Highly Active Heterogeneous Double Metal Cyanide Catalysts for Ring-Opening Polymerization of Cyclic Monomers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Optimization of DMC Catalysts
2.3. Polymerization of PO
2.4. Polymerization of Lactones
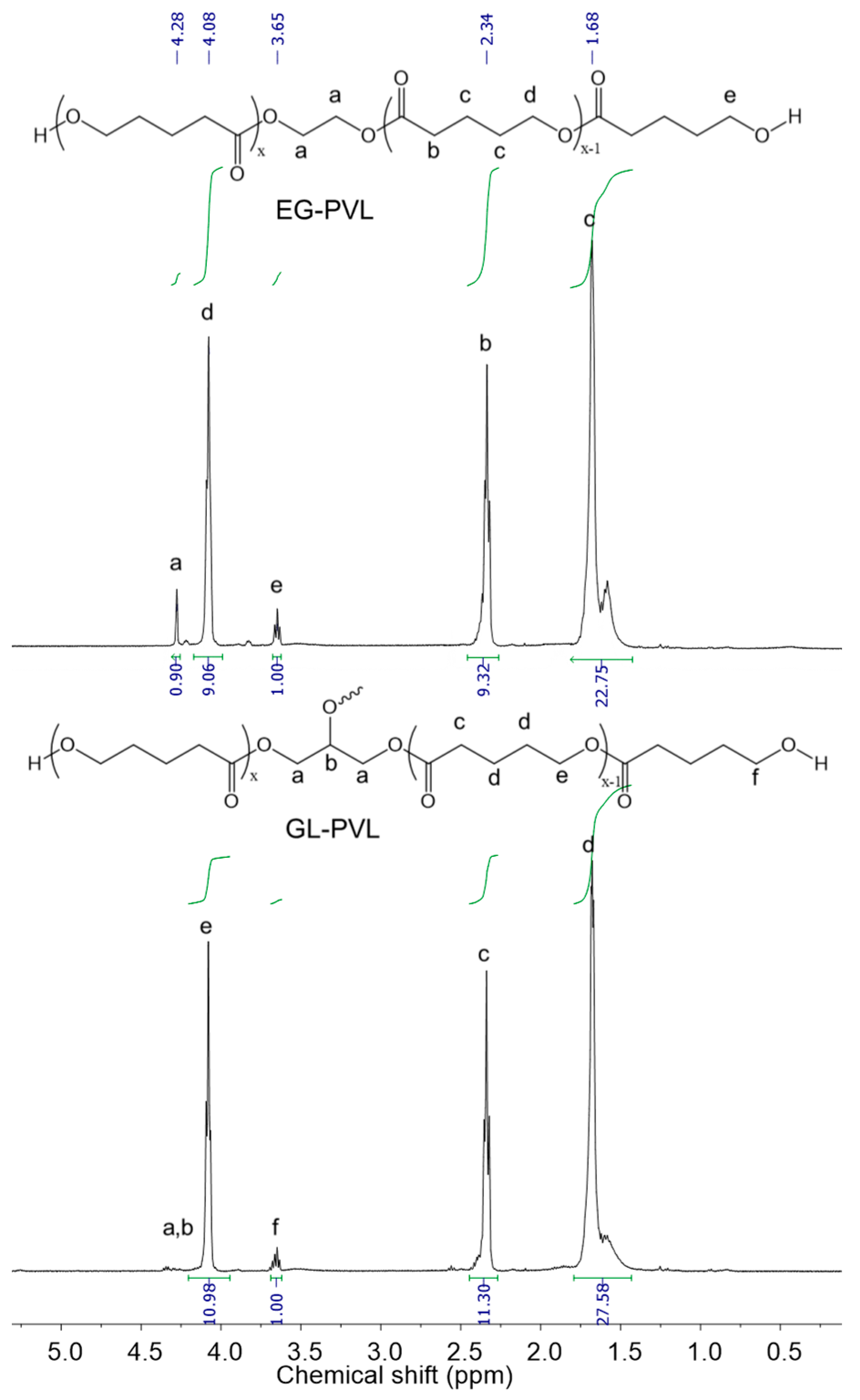
2.5. Characterization
3. Results and Discussion
3.1. Characterization of DMC Catalysts
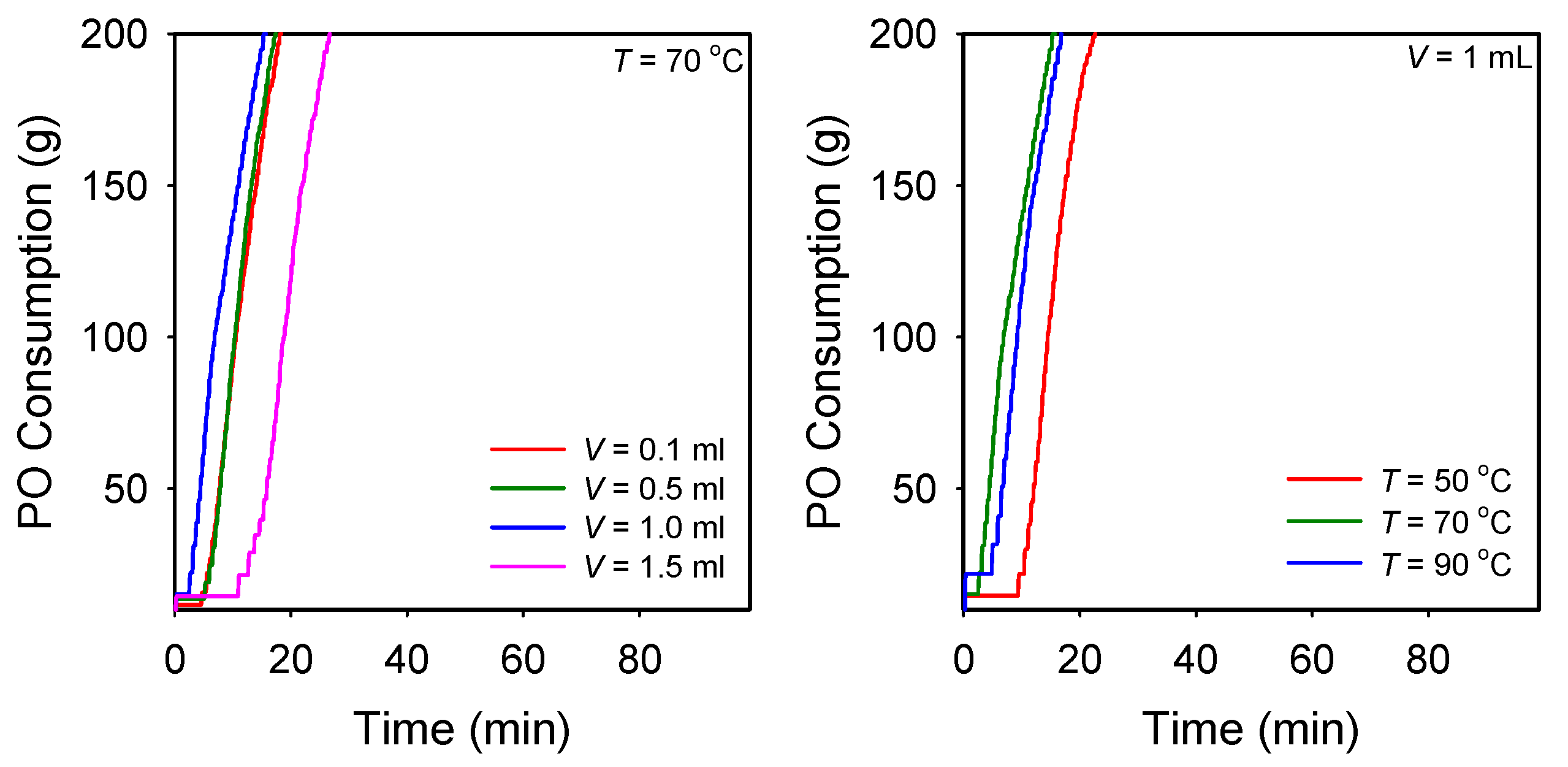
3.2. Evaluation of Catalytic Activity of DMC Catalysts for the Polymerization of PO
3.3. Polymerization of CL and VL Using DMC Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, Y.; Shen, Z.; Shen, J.; Zhang, Y.; Yao, K. Characteristics and Mechanism of ε-Caprolactone Polymerization with Rare Earth Halide Systems. Macromolecules 1996, 29, 3441–3446. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Varma, I.K. Recent Developments in Ring Opening Polymerization of Lactones for Biomedical Applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Rabnawaz, M.; Wyman, I.; Auras, R.; Cheng, S. A roadmap towards green packaging: The current status and future outlook for polyesters in the packaging industry. Green Chem. 2017, 19, 4737–4753. [Google Scholar] [CrossRef]
- Hernandez, E.; Nowack, B.; Mitrano, D.M. Polyester Textiles as a Source of Microplastics from Households: A Mechanistic Study to Understand Microfiber Release During Washing. Environ. Sci. Technol. 2017, 51, 7036–7046. [Google Scholar] [CrossRef] [PubMed]
- Boiteux, G.; Cuvé, L.; Pascault, J.-P. Synthesis and Properties of Polyurethanes Based on Polyolefin: 3. Monitoring of Phase Separation by Dielectric Relaxation Spectroscopy of Segmented Semicrystalline Polyurethane Prepared in Bulk by the Use of Emulsifiers. Polymer 1994, 35, 173–178. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Gustini, L.; Lavilla, C.; Finzel, L.; Noordover, B.A.J.; Hendrix, M.M.R.M.; Koning, C.E. Sustainable Coatings from Bio-Based, Enzymatically Synthesized Polyesters with Enhanced Functionalities. Polym. Chem. 2016, 7, 6586–6597. [Google Scholar] [CrossRef]
