Anionic Dye Removal Using a Date Palm Seed-Derived Activated Carbon/Chitosan Polymer Microbead Biocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Activated Carbon (AC)
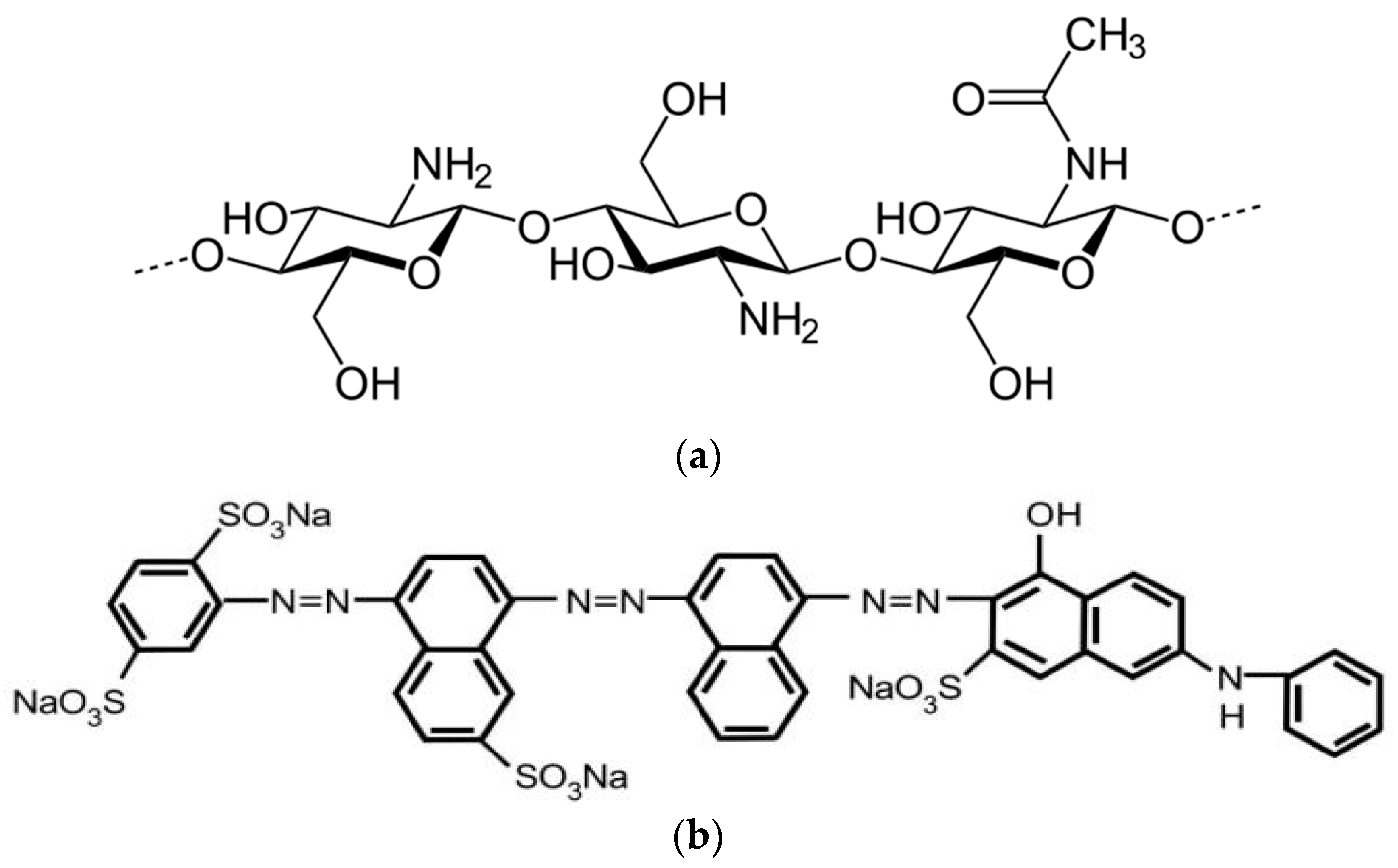
2.2. Chitosan and Dye
2.3. Preparation of Activated Carbon/Chitosan Microbeads (ACMB)
2.4. Adsorption Studies
2.5. Characterization of Materials
3. Results and Discussion
3.1. Characterization of Adsorbents
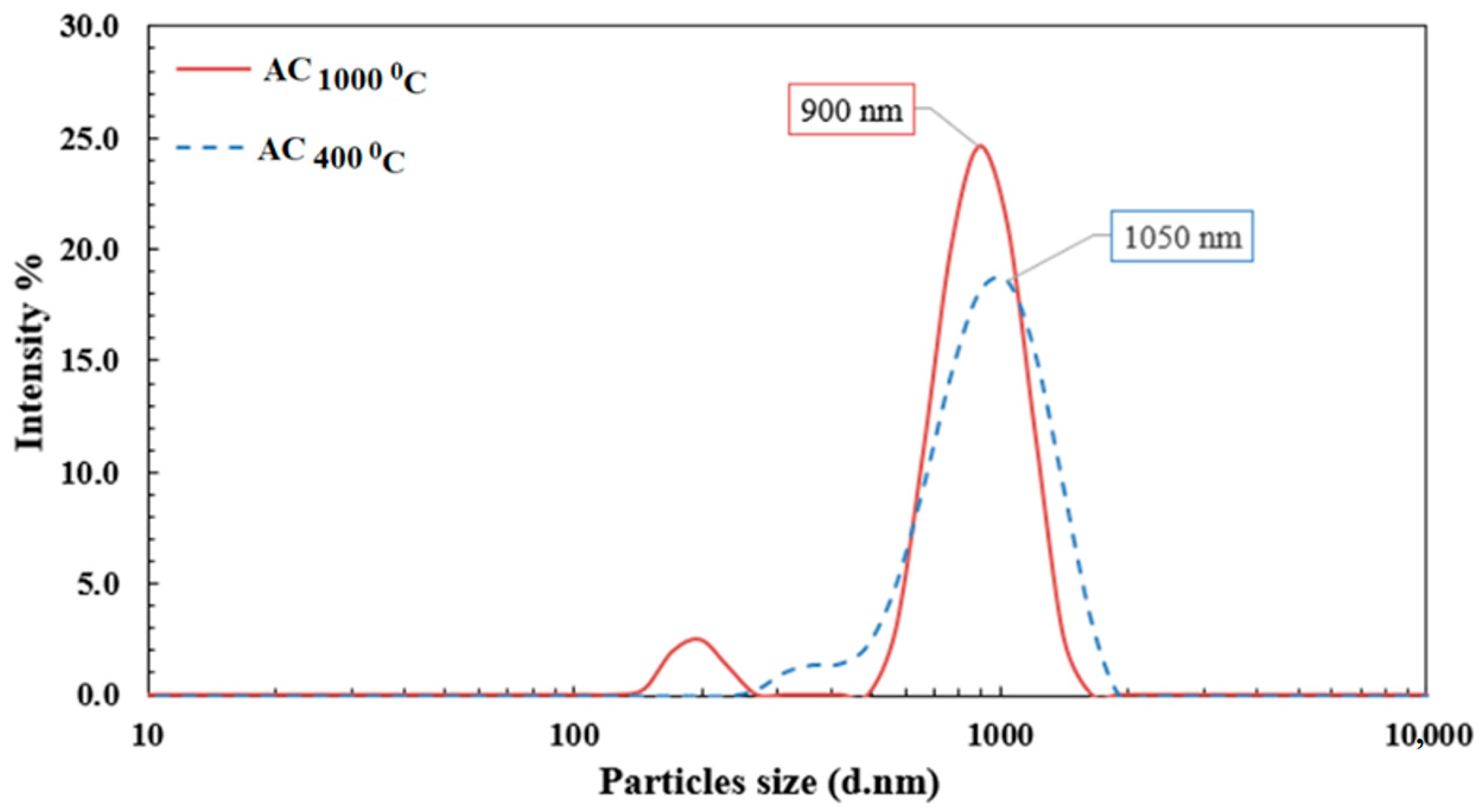
3.1.1. Zeta Sizer Analysis
3.1.2. Zeta Potential Analysis
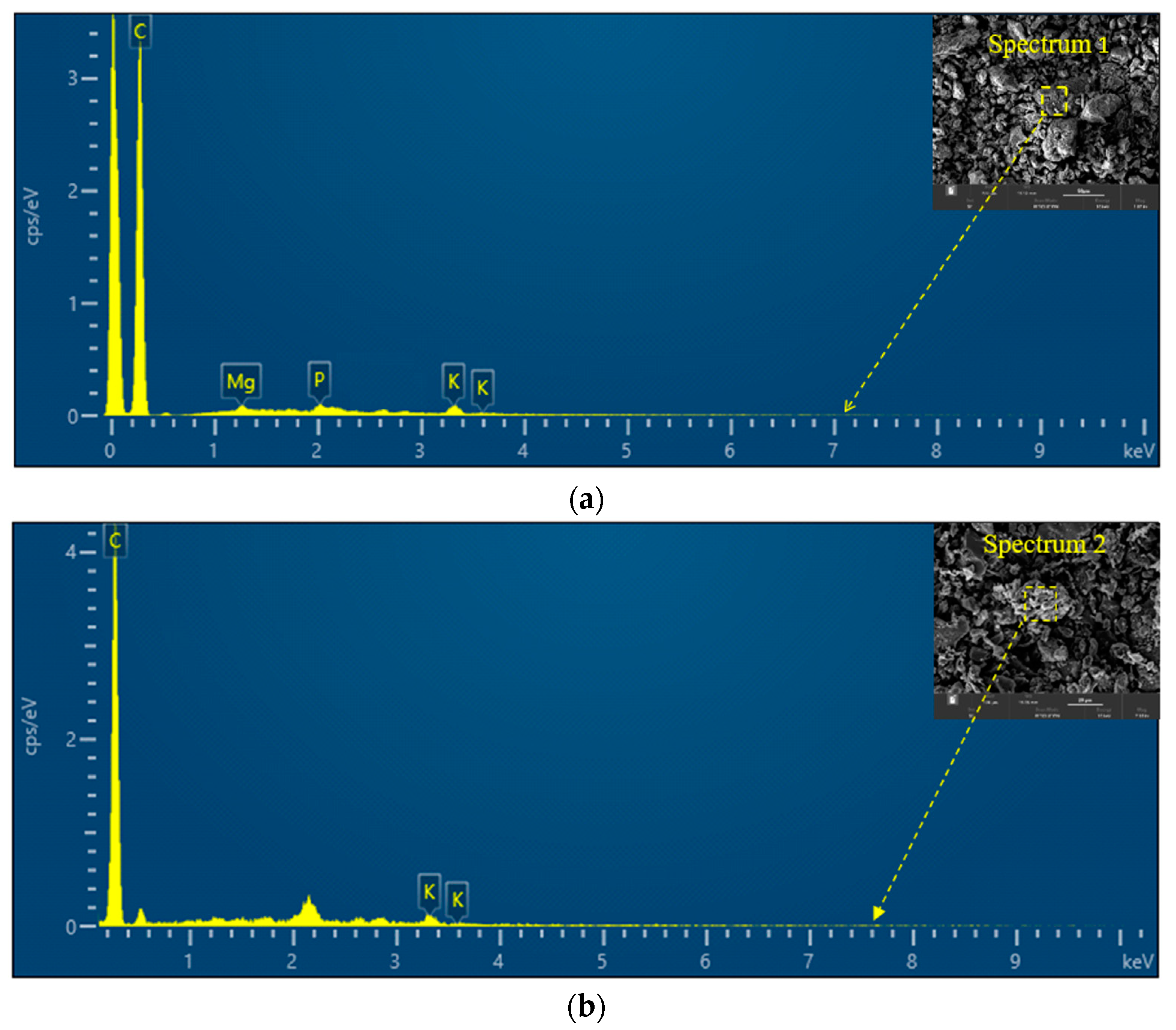
3.1.3. Energy-Dispersive X-ray (EDX) Analysis
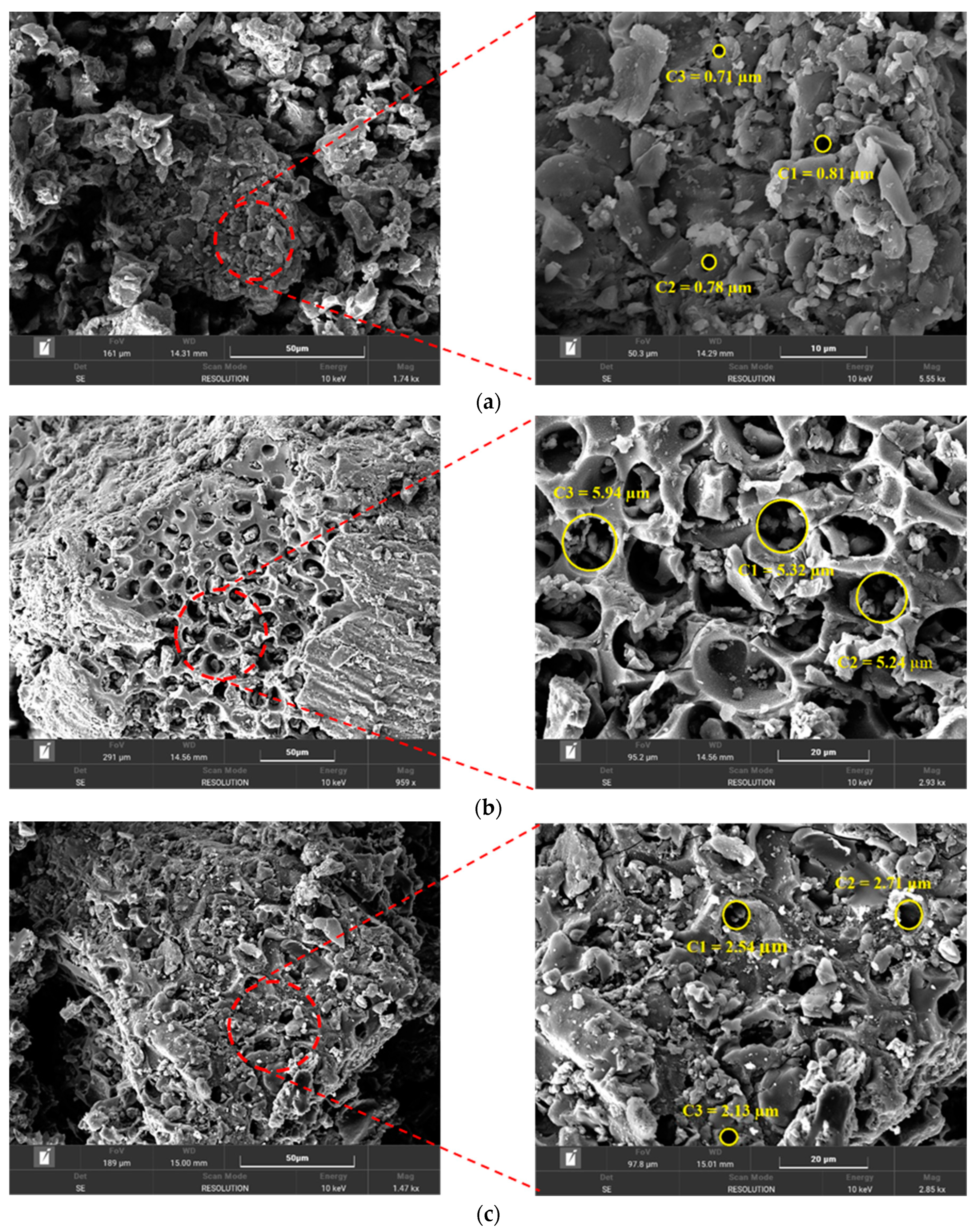
3.1.4. Scanning Electron Microscopy (SEM) Analysis
3.1.5. FTIR Analysis
3.1.6. Surface Area
3.2. Adsorption Tests
3.2.1. Effect of Microbead Dose on DB78-Dye-Removal Efficiency
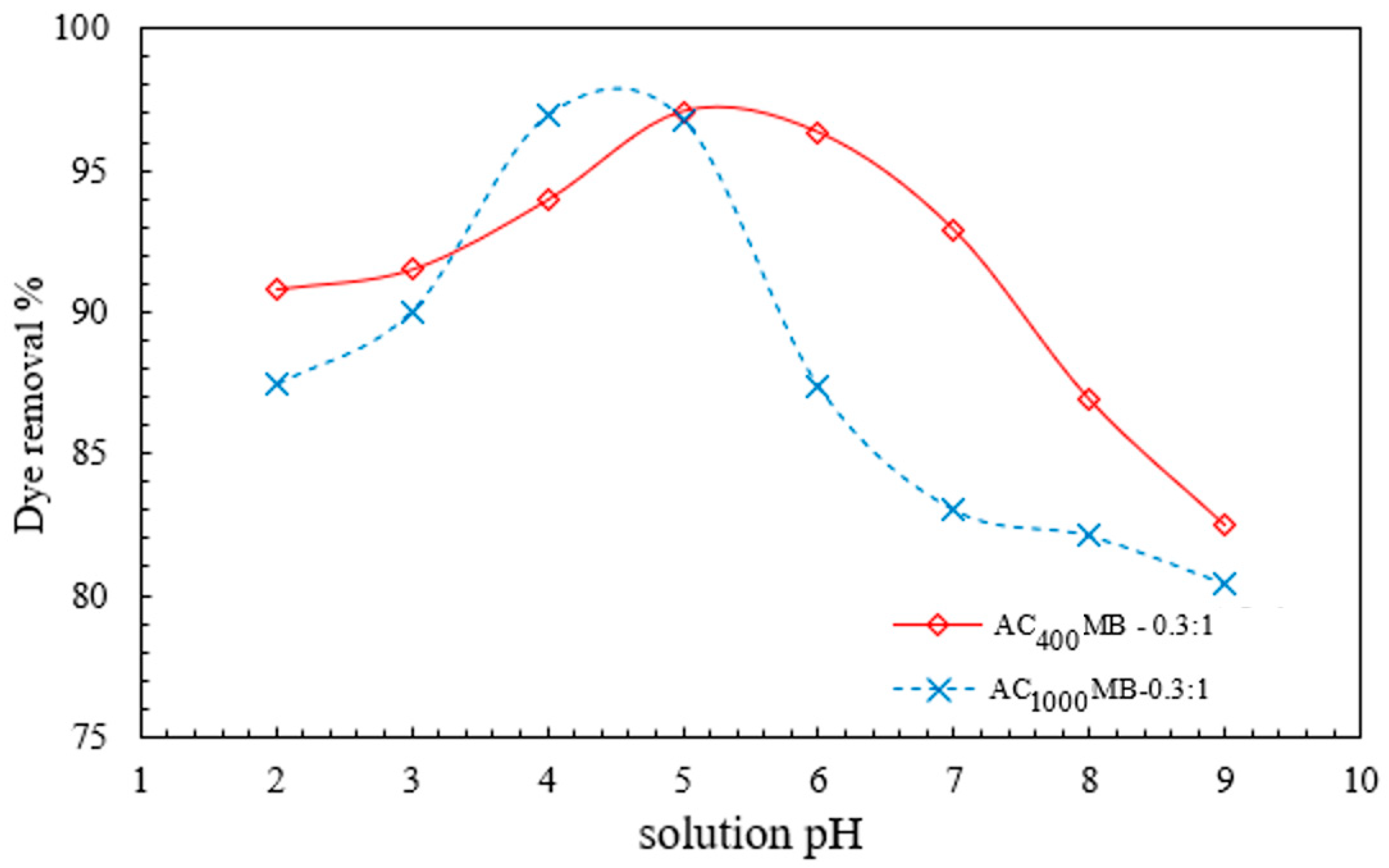
3.2.2. Effect of pH on DB78-Dye-Removal Efficiency
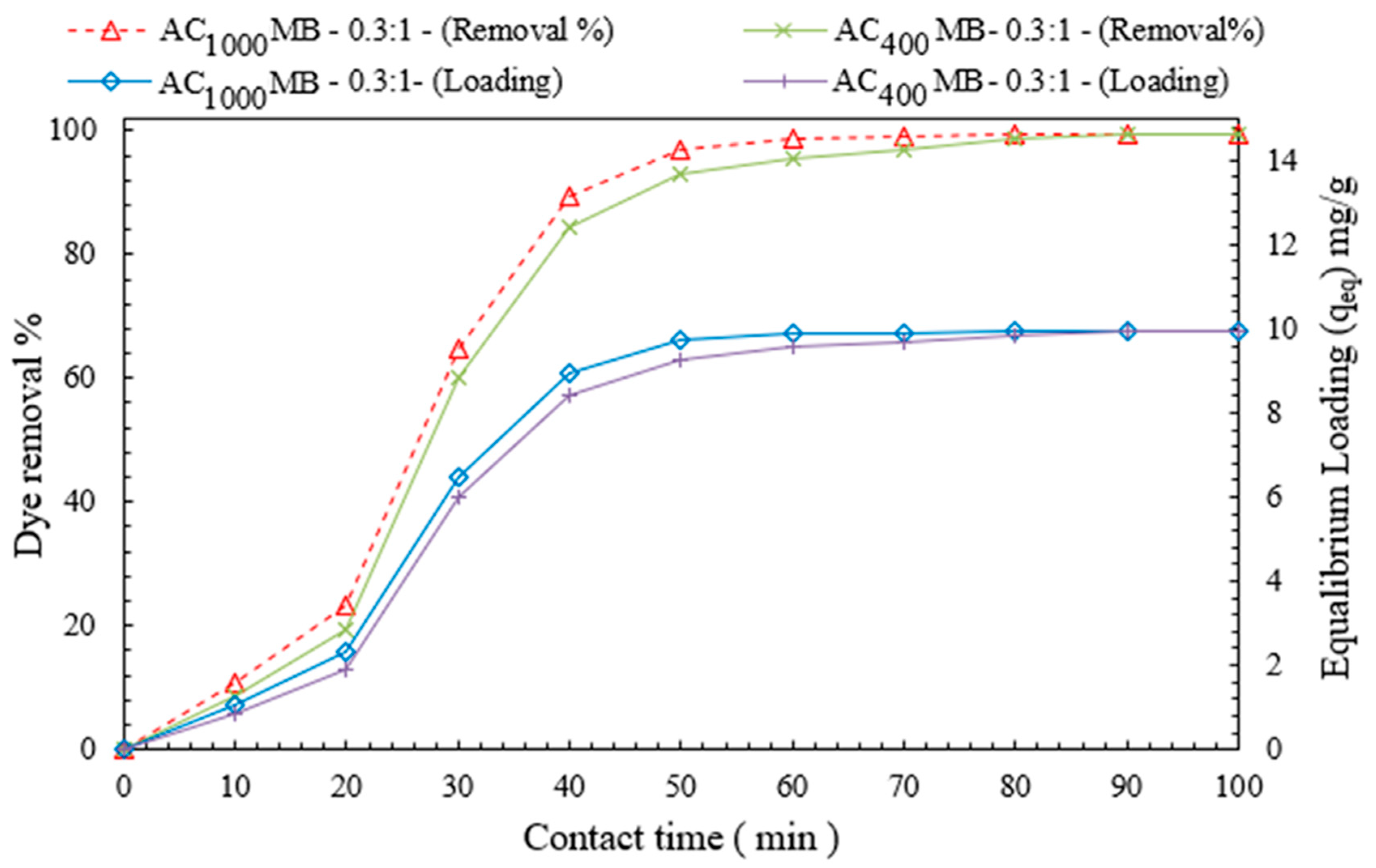
3.2.3. Effect of Contact Time on DB78-Dye-Removal Efficiency
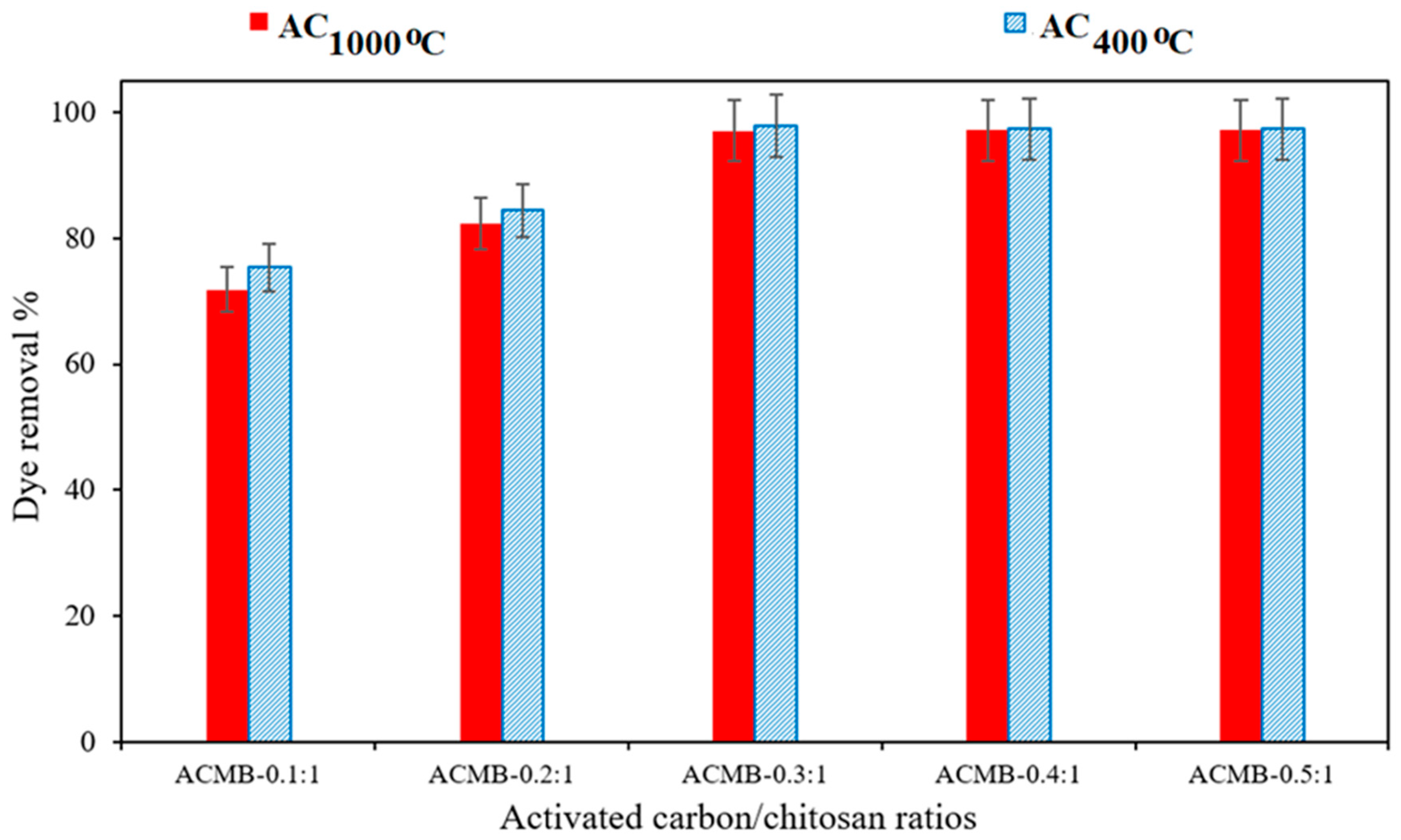
3.2.4. Effect of Activated Carbon/Chitosan Ratio on DB78 Dye Removal
3.2.5. Effect of Contact Time
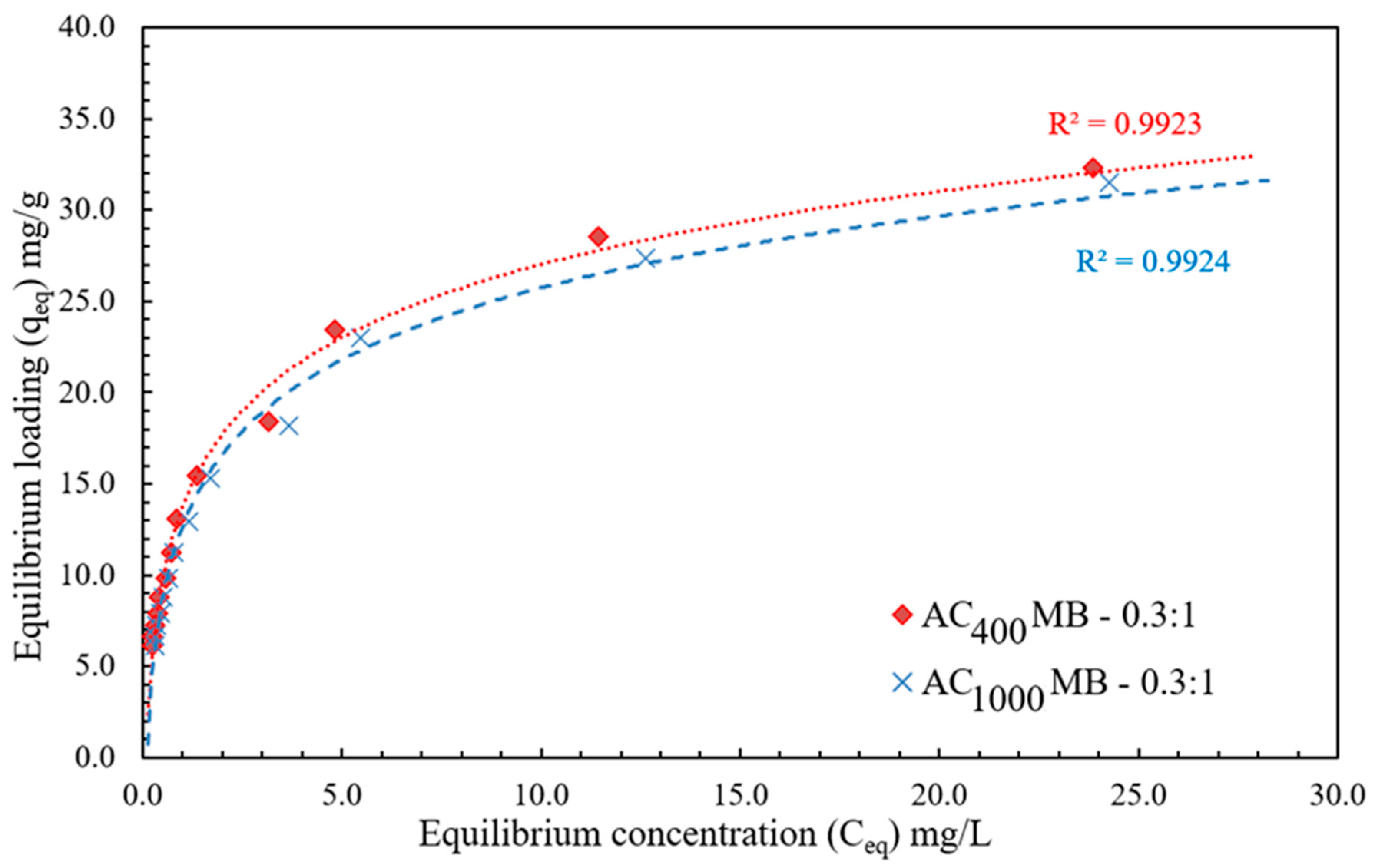
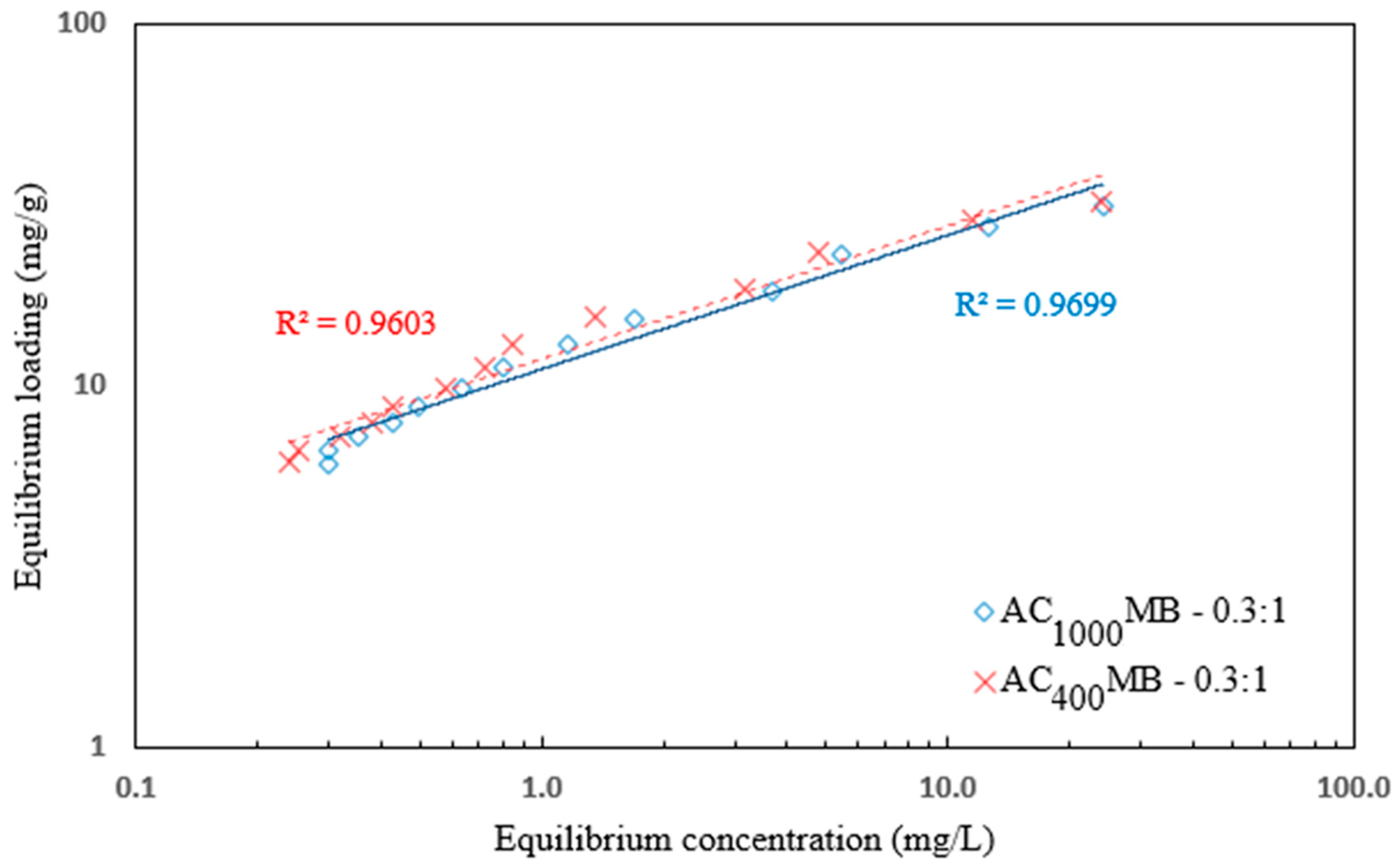
3.3. Adsorption Isotherm
