2D Zinc-Based Metal–Organic Complexes Derived N-Doped Porous Carbon Nanosheets as Durable Air Cathode for Rechargeable Zn–Air Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of Zn-MOCs
2.3. Preparation of NPCNSs
2.4. Material Characterization
2.5. Electrochemical Evaluation of Catalyst
2.6. Electrochemical Evaluation of Rechargeable Zn–Air Batteries
3. Results and Discussion
3.1. Microstructures of Zn-MOCs
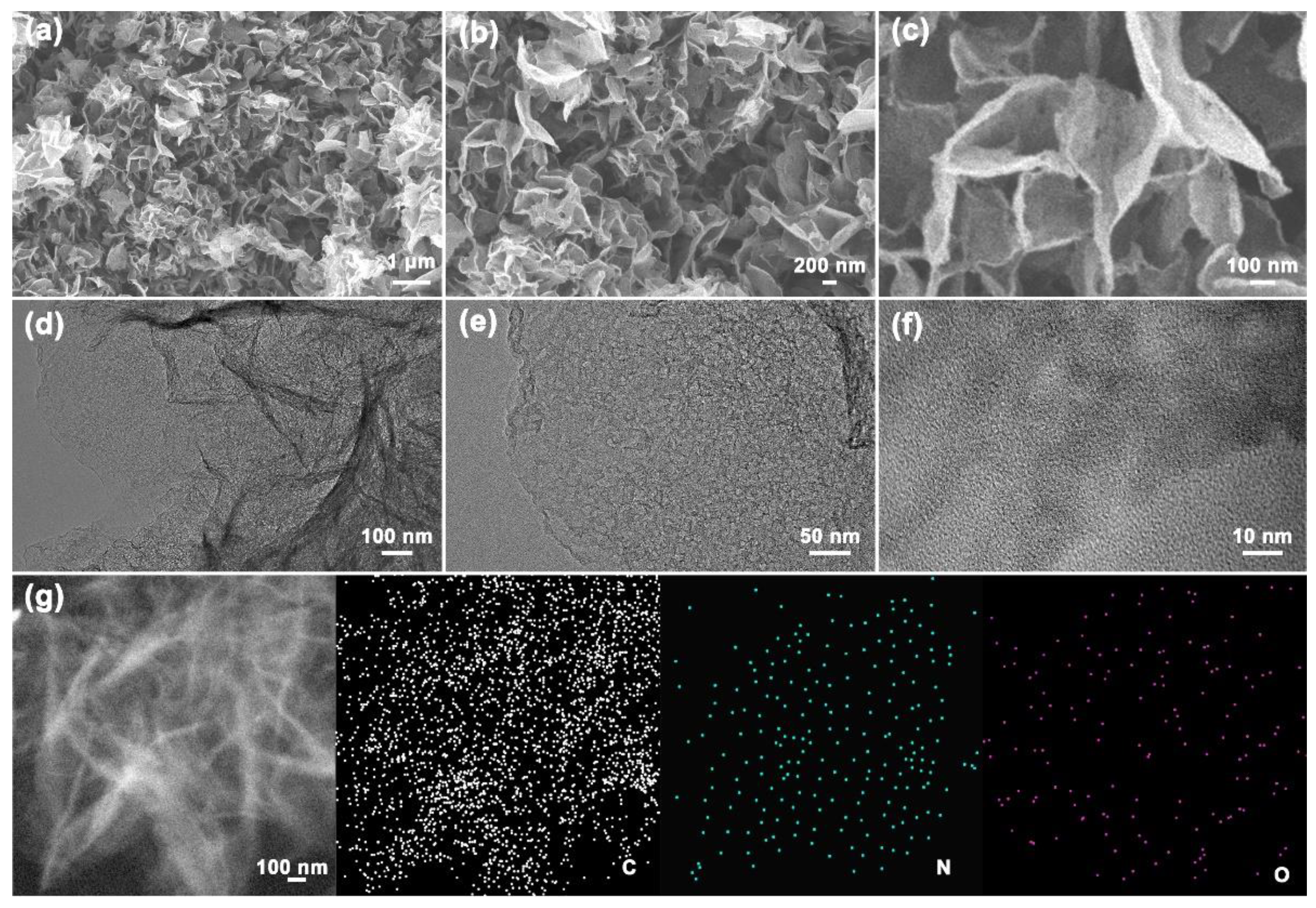
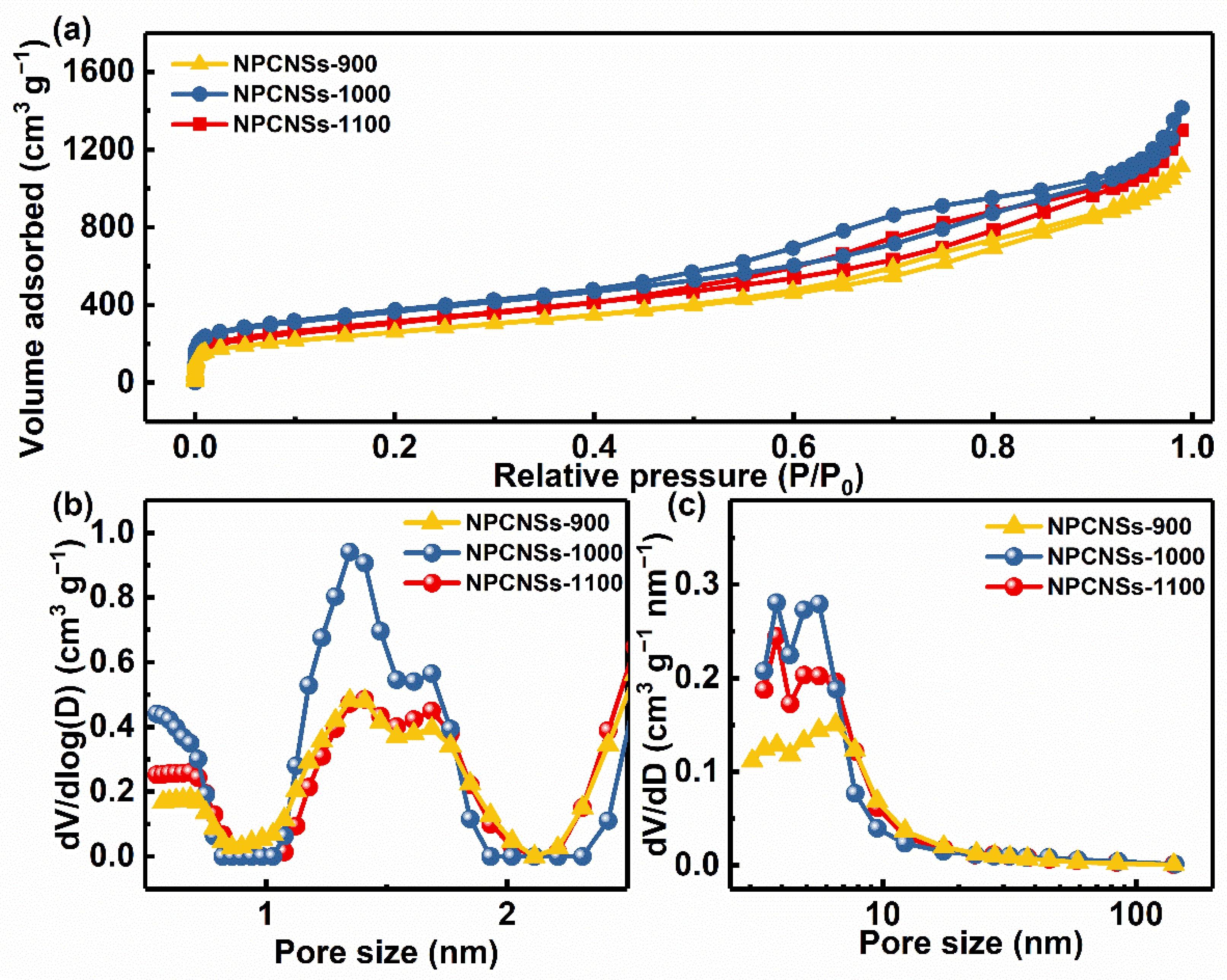
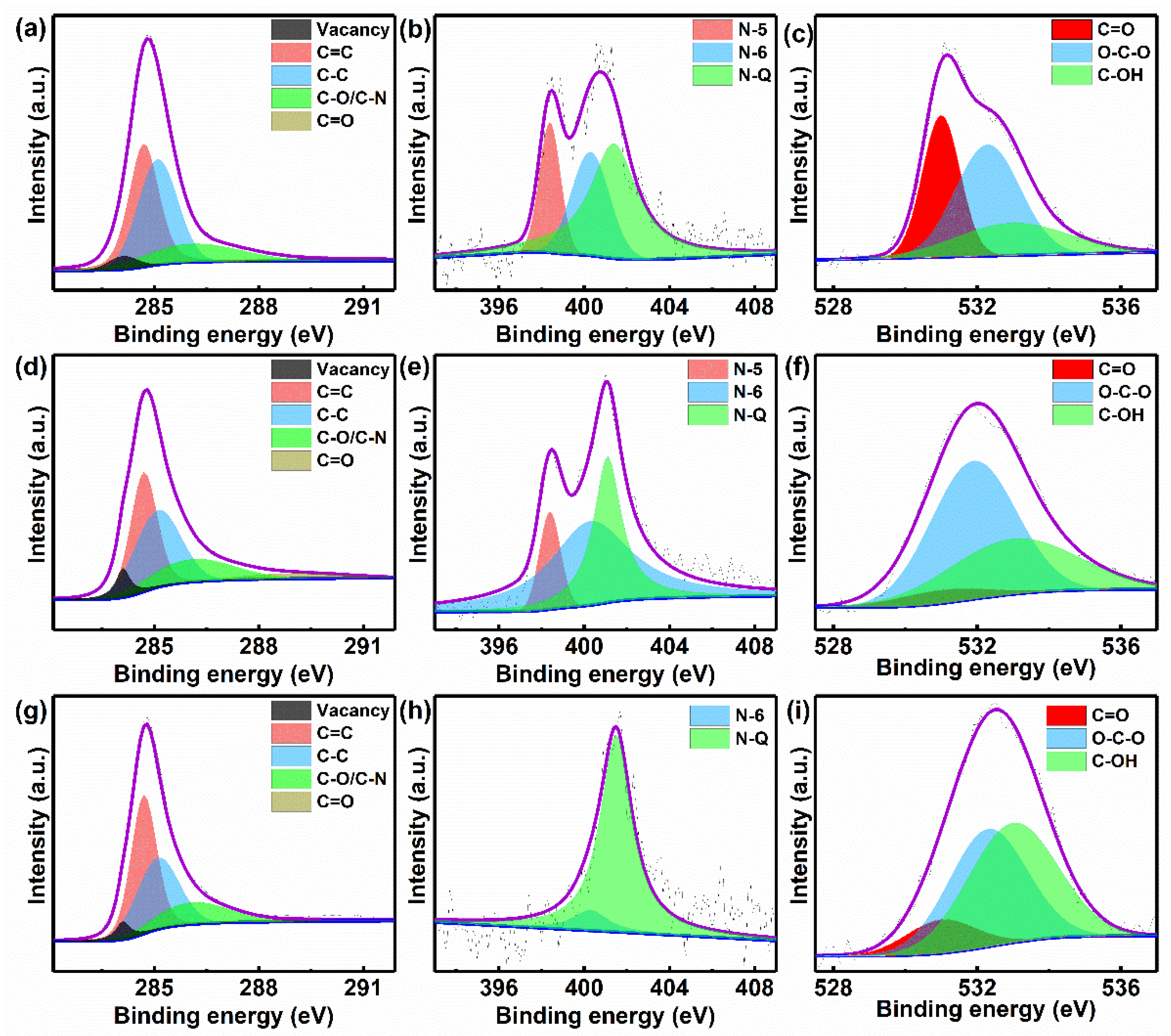
3.2. Microstructures and Physicochemical Properties of NPCNSs
- I-band (~1220 cm−1): sp2–sp3 bonds or stretching vibrations of C–C and C=C in polyene-like structures.