- Zia, K.M.; Noreen, A.; Zuber, M.; Tabasum, S.; Mujahid, M. Recent developments and future prospects on bio-based polyesters derived from renewable resources: A review. Int. J. Biol. Macromol. 2016, 82, 1028–1040. [Google Scholar] [CrossRef]
- Ji, C.; Jie, S.; Braunstein, P.; Li, B.-G. Fast and controlled ring-opening polymerization of δ-valerolactone catalyzed by benzoheterocyclic urea/MTBD catalysts. Catal. Sci. Technol. 2020, 10, 7555–7565. [Google Scholar] [CrossRef]
- Thongkham, S.; Monot, J.; Martin-Vaca, B.; Bourissou, D. Simple In-Based Dual Catalyst Enables Significant Progress in ε-Decalactone Ring-Opening (Co)polymerization. Macromolecules 2019, 52, 8103–8113. [Google Scholar] [CrossRef]
- Buntara, T.; Noel, S.; Phua, P.H.; Melián-Cabrera, I.; de Vries, J.G.; Heeres, H.J. Caprolactam from Renewable Resources: Catalytic Conversion of 5-Hydroxymethylfurfural into Caprolactone. Angew. Chem. Int. Ed. 2011, 50, 7083–7087. [Google Scholar] [CrossRef]
- Caretto, A.; Noè, M.; Selva, M.; Perosa, A. Upgrading of Biobased Lactones with Dialkylcarbonates. ACS Sustain. Chem. Eng. 2014, 2, 2131–2141. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Hakkarainen, M. Designed to degrade. Science 2017, 358, 872–873. [Google Scholar] [CrossRef]
- Kuran, W. Coordination polymerization of heterocyclic and heterounsaturated monomers. Prog. Polym. Sci. 1998, 23, 919–992. [Google Scholar] [CrossRef]
- Kowalski, A.; Duda, A.; Penczek, S. Kinetics and Mechanism of Cyclic Esters Polymerization Initiated with Tin(Ii) Octoate, 1. Polymerization of ε-Caprolactone. Macromol. Rapid Commun. 1998, 19, 567–572. [Google Scholar] [CrossRef]
- Baśko, M.; Kubisa, P. Mechanism of Propagation in the Cationic Polymerization of L,L-Lactide. J. Polym. Sci. A Polym. Chem. 2008, 46, 7919–7923. [Google Scholar] [CrossRef]