3.4. Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rai, P.; Gautam, R.K.; Banerjee, S.; Rawat, V.; Chattopadhyaya, M.C. Synthesis and characterization of a novel SnFe2O4@ activated carbon magnetic nanocomposite and its effectiveness in the removal of crystal violet from aqueous solution. J. Environ. Chem. Eng. 2015, 3, 2281–2291. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Patil, A.P.; Bhanvase, B.A.; Sonawane, S.H. Ultrasonically prepared poly (acrylamide)-kaolin composite hydrogel for removal of crystal violet dye from wastewater. J. Environ. Chem. Eng. 2015, 3, 1152–1162. [Google Scholar] [CrossRef]
- Kubra, K.T.; Salman, M.S.; Znad, H.; Hasan, M.N. Efficient encapsulation of toxic dye from wastewater using biodegradable polymeric adsorbent. J. Mol. Liq. 2021, 329, 115541. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Esvandi, Z.; Khatooni, H.; Ramavandi, B. Evaluation of two cationic dyes removal from aqueous environments using CNT/MgO/CuFe2O4 magnetic composite powder: A comparative study. J. Environ. Chem. Eng. 2021, 9, 104752. [Google Scholar] [CrossRef]
- Cheruiyot, G.K.; Wanyonyi, W.C.; Kiplimo, J.J.; Maina, E.N. Adsorption of toxic crystal violet dye using coffee husks: Equilibrium, kinetics and thermodynamics study. Sci. Afr. 2019, 5, e00116. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Aghdasinia, H.; Mohammadi, R.; Ramavandi, B. Modification of bio-hydroxyapatite generated from waste poultry bone with MgO for purifying methyl violet-laden liquids. Environ. Sci. Pollut. Res. 2020, 27, 44218–44229. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Aghamohammadi-Bavil, O.; Foroutan, R.; Arsalani, N. Removal of malachite green using carboxymethyl cellulose-g-polyacrylamide/montmorillonite nanocomposite hydrogel. Int. J. Biol. Macromol. 2020, 159, 1122–1131. [Google Scholar] [CrossRef]
- Eteba, A.; Bassyouni, M.; Saleh, M. Removal of Hazardous Organic Pollutants Using Fly Ash. Environ. Ecol. Res. 2021, 9, 196–203. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- El-Mehalmey, W.A.; Safwat, Y.; Bassyouni, M.; Alkordi, M.H. Strong interplay between polymer surface charge and MOF cage chemistry in mixed-matrix membrane for water treatment applications. ACS Appl. Mater. Interfaces 2020, 12, 27625–27631. [Google Scholar] [CrossRef]
- Fouad, K.; Alalm, M.G.; Bassyouni, M.; Saleh, M.Y. A novel photocatalytic reactor for the extended reuse of W–TiO2 in the degradation of sulfamethazine. Chemosphere 2020, 257, 127270. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, M.H.; El-Ashtoukhy, E.Z.; Zoromba, M.S.; Bassyouni, M.; Sedahmed, G.H. Removal of nitrates from water by electrocoagulation using a cell with horizontally oriented Al serpentine tube anode. J. Ind. Eng. Chem. 2020, 82, 105–112. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.H.; Bassyouni, M.; Zoromba, M.S.; Alshehri, A.A. Removal of dyes from waste solutions by anodic oxidation on an array of horizontal graphite rods anodes. Ind. Eng. Chem. Res. 2018, 58, 1004–1018. [Google Scholar] [CrossRef]