- D-band (1360 cm−1): A1g phonons at the Dirac point (K-point) resulting from the breathing mode of hexatomic rings indicating the disordered carbons and defective graphitic structures, and the broadening of which demonstrates more short-range structural defects such as domain boundaries, edges, vacancies, and doped heteroatoms or more disordered sites.
- D″-band (~1500 cm−1): the amorphous carbon, and the intensity of which is inversely proportional to the crystallinity.
- G-band (~1590 cm−1): optical E2g phonons at the Brillouin zone center (Γ-point) arising from in-plane stretching vibrations of sp2–sp2 bands, and the broadening of which suggests more disorder in the bond length and angle of sp2–sp2 bands or more sp3 carbon atoms.
- D′-band (~1680 cm−1): the intensity of which is positively correlated with the ratio in the density of states of graphite to disordered graphitic lattices.
- Extremely low relative pressure zone (P/P0 < 0.01): type I(a) isotherms with sharp adsorption for indicating lots of ultramicropores (d < 0.7 nm).
- Low relative pressure zone (0.01 < P/P0 < 0.15): type I(b) isotherms with discernible adsorption for demonstrating lots of supermicropores (0.7 < d < 2.0 nm).
- Medium relative pressure zone (0.15 < P/P0 < 0.9): type IV(a) isotherms and an H4-type hysteresis loops with a notable adsorption capacity for revealing plenty of mesopores.
- High relative pressure zone (0.9 < P/P0 < 1.0): type II isotherms with an obvious upward tendency to adsorption capacity for implying the abundant macropores.