- Lewinski, P.; Pretula, J.; Kaluzynski, K.; Kaźmierski, S.; Penczek, S. ε-Caprolactone: Activated Monomer Polymerization; Controversy over the Mechanism of Polymerization Catalyzed by Phosphorus Acids (Diarylhydrogen Phosphates). Do Acids also Act as Initiators? J. Catal. 2019, 371, 305–312. [Google Scholar] [CrossRef]
- Nederberg, F.; Connor, E.F.; Möller, M.; Glauser, T.; Hedrick, J.L. New Paradigms for Organic Catalysts: The First Organocatalytic Living Polymerization. Angew. Chem. Int. Ed. 2001, 40, 2712–2715. [Google Scholar] [CrossRef]
- Lohmeijer, B.G.G.; Pratt, R.C.; Leibfarth, F.; Logan, J.W.; Long, D.A.; Dove, A.P.; Nederberg, F.; Choi, J.; Wade, C.; Waymouth, R.M.; et al. Guanidine and Amidine Organocatalysts for Ring-Opening Polymerization of Cyclic Esters. Macromolecules 2006, 39, 8574–8583. [Google Scholar] [CrossRef]
- Martello, M.T.; Burns, A.; Hillmyer, M.A. Bulk Ring-Opening Transesterification Polymerization of the Renewable δ-Decalactone Using an Organocatalyst. ACS Macro Lett. 2012, 1, 131–135. [Google Scholar] [CrossRef]
- Kamber, N.E.; Jeong, W.; Waymouth, R.M.; Pratt, R.C.; Lohmeijer, B.G.G.; Hedrick, J.L. Organocatalytic Ring-Opening Polymerization. Chem. Rev. 2007, 107, 5813–5840. [Google Scholar] [CrossRef]
- Kiesewetter, M.K.; Shin, E.J.; Hedrick, J.L.; Waymouth, R.M. Organocatalysis: Opportunities and Challenges for Polymer Synthesis. Macromolecules 2010, 43, 2093–2107. [Google Scholar] [CrossRef]
- Fukushima, K.; Nozaki, K. Organocatalysis: A Paradigm Shift in the Synthesis of Aliphatic Polyesters and Polycarbonates. Macromolecules 2020, 53, 5018–5022. [Google Scholar] [CrossRef]
- Storey, R.F.; Sherman, J.W. Kinetics and Mechanism of the Stannous Octoate-Catalyzed Bulk Polymerization of ε-Caprolactone. Macromolecules 2002, 35, 1504–1512. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef]
- Marquez, C.; Simonov, A.; Wharmby, M.T.; Van Goethem, C.; Vankelecom, I.; Bueken, B.; Krajnc, A.; Mali, G.; De Vos, D.; De Baerdemaeker, T. Layered Zn2[Co(CN)6](CH3COO) double metal cyanide: A two-dimensional DMC phase with excellent catalytic performance. Chem. Sci. 2019, 10, 4868–4875. [Google Scholar] [CrossRef] [Green Version]