- Bassyouni, M.; Mansi, A.E.; Elgabry, A.; Ibrahim, B.A.; Kassem, O.A.; Alhebeshy, R. Utilization of carbon nanotubes in removal of heavy metals from wastewater: A review of the CNTs’ potential and current challenges. Appl. Phys. A 2020, 126, 38. [Google Scholar] [CrossRef]
- Elhenawy, Y.; Elminshawy, N.A.; Bassyouni, M.; Alanezi, A.A.; Drioli, E. Experimental and theoretical investigation of a new air gap membrane distillation module with a corrugated feed channel. J. Membr. Sci. 2020, 594, 117461. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Zheng, J.; Bu, T.; He, G.; Wu, J. Safety considerations on food protein-derived bioactive peptides. Trends Food Sci. Technol. 2020, 96, 199–207. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Chen, H.; Lu, J.; Yu, G.; Möslang, M.; Zhou, Y. Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy-metal ions. J. Hazard. Mater. 2020, 382, 121040. [Google Scholar] [CrossRef]
- Mezohegyi, G.; van der Zee, F.P.; Font, J.; Fortuny, A.; Fabregat, A. Towards advanced aqueous dye removal processes: A short review on the versatile role of activated carbon. J. Environ. Manag. 2012, 102, 148–164. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Pintar, A. Catalytic processes for the purification of drinking water and industrial effluents. Catal. Today 2003, 77, 451–465. [Google Scholar] [CrossRef]