3.3. ORR Performances of NPCNSs
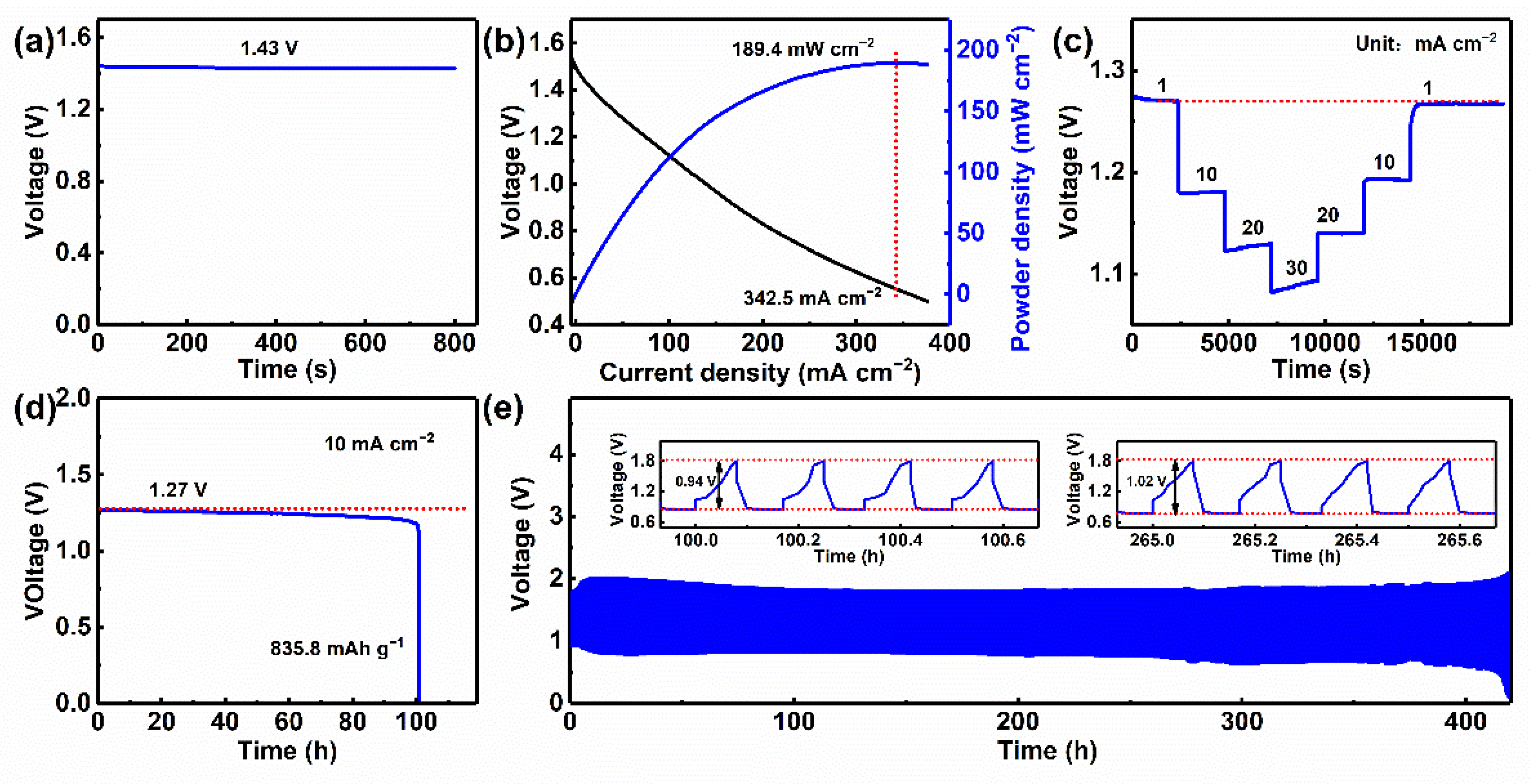
3.4. Electrochemical Performances of Assembled Rechargeable Zn–Air Batteries with NPCNSs-1000
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daskalakis, S.; Wang, M.; Carmalt, C.J.; Vernardou, D. Electrochemical investigation of phenethylammonium bismuth iodide as anode in aqueous Zn2+ electrolytes. Nanomaterials 2022, 11, 656. [Google Scholar] [CrossRef]
- Khanna, S.; Marathey, P.; Vanpariya, A.; Paneliya, S.; Mukhopadhyay, I. In-situ preparation of titania/graphene nanocomposite via a facile sol–gel strategy: A promising anodic material for Li-ion batteries. Mater. Lett. 2021, 300, 130143. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, H.X.; Qin, Y.L.; Zhang, X.B. An efficient three-dimensional oxygen evolution electrode. Angew. Chem. Int. Ed. 2013, 52, 5248–5253. [Google Scholar] [CrossRef]
- Zhong, H.X.; Zhang, Q.; Wang, J.; Zhang, X.B.; Wei, X.L.; Wu, Z.J.; Li, K.; Meng, F.L.; Bao, D.; Yan, J.M. Engineering ultrathin C3N4 quantum dots on graphene as a metal-free water reduction electrocatalyst. ACS Catal. 2018, 8, 3965–3970. [Google Scholar] [CrossRef]
- Lai, Q.; Zheng, J.; Tang, Z.; Bi, D.; Zhao, J.; Liang, Y. Optimal configuration of N-doped carbon defects in 2D turbostratic carbon nanomesh for advanced oxygen reduction electrocatalysis. Angew. Chem. Int. Ed. 2020, 59, 11999–12006. [Google Scholar] [CrossRef]
- Dong, M.Y.; Liu, X.; Jiang, L.X.; Zhu, Z.J.; Shu, Y.J.; Chen, S.; Dou, Y.H.; Liu, P.R.; Yin, H.J.; Zhao, H.J. Cobalt-doped Mn3O4 nanocrystals embedded in graphene nanosheets as a high-performance bifunctional oxygen electrocatalyst for rechargeable Zn−Air batteries. Green Energy Environ. 2020, 5, 499–505. [Google Scholar] [CrossRef]
- Liu, G.; Wu, Y.; Yao, R.; Zhao, F.; Zhao, Q.; Li, J.P. Amorphous iron-nickel phosphide nanocone arrays as efficient bifunctional electrodes for overall water splitting. Green Energy Environ. 2021, 6, 496–505. [Google Scholar] [CrossRef]
- Qiang, F.; Feng, J.; Wang, H.; Yu, J.; Shi, J.; Huang, M.; Shi, Z.; Liu, S.; Li, P.; Dong, L. Oxygen engineering enables N-doped porous carbon nanofibers as oxygen reduction/evolution reaction electrocatalysts for flexible zinc–air batteries. ACS Catal. 2022, 12, 4002–4015. [Google Scholar] [CrossRef]
- Gui, F.; Jin, Q.; Xiao, D.; Xu, X.; Tan, Q.; Yang, D.; Li, B.; Ming, P.; Zhang, C.; Chen, Z.; et al. High-prformance zinc–air batteries based on bifunctional hierarchically porous Nitrogen-doped carbon. Small 2022, 18, 2105928. [Google Scholar] [CrossRef]
- Li, P.; Qi, X.; Zhao, L.; Wang, J.; Wang, M.; Shao, M.; Chen, J.S.; Wu, R.; Wei, Z. Hierarchical 3D porous carbon with facilely accessible Fe–N4 single-atom sites for Zn–Air batteries. J. Mater. Chem. A 2022, 10, 5925–5929. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Zheng, Y.; Fu, G.T.; Sun, D.M.; Li, Y.F.; Tang, Y.W.; Ma, T.Y. Gadolinium-induced valence structure engineering for enhanced oxygen electrocatalysis. Adv. Energy Mater. 2020, 10, 1903833. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Zhu, J.; Kou, Z.; Hu, P.; Liu, L.; Li, S.; Mu, S.; Huang, Y. Defect and pyridinic nitrogen engineering of carbon-based metal-free nanomaterial toward oxygen reduction. Nano Energy 2018, 52, 307–314. [Google Scholar] [CrossRef]