- Sreeprasanth, P.S.; Srivastava, R.; Srinivas, D.; Ratnasamy, P. Hydrophobic, Solid Acid Catalysts for Production of Biofuels and Lubricants. Appl. Catal. A 2006, 314, 148–159. [Google Scholar] [CrossRef]
- Milgrom, J. Method of Making a Polyether Using a Double Metal Cyanide Complex. To General Tire and Rubber Company. U.S. Patent 3278457, 11 October 1966. [Google Scholar]
- Kim, I.; Ahn, J.-T.; Ha, C.S.; Yang, C.S.; Park, I. Polymerization of Propylene Oxide by Using Double Metal Cyanide Catalysts and the Application to Polyurethane Elastomer. Polymer 2003, 44, 3417–3428. [Google Scholar] [CrossRef]
- Tran, C.H.; Kim, S.A.; Moon, Y.; Lee, Y.; Ryu, H.M.; Baik, J.H.; Hong, S.C.; Kim, I. Effect of Dicarbonyl Complexing Agents on Double Metal Cyanide Catalysts Toward Copolymerization of CO2 and Propylene Oxide. Catal. Today 2020, 375, 335–342. [Google Scholar] [CrossRef]
- Dharman, M.M.; Ahn, J.-Y.; Lee, M.-K.; Shim, H.-L.; Kim, K.-H.; Kim, I.; Park, D.-W. Moderate Route for the Utilization of CO2-Microwave Induced Copolymerization with Cyclohexene Oxide Using Highly Efficient Double Metal Cyanide Complex Catalysts Based on Zn3[Co(CN)6]. Green Chem. 2008, 10, 678–684. [Google Scholar] [CrossRef]
- An, N.; Li, Q.; Yin, N.; Kang, M.; Wang, J. Effects of Addition Mode on Zn–Co Double Metal Cyanide Catalyst for Synthesis of Oligo(Propylene-Carbonate) Diols. Appl. Organomet. Chem. 2018, 32, e4509. [Google Scholar] [CrossRef]
- Srivastava, R.; Srinivas, D.; Ratnasamy, P. Fe–Zn Double-Metal Cyanide Complexes as Novel, Solid Transesterification Catalysts. J. Catal. 2006, 241, 34–44. [Google Scholar] [CrossRef]
- Satyarthi, J.K.; Srinivas, D.; Ratnasamy, P. Influence of Surface Hydrophobicity on the Esterification of Fatty Acids over Solid Catalysts. Energy Fuels 2010, 24, 2154–2161. [Google Scholar] [CrossRef]
- Peeters, A.; Valvekens, P.; Ameloot, R.; Sankar, G.; Kirschhock, C.E.A.; De Vos, D.E. Zn–Co Double Metal Cyanides as Heterogeneous Catalysts for Hydroamination: A Structure–Activity Relationship. ACS Catal. 2013, 3, 597–607. [Google Scholar] [CrossRef]