- El-Sheikh, A.H.; Newman, A.P.; Al-Daffaee, H.K.; Phull, S.; Cresswell, N. Characterization of activated carbon prepared from a single cultivar of Jordanian Olive stones by chemical and physicochemical techniques. J. Anal. Appl. Pyrolysis 2004, 71, 151–164. [Google Scholar] [CrossRef]
- Hesas, R.H.; Daud, W.M.A.W.; Sahu, J.N.; Arami-Niya, A. The effects of a microwave heating method on the production of activated carbon from agricultural waste: A review. J. Anal. Appl. Pyrolysis 2013, 100, 1–11. [Google Scholar] [CrossRef]
- Li, W.; Peng, J.; Zhang, L.; Yang, K.; Xia, H.; Zhang, S.; Guo, S.H. Preparation of activated carbon from coconut shell chars in pilot-scale microwave heating equipment at 60 kW. Waste Manag. 2009, 29, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, J.; Zhang, H.; Yang, S.; Qi, J.; Wang, Z.; Xu, H. Use of rice husk-based porous carbon for adsorption of Rhodamine B from aqueous solutions. Dyes Pigments 2005, 66, 123–128. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Optimization of preparation conditions for activated carbons from coconut husk using response surface methodology. Chem. Eng. J. 2008, 137, 462–470. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Hameed, B.H.; Ahmad, A.L. Equilibrium and kinetic studies on basic dye adsorption by oil palm fibre activated carbon. Chem. Eng. J. 2007, 127, 111–119. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of chitosan—A challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Ruan, C.Q.; Strømme, M.; Lindh, J. Preparation of porous 2, 3-dialdehyde cellulose beads crosslinked with chitosan and their application in adsorption of Congo red dye. Carbohydr. Polym. 2018, 181, 200–207. [Google Scholar] [CrossRef]
- Chiu, C.-W.; Wu, M.-T.; Lee, J.C.-M.; Cheng, T.-Y. Isothermal adsorption properties for the adsorption and removal of reactive blue 221 dye from aqueous solutions by cross-linked β-chitosan glycan as acid-resistant adsorbent. Polymers 2018, 10, 1328. [Google Scholar] [CrossRef] [Green Version]
- Sait, H.H.; Hussain, A.; Bassyouni, M.; Ali, I.; Kanthasamy, R.; Ayodele, B.V.; Elhenawy, Y. Hydrogen-Rich Syngas and Biochar Production by Non-Catalytic Valorization of Date Palm Seeds. Energies 2022, 15, 2727. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Adsorption of phenolic compounds on low-cost adsorbents: A review. Adv. Colloid Interface Sci. 2008, 143, 48–67. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, F.P.; Carvalho, A.M.M.L.; Carneiro, A.D.C.O.; de Magalhães, M.A.; Xisto, M.F.; Canal, W.D. Adsorption of neutral red dye by chitosan and activated carbon composite films. Heliyon 2021, 7, e07629. [Google Scholar] [CrossRef]
- Nasir, M.; Hashim, R.; Sulaiman, O.; Nordin, N.A.; Lamaming, J.; Asim, M. Laccase, an emerging tool to fabricate green composites: A review. BioResources 2015, 10, 6262–6284. [Google Scholar] [CrossRef] [Green Version]
