for tissue engineering. J. Sci.-Adv. Mater. Dev. 2022, 7, 100398–100410. [Google Scholar] [CrossRef]
- Jus, S.; Stachel, I.; Schloegl, W.; Pretzler, M.; Friess, W.; Meyer, M.; Birner-Gruenberger, R.; Guebitz, G.M. Cross-linking of collagen with laccases and tyrosinases. Mat. Sci. Eng. C 2011, 31, 1068–1077. [Google Scholar] [CrossRef]
- Stachel, I.; Schwarzenbolz, U.; Henle, T.; Meyer, M. Cross-linking of type I collagen with microbial transglutaminase: Identification of cross-linking sites. Biomacromolecules 2010, 11, 698–705. [Google Scholar] [CrossRef]
- Fan, L.; Wu, H.; Cao, M.; Zhou, X.; Peng, M.; Xie, W.; Liu, S. Enzymatic synthesis of collagen peptide-carboxymethylated chitosan copolymer and its characterization. React. Funct. Polym. 2014, 76, 26–31. [Google Scholar] [CrossRef]
- Zeugolis, D.I.; Panengad, P.P.; Yew, E.S.Y.; Sheppard, C.; Phan, T.T.; Raghunath, M. An in situ and in vitro investigation for the transglutaminase potential in tissue engineering. J. Biomed. Mater. Res. Part A 2009, 92, 1310–1320. [Google Scholar]
- Wang, R.R.; Ban, S.; Zhao, Q. Appreciation of waste yak hair: Preparation and characterisation of keratin from yak hair using urea and sodium sulfide. J. Soc. Leather Technol. Chem. 2020, 104, 283–288. [Google Scholar]
- Barone, J.R.; Arikan, O. Composting and biodegradation of thermally processed feather keratin polymer. Polym. Degrad. Stab. 2007, 92, 859–867. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, J.; Chen, X.; Luo, M.; Liu, H.; Shao, P. Fabrication and characterization of multilayered kafirin/gelatin film with one-way water barrier property. Food Hydrocoll. 2018, 81, 159–168. [Google Scholar] [CrossRef]
- Mi, X.; Xu, H.; Yang, Y. Submicron amino acid particles reinforced 100% keratin biomedical films with enhanced wet properties via interfacial strengthening. Colloids Surf. B 2019, 177, 33–40. [Google Scholar] [CrossRef]
- Ramakrishnan, N.; Sharma, S.; Gupta, A.; Alashwal, B.Y. Keratin based bioplastic film from chicken feathers and its characterization. Int. J. Biol. Macromol. 2018, 111, 352–358. [Google Scholar] [CrossRef]
- Das, A.; Das, A.; Basu, A.; Datta, P.; Gupta, M.; Mukherjee, A. Newer guar gum ester/chicken feather keratin interact films for tissue engineering. Int. J. Biol. Macromol. 2021, 180, 339–354. [Google Scholar] [CrossRef]
- Wongphan, P.; Panrong, T.; Harnkarnsujarit, N. Effect of different modified starches on physical, morphological, thermomechanical, barrier and biodegradation properties of cassava starch and polybutylene adipate terephthalate blend film. Food Packag. Shelf Life 2022, 32, 100844–100855. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Biodegradable poly(butylene adipate-co-terephthalate) and thermoplastic starch-blended TiO2 nanocomposite blown films as functional active packaging of fresh fruit. Polymers 2021, 13, 4192–4207. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Wongphan, P.; Promhuad, K.; Leelaphiwat, P.; Harnkarnsujarit, N. Morphology and permeability of bio-based poly(butylene adipate-co-terephthalate) (PBAT), poly(butylene succinate) (PBS) and linear low-density polyethylene (LLDPE) blend films control shelf-life of packaged bread. Food Control. 2022, 132, 108541–108552. [Google Scholar] [CrossRef]
- Mariniello, L.; Pierro, P.D.; Esposito, C.; Sorrentino, A.; Masi, P.; Porta, R. Preparation and mechanical properties of edible pectin–soy flour films obtained in the absence or presence of transglutaminase. J. Biotechnol. 2003, 102, 191–198. [Google Scholar] [CrossRef]
- Bae, H.J.; Darby, D.O.; Kimmel, R.M.; Park, H.J.; Whiteside, W.S. Effects of transglutaminase-induced cross-linking on properties of fish gelatin–nanoclay composite film. Food Chem. 2009, 114, 180–189. [Google Scholar] [CrossRef]
- Wadaugsorn, K.; Panrong, T.; Wongphan, P.; Harnkarnsujarit, N. Plasticized hydroxypropyl cassava starch blended PBAT for improved clarity blown films: Morphology and properties. Ind. Crop. Prod. 2022, 176, 114311–114320. [Google Scholar] [CrossRef]
- Katekhong, W.; Wongphan, P.; Klinmalai, P.; Harnkarnsujarit, N. Thermoplastic starch blown films functionalized by plasticized nitrite blended with PBAT for superior oxygen barrier and active biodegradable meat packaging. Food Chem. 2022, 374, 131709–131720. [Google Scholar] [CrossRef]
- Leelaphiwat, P.; Pechprankan, C.; Siripho, P.; Bumbudsanpharoke, N.; Harnkarnsujarit, N. Effects of nisin and EDTA on morphology and properties of thermoplastic starch and PBAT biodegradable films for meat packaging. Food Chem. 2022, 369, 130956–130967. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.R.; Wang, H.R.; Yao, Y.J.; Ji, W.L. Soy protein isolate reinforced yak skin collagen edible films for ultraviolet barring function. J. Soc. Leather Technol. Chem. 2019, 103, 173–179. [Google Scholar]
- Zheng, T.; Yu, X.; Pilla, S. Mechanical and moisture sensitivity of fully bio-based dialdehyde carboxymethyl cellulose cross-linked soy protein isolate films. Carbohydr. Polym. 2017, 157, 1333–1340. [Google Scholar] [CrossRef]





| Volume Ratios (SPI/USYK) | 5/1 | 4/1 | 3/1 | 2/1 | 1/1 |
|---|---|---|---|---|---|
| Thickness (mm) | 0.1 ± 0.006 | 0.08 ± 0.007 | 0.09 ± 0.009 | 0.09 ± 0.006 | 0.08 ± 0.009 |
| EWC (%) | 19.24 ± 0.23 | 19.54 ± 0.15 | 19.08 ± 0.16 | 19.52 ± 0.11 | 19.82 ± 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R. Performance and Structure Evaluation of Gln-Lys Isopeptide Bond Crosslinked USYK-SPI Bioplastic Film Derived from Discarded Yak Hair. Polymers 2022, 14, 2471. https://doi.org/10.3390/polym14122471
Wang R. Performance and Structure Evaluation of Gln-Lys Isopeptide Bond Crosslinked USYK-SPI Bioplastic Film Derived from Discarded Yak Hair. Polymers. 2022; 14(12):2471. https://doi.org/10.3390/polym14122471
Chicago/Turabian StyleWang, Ruirui. 2022. "Performance and Structure Evaluation of Gln-Lys Isopeptide Bond Crosslinked USYK-SPI Bioplastic Film Derived from Discarded Yak Hair" Polymers 14, no. 12: 2471. https://doi.org/10.3390/polym14122471
APA StyleWang, R. (2022). Performance and Structure Evaluation of Gln-Lys Isopeptide Bond Crosslinked USYK-SPI Bioplastic Film Derived from Discarded Yak Hair. Polymers, 14(12), 2471. https://doi.org/10.3390/polym14122471