- Patil, M.V.; Yadav, M.K.; Jasra, R.V. Prins Condensation for Synthesis of Nopol from β-Pinene and Paraformaldehyde on Novel Fe–Zn Double Metal Cyanide Solid Acid Catalyst. J. Mol. Catal. A Chem. 2007, 273, 39–47. [Google Scholar] [CrossRef]
- García-Ortiz, A.; Grirrane, A.; Reguera, E.; García, H. Mixed (Fe2+ and Cu2+) Double Metal Hexacyanocobaltates as Solid Catalyst for the Aerobic Oxidation of Oximes to Carbonyl Compounds. J. Catal. 2014, 311, 386–392. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Li, Y.; Zhang, C.-J.; Zhang, Y.-Y.; Cao, X.-H.; Zhang, X.-H. Synthesis of high-molecular-weight poly(ε-caprolactone) via heterogeneous zinc-cobalt(III) double metal cyanide complex. Giant 2020, 3, 100030. [Google Scholar] [CrossRef]
- Tran, C.H.; Lee, M.W.; Park, S.W.; Jeong, J.E.; Lee, S.J.; Song, W.; Huh, P.; Kim, I. Heterogeneous Double Metal Cyanide Catalyzed Synthesis of Poly(ε-caprolactone) Polyols for the Preparation of Thermoplastic Elastomers. Catalysts 2021, 11, 1033. [Google Scholar] [CrossRef]
- Tran, C.H.; Pham, L.T.T.; Jang, H.B.; Kim, S.A.; Kim, I. Effect of α-, β-, γ-, and δ-Dicarbonyl Complexing Agents on the Double Metal Cyanide-Catalyzed Ring-Opening Polymerization of Propylene Oxide. Catal. Today 2020, 375, 429–440. [Google Scholar] [CrossRef]
- ASTM Standard E 1899-97; Standard Test Method for Hydroxyl Groups Using Reaction with p-Toluenesulfonyl Isocyanate (TSI) and Potentiometric Titration with Tetrabutylammonium Hydroxide. American Society for Testing and Materials: West Conshohocken, PA, USA, 1997.
- Cameron, D.J.A.; Shaver, M.P. Aliphatic polyester polymer stars: Synthesis, properties and applications in biomedicine and nanotechnology. Chem. Soc. Rev. 2011, 40, 1761–1776. [Google Scholar] [CrossRef] [Green Version]
- Tran, C.H.; Pham, L.T.T.; Lee, Y.; Jang, H.B.; Kim, S.; Kim, I. Mechanistic Insights on Zn(II)–Co(III) Double Metal Cyanide-Catalyzed Ring-Opening Polymerization of Epoxides. J. Catal. 2019, 372, 86–102. [Google Scholar] [CrossRef]