- Abraham, E.; Deepa, B.; Pothan, L.A.; Jacob, M.; Thomas, S.; Cvelbar, U.; Anandjiwala, R. Extraction of nanocellulose fibrils from lignocellulosic fibres: A novel approach. Carbohydr. Polym. 2011, 86, 1468–1475. [Google Scholar] [CrossRef]
- Staroń, P.; Chwastowski, J. Raphia-Microorganism Composite Biosorbent for Lead Ion Removal from Aqueous Solutions. Materials 2021, 14, 7482. [Google Scholar] [CrossRef] [PubMed]
- Bassyouni, M.; Zoromba, M.S.; Abdel-Aziz, M.H.; Mosly, I. Extraction of Nanocellulose for Eco-Friendly Biocomposite Adsorbent for Wastewater Treatment. Polymers 2022, 14, 1852. [Google Scholar] [CrossRef]




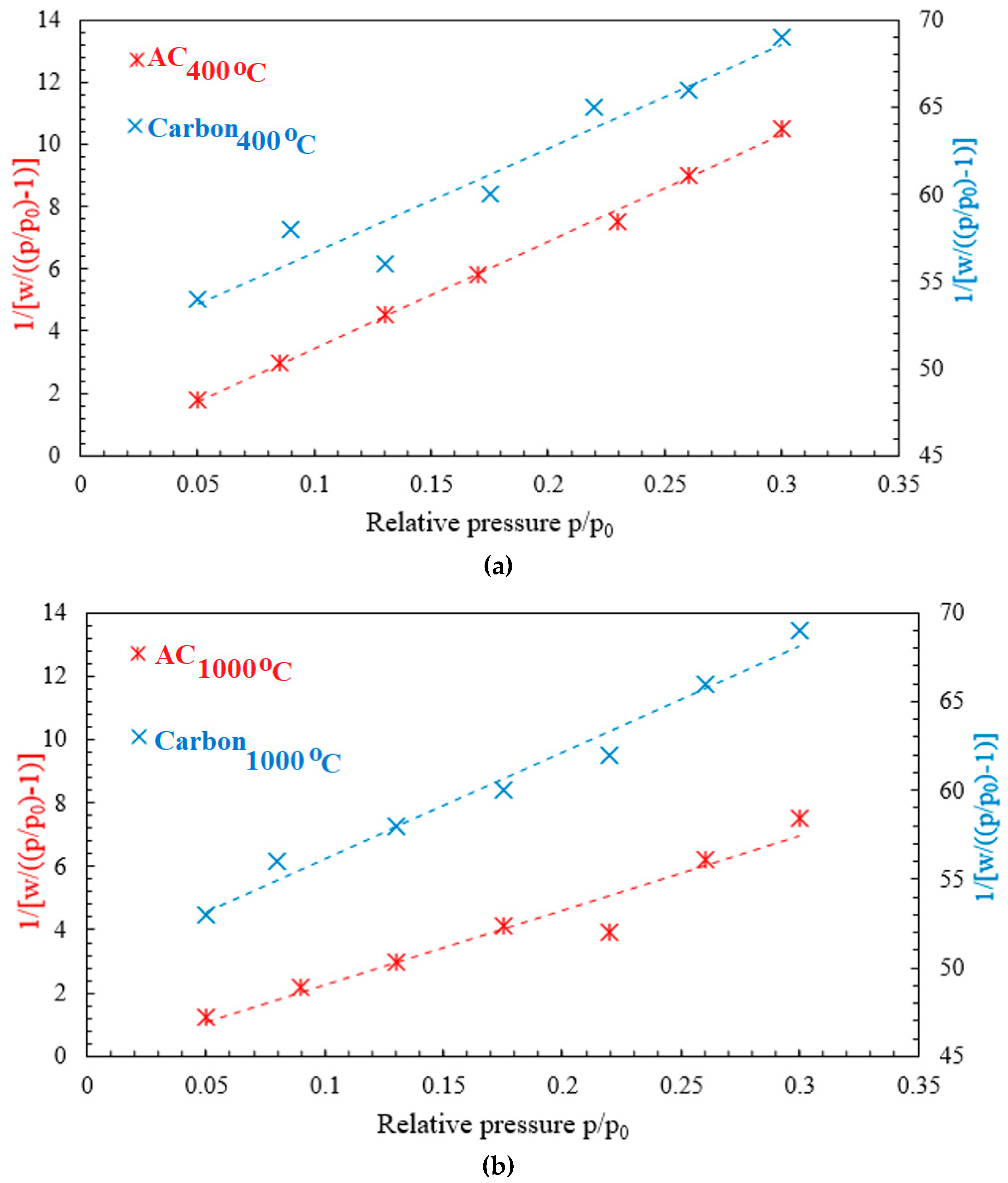





| Parameter | C400°C | AC400°C | C1000°C | AC1000°C |
|---|---|---|---|---|
| Average pore radius (nm) | 1.67 | 1.63 | 1.78 | 1.57 |
| BET surface area (m2/g) | 32.59 | 99.91 | 33.08 | 138 |
| Pore volume (cm3/g) | 0.023 | 0.037 | 0.025 | 0.045 |
| Freundlich Isothermal | Langmuir Isothermal | Adsorbent | ||||
|---|---|---|---|---|---|---|
| R2 | 1/n | Kf (mg/g) | R2 | B (L/mg) | Q0 (mg/g) | |
| 0.960 | 0.36 | 11.86 | 0.995 | 1.47 | 33.5 | AC400°C MB-0.3:1 |
| 0.969 | 0.37 | 11.13 | 0.993 | 1.68 | 32.7 | AC1000°C MB-0.3:1 |
| Kinetic Model Parameters | Pseudo First-Order Model | Pseudo Second-Order Model | ||||
|---|---|---|---|---|---|---|