- Alonso-Vante, N.; Feng, Y.J.; Yang, H. Novel non-precious metal electrocatalysts for oxygen electrode reactions. Catalysts 2019, 9, 731. [Google Scholar] [CrossRef] [Green Version]
- Lyu, D.; Yao, S.; Ali, A.; Tian, Z.Q.; Tsiakaras, P.; Shen, P.K. N, S codoped carbon matrix-encapsulated Co9S8 nanoparticles as a highly efficient and durable bifunctional oxygen redox electrocatalyst for rechargeable Zn–Air batteries. Adv. Energy Mater. 2021, 11, 2101249. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [Green Version]
- Xie, Q.; Si, W.; Shen, Y.; Wang, Z.; Uyama, H. N-and O-doped hollow carbons constructed by self-and extrinsic activation for the oxygen reduction reaction and flexible zinc-air batteries. Nanoscale 2021, 13, 16296–16306. [Google Scholar] [CrossRef]
- Zheng, X.J.; Wu, J.; Cao, X.C.; Abbott, J.; Jin, C.; Wang, H.B.; Strasser, P.; Yang, R.Z.; Chen, X.; Wu, G. N-, P-, and S-doped graphene-like carbon catalysts derived from onium salts with enhanced oxygen chemisorption for Zn–Air battery cathodes. Appl. Catal. B 2019, 241, 442–451. [Google Scholar] [CrossRef]
- Lyu, D.D.; Yao, S.X.; Bahari, Y.; Hasan, S.W.; Pan, C.; Zhang, X.R.; Yu, F.; Tian, Z.Q.; Shen, P.K. In situ molecular-level synthesis of N, S co-doped carbon as efficient metal-free oxygen redox electrocatalysts for rechargeable Zn–Air batteries. Appl. Mater. Today 2020, 20, 100737. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Wang, J.; Huang, S.F.; Hou, Y.L.; Li, Y.G.; Zhao, Y.P.; Mu, S.C.; Zhang, J.J.; Zhao, Y.F. N, B-codoped defect-rich graphitic carbon nanocages as high performance multifunctional electrocatalysts. Nano Energy 2017, 42, 334–340. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, J.X.; Jia, J.C.; Hu, X.; Yang, H.J.; Jia, M.L.; Wen, Z.H. The fluorine-doped and defects engineered carbon nanosheets as advanced electrocatalysts for oxygen electroreduction. Appl. Catal. B 2021, 284, 119721. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sui, X.-L.; Zhou, Q.-Y.; Li, J.-Z.; Zhang, J.-J.; Huang, G.-S.; Wang, Z.-B. 1D N-doped hierarchically porous hollow carbon tubes derived from a supramolecular template as metal-free electrocatalysts for a highly efficient oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 6212–6219. [Google Scholar] [CrossRef]
- Tang, C.; Wang, H.F.; Chen, X.; Li, B.Q.; Hou, T.Z.; Zhang, B.; Zhang, Q.; Titirici, M.M.; Wei, F. Topological defects in metal-free nanocarbon for oxygen electrocatalysis. Adv. Mater. 2016, 28, 6845–6851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, Z.; Wang, Y.; Dou, S.; Yan, D.; Liu, D.; Xia, Z.; Wang, S. In situ exfoliated, edge-rich, oxygen-functionalized graphene from carbon fibers for oxygen electrocatalysis. Adv. Mater. 2017, 29, 1606207. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Iqbal, N.; Noor, T.; Imtiaz, U. Nanostructured Mn-doped Zn-N-C @reduced graphene oxide as high performing electrocatalyst for oxygen reduction reaction. J. Electroanal. Chem. 2022, 914, 116324. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Zhao, Q.; Zhang, X.; Liu, R.; Ge, X.; Wang, G.; Zhao, H.; Cai, W. Metal-organic framework derived nitrogen-doped porous carbon@graphene sandwich-like structured composites as bifunctional electrocatalysts for oxygen reduction and evolution reactions. Carbon 2016, 106, 74–83. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Ji, Y.; Lei, Y.; Wang, Y.; Wang, Y.; Li, Y.; Wang, S. Pyridinic-N-dominated doped defective graphene as a superior oxygen electrocatalyst for ultrahigh-energy-density Zn-Air batteries. ACS Energy Lett. 2018, 3, 1183–1191. [Google Scholar] [CrossRef]