- Tran, C.H.; Lee, M.W.; Kim, S.A.; Jang, H.B.; Kim, I. Kinetic and Mechanistic Study of Heterogeneous Double Metal Cyanide-Catalyzed Ring-Opening Multibranching Polymerization of Glycidol. Macromolecules 2020, 53, 2051–2060. [Google Scholar] [CrossRef]
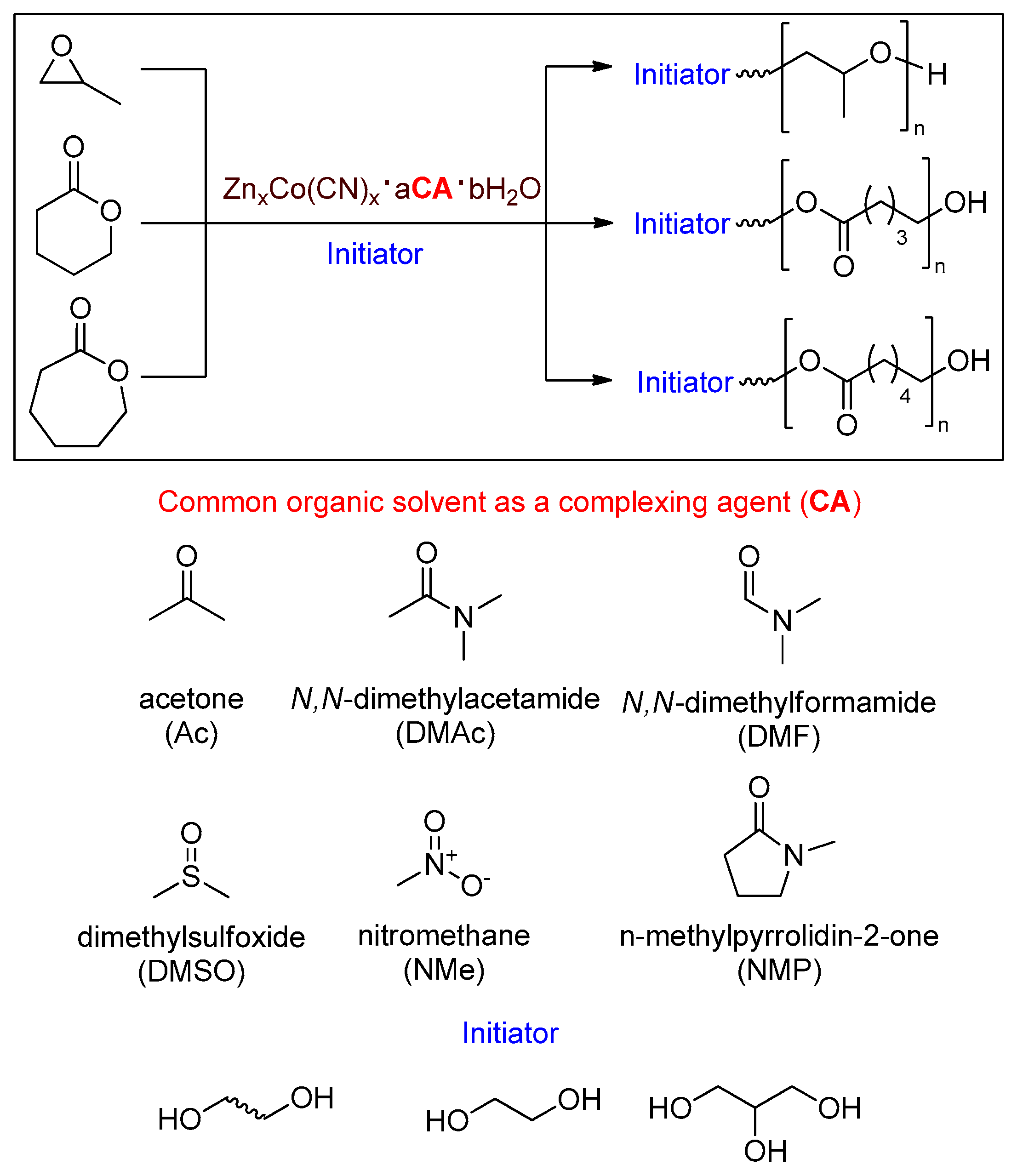
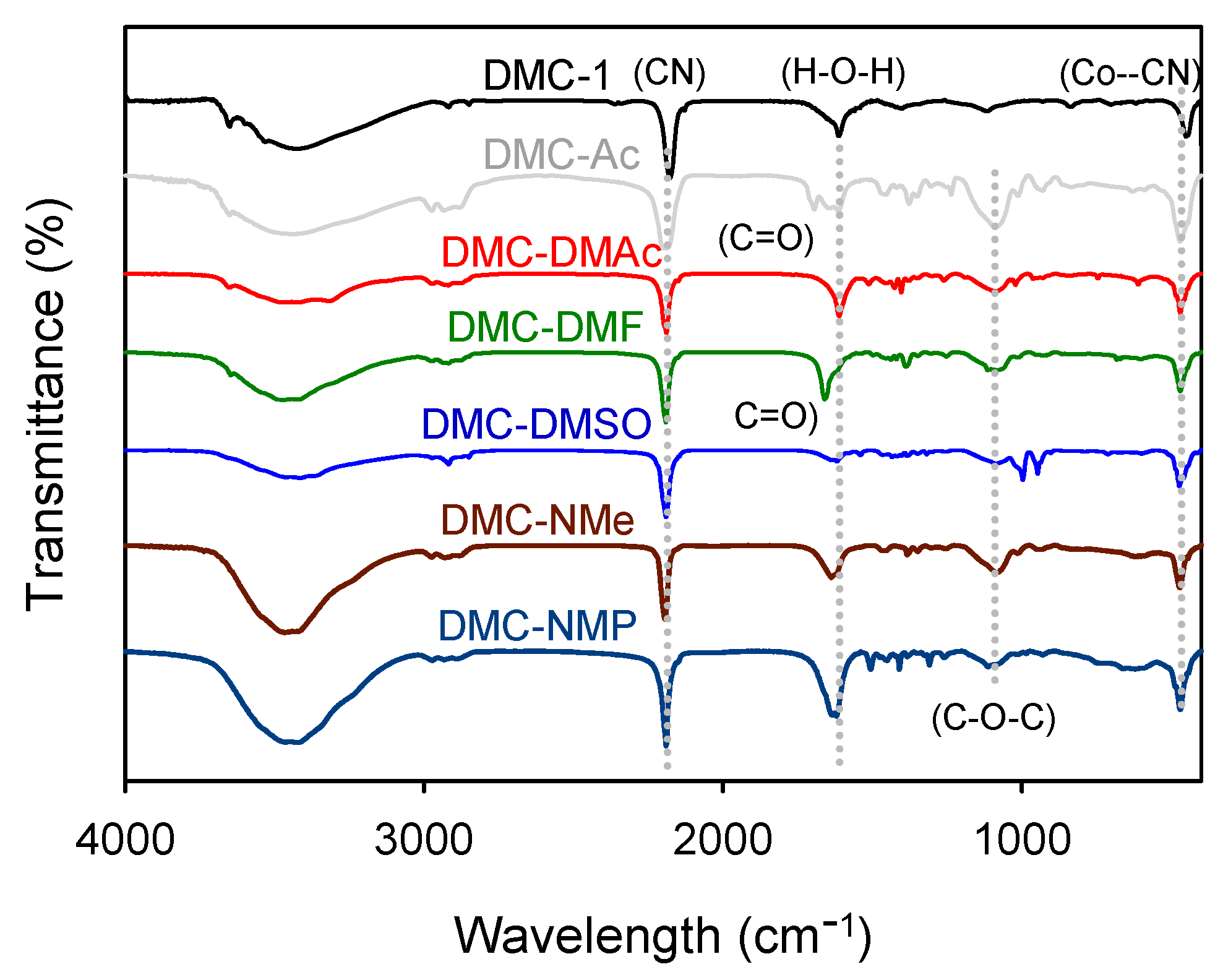
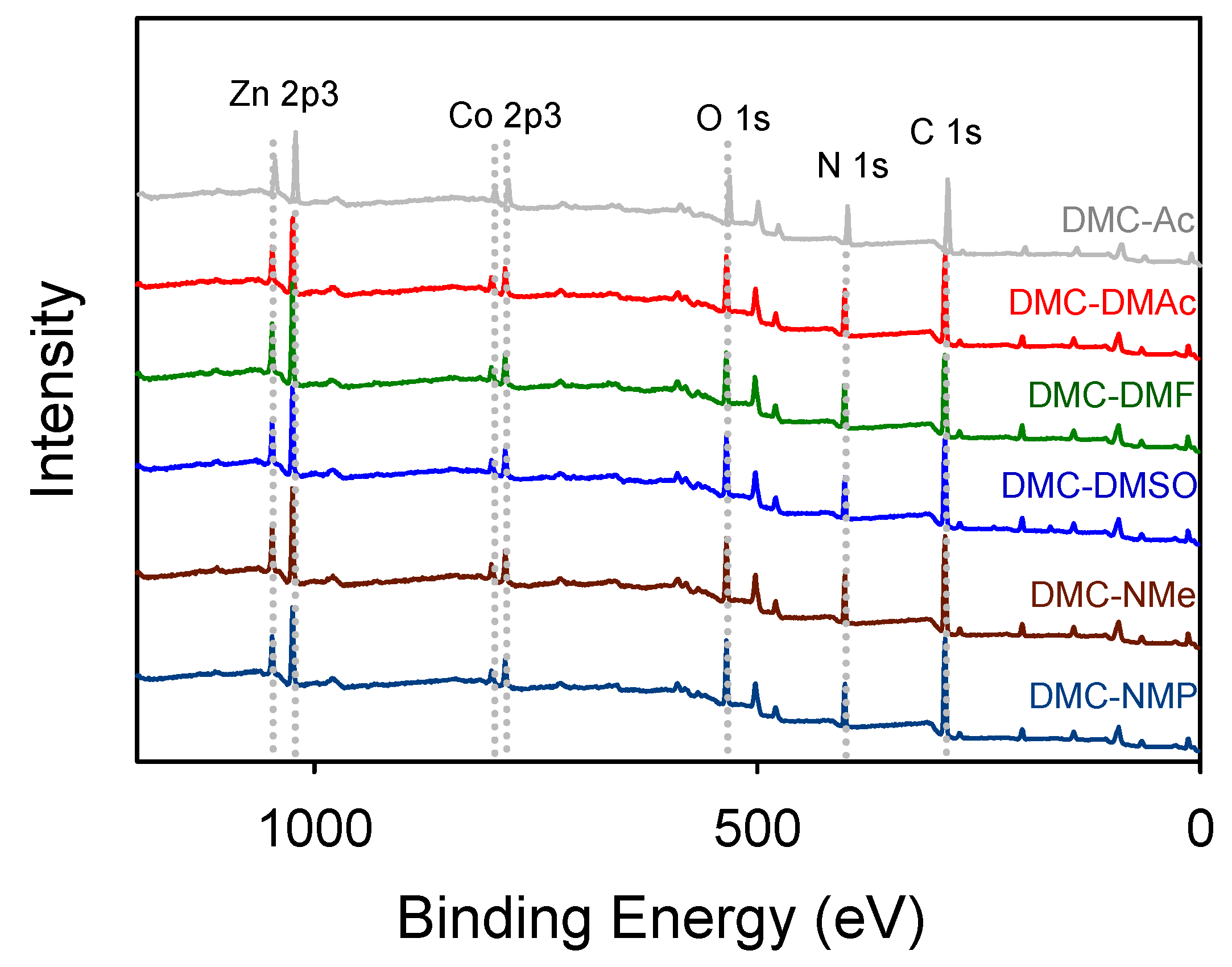
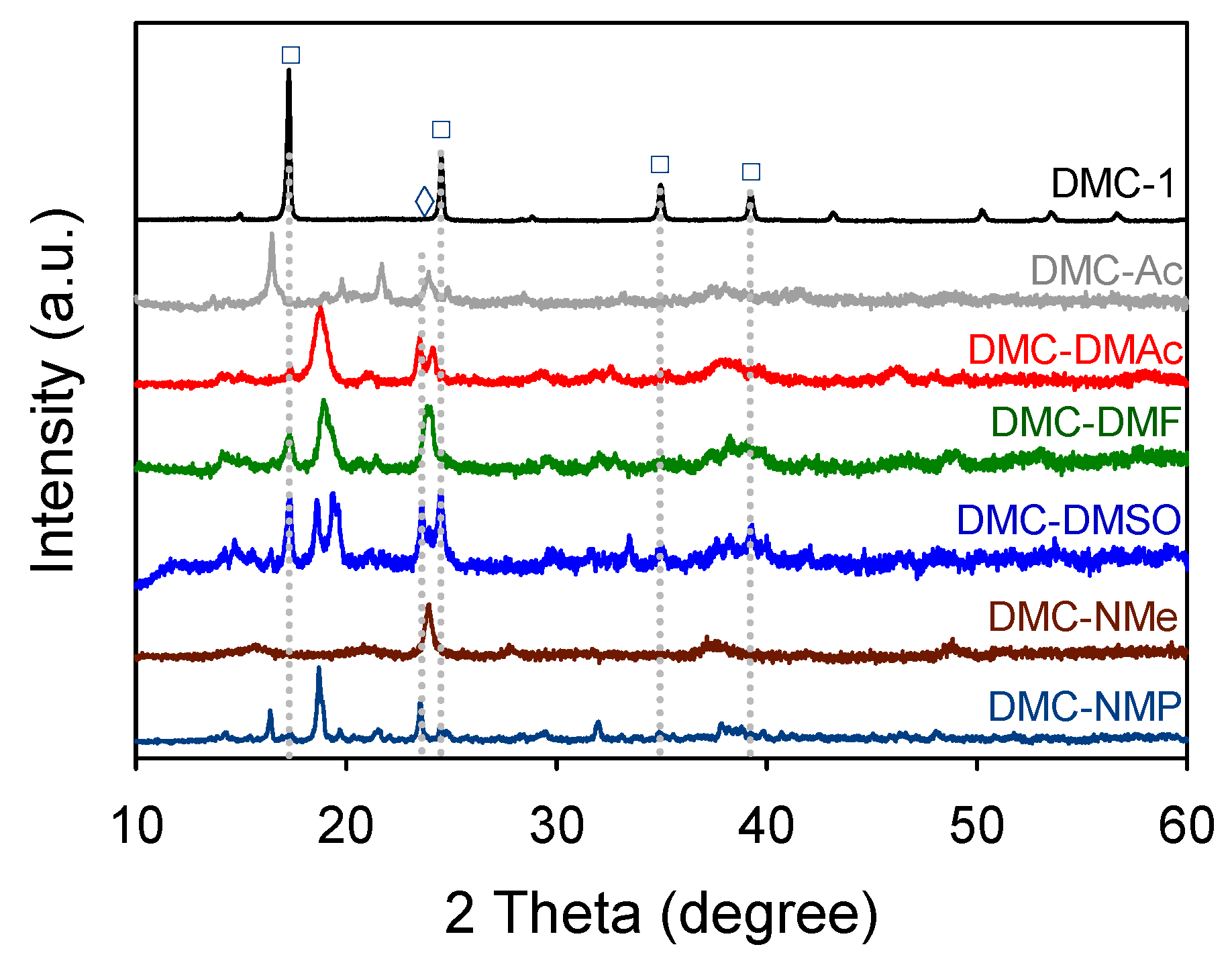

| Catalyst | Vibration Frequency (cm−1) | |||||
|---|---|---|---|---|---|---|
| v (OH) | v( C≡N) | v (C=O) | δ (H−O−H) | v (C−O−C) | δ (Co−CN) | |
| DMC-1 | 3423 | 2178 | − | 1615 | − | 448 |
| DMC-TBA | 3436 | 2193 | − | 1630 | 1085 | 473 |
| DMC-Ac | 3442 | 2196 | 1701 | 1621 | 1086 | 471 |
| DMC-DMAc | 3417 | 2190 | − | 1616 | 1089 | 470 |
| DMC-DMF | 3471 | 2191 | 1660 | 1620 | 1081 | 470 |
| DMC-DMSO | 3428 | 2191 | − | 1630 | 1085 | 474 |
| DMC-NMe | 3453 | 2195 | − | 1639 | 1081 | 471 |
| DMC-NMP | 3446 | 2191 | − | 1630 | 1089 | 471 |
| Catalyst | ICP-Mass (wt%) | Elemental Analysis (wt%) | TGA (wt%) | Estimated Catalyst Formulation | |||||
|---|---|---|---|---|---|---|---|---|---|
| Zn | Co | C | H | N | CA | P123 | H2O | ||
| DMC-1 | 27.6 | 16.6 | 20.2 | 1.3 | 23.6 | − | − | 12.0 | Zn1.5Co(CN)6·2.37H2O |
| DMC-Ac | 22.3 | 4.13 | 29.2 | 3.5 | 16.1 | 1.7 | 26.8 | 1.0 | Zn4.87Co(CN)6.01·0.32Ac·0.07P123·0.18H2O·13.3Cl− |
| DMC-DMAc | 24.3 | 6.35 | 28.4 | 3.1 | 10.34 | 4.8 | 24.7 | 2.8 | Zn3.45Co(CN)5.99·0.30DMAc·0.04P123·1.44H2O·6.56Cl− |
| DMC-DMF | 27.9 | 11.9 | 29.7 | 3.4 | 19.4 | 4.8 | 20.5 | 2.6 | Zn2.11Co(CN)6.12·0.14DMF·0.02P123·0.71H2O·0.78Cl− |
| DMC-DMSO | 26.9 | 11.0 | 29.5 | 3.3 | 17.9 | 6.8 | 21.0 | 2.0 | Zn2.20Co(CN)6.05·0.11DMSO·0.02P123·0.59H2O·1.51Cl− |
| DMC-NMe | 24.9 | 10.7 | 29.3 | 3.2 | 17.4 | 7.2 | 26.9 | 1.6 | Zn2.10Co(CN)6.01·0.65NMe·0.03P123·0.49H2O·0.06Cl− |
| DMC-NMP | 27.3 | 12.4 | 27.1 | 2.9 | 20.2 | 5.8 | 17.2 | 1.8 | Zn1.98Co(CN)6.22·0.28NMP·0.01P123·0.47H2O·1.14Cl− |