| K1 (1/min) | qe (mg/g) | R2 | K2 (g/mg min) | qe (mg/g) | R2 | |
| AC400°C MB-0.3:1 | 0.065 | 29.5 | 0.985 | 5.2 × 10−5 | 57.66 | 0.101 |
| AC1000°C MB-0.3:1 | 0.08 | 32.45 | 0.965 | 6.11 × 10−5 | 60.24 | 0.107 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sait, H.H.; Hussain, A.; Bassyouni, M.; Ali, I.; Kanthasamy, R.; Ayodele, B.V.; Elhenawy, Y. Anionic Dye Removal Using a Date Palm Seed-Derived Activated Carbon/Chitosan Polymer Microbead Biocomposite. Polymers 2022, 14, 2503. https://doi.org/10.3390/polym14122503
Sait HH, Hussain A, Bassyouni M, Ali I, Kanthasamy R, Ayodele BV, Elhenawy Y. Anionic Dye Removal Using a Date Palm Seed-Derived Activated Carbon/Chitosan Polymer Microbead Biocomposite. Polymers. 2022; 14(12):2503. https://doi.org/10.3390/polym14122503
Chicago/Turabian StyleSait, Hani Hussain, Ahmed Hussain, Mohamed Bassyouni, Imtiaz Ali, Ramesh Kanthasamy, Bamidele Victor Ayodele, and Yasser Elhenawy. 2022. "Anionic Dye Removal Using a Date Palm Seed-Derived Activated Carbon/Chitosan Polymer Microbead Biocomposite" Polymers 14, no. 12: 2503. https://doi.org/10.3390/polym14122503
APA StyleSait, H. H., Hussain, A., Bassyouni, M., Ali, I., Kanthasamy, R., Ayodele, B. V., & Elhenawy, Y. (2022). Anionic Dye Removal Using a Date Palm Seed-Derived Activated Carbon/Chitosan Polymer Microbead Biocomposite. Polymers, 14(12), 2503. https://doi.org/10.3390/polym14122503