- Zhu, J.; Mu, S. Defect engineering in carbon-based electrocatalysts: Insight into intrinsic carbon defects. Adv. Funct. Mater. 2020, 30, 2001097. [Google Scholar] [CrossRef]
- Chen, G.; Liu, P.; Liao, Z.; Sun, F.; He, Y.; Zhong, H.; Zhang, T.; Zschech, E.; Chen, M.; Wu, G.; et al. Zinc-mediated template synthesis of Fe-N-C electrocatalysts with densely accessible Fe-Nx active sites for efficient oxygen reduction. Adv. Mater. 2020, 32, 1907399. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; He, Z.; Liu, Y.; Wang, S.; Wang, H.; Zhu, G. Facile preparation of N-doped hierarchically porous carbon derived from pitch-based hyper-cross-linked polymers as an efficient metal-free catalyst for oxygen-reduction. Appl. Surf. Sci. 2021, 565, 150579. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, W.; Gao, J.; Sun, F.; Zhao, G. H2O2 electrogeneration from O2 electroreduction by N-doped carbon materials: A mini-review on preparation methods, selectivity of N sites, and prospects. Adv. Mater. Interfaces 2021, 8, 2002091. [Google Scholar] [CrossRef]
- Jiang, H.; Gu, J.; Zheng, X.; Liu, M.; Qiu, X.; Wang, L.; Li, W.; Chen, Z.; Ji, X.; Li, J. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR, OER and HER. Energy Environ. Sci. 2019, 12, 322–333. [Google Scholar] [CrossRef]
- Lei, J.; Wang, K.; Deng, B.; Li, Y.; Zhang, S.; Cao, Y. Enhanced oxygen reduction of porous N-doped carbon nanosheets with graphitic N and defects obtained from coal-based graphene quantum dots. J. Alloys Compd. 2022, 914, 165359. [Google Scholar] [CrossRef]
- Jia, P.; Li, X.; Zhang, J.; Zhang, K.; Teng, X.; Hu, X.; Yang, C.; Zhao, D. Liquid-liquid structure transition and its effect on the solidification behaviors and microstructure of Sn75Bi25 alloy. J. Mol. Liq. 2018, 263, 218–227. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X.; Liu, Y.; Hou, L.; Yuan, C. Design and construction of bi-metal MOF-derived yolk–shell Ni2P/ZnP2 hollow microspheres for efficient electrocatalytic oxygen evolution. Mater. Chem. Front. 2020, 4, 1366–1374. [Google Scholar] [CrossRef]
- Zhang, C.L.; Xie, Y.; Liu, J.T.; Cao, F.H.; Cong, H.P.; Li, H. 1D core−shell MOFs derived CoP nanoparticles-embedded N-doped porous carbon nanotubes anchored with MoS2 nanosheets as efficient bifunctional electrocatalysts. Chem. Eng. J. 2021, 419, 129977. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X.P.; Lin, X.; Hu, K.; Zhao, W.; Xie, G.; Lin, X.; Liu, X.; Ito, Y.; Qiu, H.J. Enhanced bifunctional catalytic activities of N-doped graphene by Ni in a 3D trimodal nanoporous nanotubular network and its ultralong cycling performance in Zn−Air batteries. J. Energy Chem. 2022, 66, 466–473. [Google Scholar] [CrossRef]
- Jiang, Y.; Deng, Y.P.; Liang, R.; Chen, N.; King, G.; Yu, A.; Chen, Z. Linker-compensated metal-organic framework with electron delocalized metal sites for bifunctional oxygen electrocatalysis. J. Am. Chem. Soc. 2022, 144, 4783–4791. [Google Scholar] [CrossRef]
- Marques Cordeiro-Junior, P.J.; Kronka, M.S.; Goulart, L.A.; Verissimo, N.C.; Mascaro, L.H.; dos Santos, M.C.; Bertazzoli, R.; de Vasconcelos Lanza, M.R. Catalysis of oxygen reduction reaction for H2O2 electrogeneration: The impact of different conductive carbon matrices and their physicochemical properties. J. Catal. 2020, 392, 56–68. [Google Scholar] [CrossRef]