| Catalyst | Preparation Condition a | Catalytic Activity | Polymer Properties | |||||
|---|---|---|---|---|---|---|---|---|
| CA | T (°C) | tindb (min) | Rp,avgc | GPC | Unsat. (meq g−1) d | |||
| Type | V (mL) | Mn | Ð | |||||
| DMC-1 | − | − | rt | − | − | |||
| DMC-TBA | TBA | 0.5 | 50 | 31 | 2200 | 3200 | 1.12 | 0.0065 |
| DMC-Ac | Ac | 0.1 | 50 | 11 | 1620 | |||
| 0.5 | 50 | 10 | 3880 | |||||
| 1.0 | 50 | 9 | 4770 | 3900 | 1.10 | 0.01667 | ||
| 1.5 | 50 | 11 | 4390 | |||||
| DMC-DMAc | DMAc | 0.1 | 70 | 4 | 6000 | |||
| 0.5 | 70 | 5 | 6320 | |||||
| 1.0 | 70 | 2 | 7200 | 4200 | 1.09 | 0.0063 | ||
| 1.5 | 70 | 10 | 4200 | |||||
| 1.0 | 50 | 9 | 4880 | |||||
| 1.0 | 90 | 4 | 6320 | |||||
| DMC-DMF | DMF | 0.1 | 70 | 8 | 4570 | |||
| 0.5 | 70 | 13 | 3430 | |||||
| 1.0 | 70 | 11 | 3720 | |||||
| 1.5 | 70 | − | − | |||||
| 0.1 | 50 | 9 | 5100 | 4400 | 1.14 | 0.0030 | ||
| 0.1 | 90 | 9 | 4620 | |||||
| DMC-DMSO | DMSO | 0.1 | 70 | 11 | 4480 | 4700 | 1.18 | 0.0091 |
| 0.5 | 70 | 12 | 3680 | |||||
| 1.0 | 70 | 13 | 2470 | |||||
| 1.5 | 70 | − | − | |||||
| 0.1 | 50 | 10 | 4400 | |||||
| 0.1 | 90 | 17 | 2780 | |||||
| DMC-NMe | NMe | 0.1 | 70 | − | − | |||
| 0.5 | 70 | 8 | 5110 | |||||
| 1.0 | 70 | 8 | 5430 | |||||
| 1.5 | 70 | 6 | 6350 | 4500 | 1.14 | 0.0050 | ||
| 1.5 | 50 | 6 | 6000 | |||||
| 1.5 | 90 | − | − | |||||
| DMC-NMP | NMP | 0.1 | 70 | 6 | 5650 | |||
| 0.5 | 70 | 5 | 5750 | |||||
| 1.0 | 70 | 23 | 2320 | |||||
| 1.5 | 70 | − | − | |||||
| 0.1 | 50 | 7 | 5370 | |||||
| 0.1 | 90 | 9 | 5970 | 3800 | 1.08 | 0.0110 | ||
| Catalyst | t (h) | Monomer Conversion (%) | NMR | GPC | |
|---|---|---|---|---|---|
| Mn | Mn | Ð | |||
| DMC-1 | 5 | 25.9 | − | − | − |
| DMC-TBA | 5 | 86.7 | 1120 | 620 | 1.16 |
| DMC-DMAc | 5 | 72.7 | 800 | 500 | 1.20 |
| DMC-DMF | 5 | 80.0 | 900 | 600 | 1.23 |
| DMC-DMSO | 5 | 38.7 | 620 | − | − |
| DMC-NMe | 5 | 95.4 | 1170 | 1200 | 1.15 |
| DMC-NMP | 5 | 95.3 | 1190 | 1200 | 1.17 |
| Catalyst | Monomer Conversion (%) | NMR | GPC | ||
|---|---|---|---|---|---|
| 4 h | 6 h | Mn | Mn | Ð | |
| DMC-1 | 81.3 | 89.3 | 1200 | 1080 | 1.18 |
| DMC-TBA | 62.7 | 86.6 | 960 | 960 | 1.16 |
| DMC-DMAc | 87.9 | 89.8 | 1090 | 1250 | 1.25 |
| DMC-DMF | 87.2 | 89.1 | 1320 | 1310 | 1.19 |
| DMC-DMSO | 83.6 | 88.8 | 890 | 1150 | 1.26 |
| DMC-NMe | 89.6 | 90.3 | 1300 | 1350 | 1.21 |
| DMC-NMP | 85.9 | 90.7 | 1250 | 1230 | 1.24 |
| Tp (°C) | [VL]0/[GL]0 | Monomer Conversion (%) | kappa × 103 (min−1) | Eab (kJ mol−1) | GPC | |
|---|---|---|---|---|---|---|
| Mn | Ð | |||||
| 130 | 10 | 95.3 | 4.12 | 34.66 | 1130 | 1.14 |
| 140 | 10 | 92.6 | 5.74 | 1050 | 1.15 | |
| 150 | 10 | 94.8 | 7.02 | 1120 | 1.15 | |
| 160 | 10 | 96.7 | 8.52 | 1150 | 1.15 | |
| 160 | 5 | 97.1 | 10.10 | 650 | 1.05 | |
| 160 | 20 | 95.4 | 6.73 | 2040 | 1.26 | |
| 160 | 50 | 89.5 | 4.57 | 4600 | 1.41 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, C.-H.; Lee, M.-W.; Lee, S.-J.; Choi, J.-H.; Lee, E.-G.; Choi, H.-K.; Kim, I. Highly Active Heterogeneous Double Metal Cyanide Catalysts for Ring-Opening Polymerization of Cyclic Monomers. Polymers 2022, 14, 2507. https://doi.org/10.3390/polym14122507
Tran C-H, Lee M-W, Lee S-J, Choi J-H, Lee E-G, Choi H-K, Kim I. Highly Active Heterogeneous Double Metal Cyanide Catalysts for Ring-Opening Polymerization of Cyclic Monomers. Polymers. 2022; 14(12):2507. https://doi.org/10.3390/polym14122507
Chicago/Turabian StyleTran, Chinh-Hoang, Min-Woong Lee, Soo-Jeong Lee, Jin-Hyeok Choi, Eun-Gyeong Lee, Ha-Kyung Choi, and Il Kim. 2022. "Highly Active Heterogeneous Double Metal Cyanide Catalysts for Ring-Opening Polymerization of Cyclic Monomers" Polymers 14, no. 12: 2507. https://doi.org/10.3390/polym14122507
APA StyleTran, C.-H., Lee, M.-W., Lee, S.-J., Choi, J.-H., Lee, E.-G., Choi, H.-K., & Kim, I. (2022). Highly Active Heterogeneous Double Metal Cyanide Catalysts for Ring-Opening Polymerization of Cyclic Monomers. Polymers, 14(12), 2507. https://doi.org/10.3390/polym14122507