- Mei, Z.Y.; Cai, S.; Zhao, G.; Jing, Q.; Sheng, X.; Jiang, J.; Guo, H. Understanding electronic configurarions and coordination environment for enhanced ORR process and improved Zn-Air battery performance. Energy Storage Mater. 2022, 50, 12–20. [Google Scholar] [CrossRef]
- Zhu, W.G.; Zheng, Y.Q.; Zhu, H.L.; Wang, J.J. Structural diversity controlled by alkali/alkaline earth metals for heterometallic AeI/AeII-ZnII coordination polymers based on 4-nitrobenzene-1,2-dicarboxylate. J. Coord. Chem. 2016, 69, 3115–3129. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Wang, G.; Hou, L.; Yuan, C. Eco-friendly and scalable synthesis of micro-/mesoporous carbon sub-microspheres as competitive electrodes for supercapacitors and sodium-ion batteries. Appl. Surf. Sci. 2020, 533, 147511. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Zhang, Y.; Zaman, F.U.; Hou, L.; Yuan, C. Construction of mesoporous bimetallic (Ni, Co) organic framework microspheres for lithium-ion capacitors. Electrochem. Commun. 2021, 125, 107006. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, Q.; Wu, D.; Zhang, Y.; Jia, P.; Hou, L.; Yuan, C. Efficient activation engineering from the inside out toward hierarchically porous carbon framework as electrode materials for supercapacitors. ACS Appl. Energy Mater. 2022, 5, 5719–5729. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Cychosz, K.A.; Guillet-Nicolas, R.; Garcia-Martinez, J.; Thommes, M. Recent advances in the textural characterization of hierarchically structured nanoporous materials. Chem. Soc. Rev. 2017, 46, 389–414. [Google Scholar] [CrossRef]
- Ganesan, K.; Ghosh, S.; Krishna, N.G.; Ilango, S.; Kamruddin, M.; Tyagi, A.K. A comparative study on defect estimation using XPS and Raman spectroscopy in few layer nanographitic structures. Phys. Chem. Chem. Phys. 2016, 18, 22160–22167. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Miao, H.; Hu, R.; Chen, B.; Yin, M.; Zhang, H.; Xia, L.; Zhang, C.; Yuan, J. A-site deficient perovskite nanofibers boost oxygen evolution reaction for zinc–air batteries. Appl. Surf. Sci. 2021, 536, 147806. [Google Scholar] [CrossRef]
- Yang, H.; Miao, J.; Hung, S.F.; Chen, J.; Tao, H.B.; Wang, X.; Zhang, L.; Chen, R.; Gao, J.; Chen, H.M.; et al. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: Development of highly efficient metal-free bifunctional electrocatalyst. Sci. Adv. 2016, 2, e1501122. [Google Scholar] [CrossRef] [Green Version]
- Kramm, U.I.; Herrmann-Geppert, I.; Behrends, J.; Lips, K.; Fiechter, S.; Bogdanoff, P. On an easy way to prepare metal–nitrogen doped carbon with exclusive presence of MeN4-type sites active for the ORR. J. Am. Chem. Soc. 2016, 138, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, X.; Zheng, L.; Li, Y.; Guo, X.; Wan, X.; Liu, Q.; Shang, J.; Shui, J. Insights into the role of active site density in the fuel cell performance of Co-N-C catalysts. Appl. Catal. B 2019, 256, 117849. [Google Scholar] [CrossRef]
- Luo, F.; Roy, A.; Silvioli, L.; Cullen, D.A.; Zitolo, A.; Sougrati, M.T.; Oguz, I.C.; Mineva, T.; Teschner, D.; Wagner, S.; et al. P-block single-metal-site tin/nitrogen-doped carbon fuel cell cathode catalyst for oxygen reduction reaction. Nat. Mater. 2020, 19, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Zhu, J.; Ma, L.; Jin, Z.; Ge, J.; Deng, X.; Hou, Y.; He, Q.; Li, J.; Jia, Q.; et al. Microporous framework induced synthesis of single-atom dispersed Fe-N-C acidic ORR catalyst and its in situ reduced Fe-N4 active site identification revealed by x-ray absorption spectroscopy. ACS Catal. 2018, 8, 2824–2832. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Gontarek, E.; Lieder, M.; Titirici, M.M. Salt melt synthesis of curved nitrogen-doped carbon nanostructures: ORR kinetics boost. Appl. Surf. Sci. 2018, 435, 543–551. [Google Scholar] [CrossRef]
- Douka, A.I.; Xu, Y.; Yang, H.; Zaman, S.; Yan, Y.; Liu, H.; Salam, M.A.; Xia, B.Y. A zeolitic-imidazole frameworks-derived interconnected macroporous carbon matrix for efficient oxygen electrocatalysis in rechargeable Zinc-Air batteries. Adv. Mater. 2020, 32, 2002170. [Google Scholar] [CrossRef]
- Nam, G.; Park, J.; Choi, M.; Oh, P.; Park, S.; Kim, M.G.; Park, N.; Cho, J.; Lee, J.S. Carbon-coated core-shell Fe-Cu nanoparticles as highly active and durable electrocatalysts for a Zn-air battery. ACS Nano 2015, 9, 6493–6501. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, X.; Xi, S.; Zhang, L.; Chen, Z.; Zeng, Z.; Huang, M.; Yang, H.; Liu, B.; Pennycook, S.J.; et al. Atomically dispersed cobalt trifunctional electrocatalysts with tailored coordination environment for flexible rechargeable Zn-air battery and self-driven water splitting. Adv. Energy Mater. 2020, 10, 2002896. [Google Scholar] [CrossRef]
- Wu, J.; Hu, L.; Wang, N.; Li, Y.; Zhao, D.; Li, L.; Peng, X.; Cui, Z.; Ma, L.J.; Tian, Y.; et al. Surface confinement assisted synthesis of nitrogen-rich hollow carbon cages with Co nanoparticles as breathable electrodes for Zn-air batteries. Appl. Catal. B 2019, 254, 55–65. [Google Scholar] [CrossRef]
- Shinde, S.S.; Lee, C.H.; Sami, A.; Kim, D.H.; Lee, S.U.; Lee, J.H. Scalable 3-D carbon nitride sponge as an efficient metal-free bifunctional oxygen electrocatalyst for rechargeable Zn-Air batteries. ACS Nano 2017, 11, 347–357. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Hu, W.; Ning, J.; Zhong, Y.; Hu, Y. Formation of sandwiched leaf-like CNTs-Co/ZnCo2O4@NC-CNTs nanohybrids for high-power-density rechargeable Zn-Air batteries. Nano Energy 2021, 82, 105710. [Google Scholar] [CrossRef]
- Deng, W.; Li, G.; Wu, T.; He, L.; Wu, J.; Liu, J.; Zheng, H.; Li, X.; Yang, Y.; Jing, M.; et al. Heteroatom functionalized double-layer carbon nanocages as highly efficient oxygen electrocatalyst for Zn-Air batteries. Carbon 2022, 186, 589–598. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, P.; Zhang, J.; Xia, G.; Yu, Z.; Sun, J.; Ji, X. 2D Zinc-Based Metal–Organic Complexes Derived N-Doped Porous Carbon Nanosheets as Durable Air Cathode for Rechargeable Zn–Air Batteries. Polymers 2022, 14, 2581. https://doi.org/10.3390/polym14132581
Jia P, Zhang J, Xia G, Yu Z, Sun J, Ji X. 2D Zinc-Based Metal–Organic Complexes Derived N-Doped Porous Carbon Nanosheets as Durable Air Cathode for Rechargeable Zn–Air Batteries. Polymers. 2022; 14(13):2581. https://doi.org/10.3390/polym14132581
Chicago/Turabian StyleJia, Peng, Jiawei Zhang, Guangmei Xia, Zhenjiang Yu, Jiazhen Sun, and Xingxiang Ji. 2022. "2D Zinc-Based Metal–Organic Complexes Derived N-Doped Porous Carbon Nanosheets as Durable Air Cathode for Rechargeable Zn–Air Batteries" Polymers 14, no. 13: 2581. https://doi.org/10.3390/polym14132581








