Bevacizumab-Controlled Delivery from Polymeric Microparticle Systems as Interesting Tools for Pathologic Angiogenesis Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
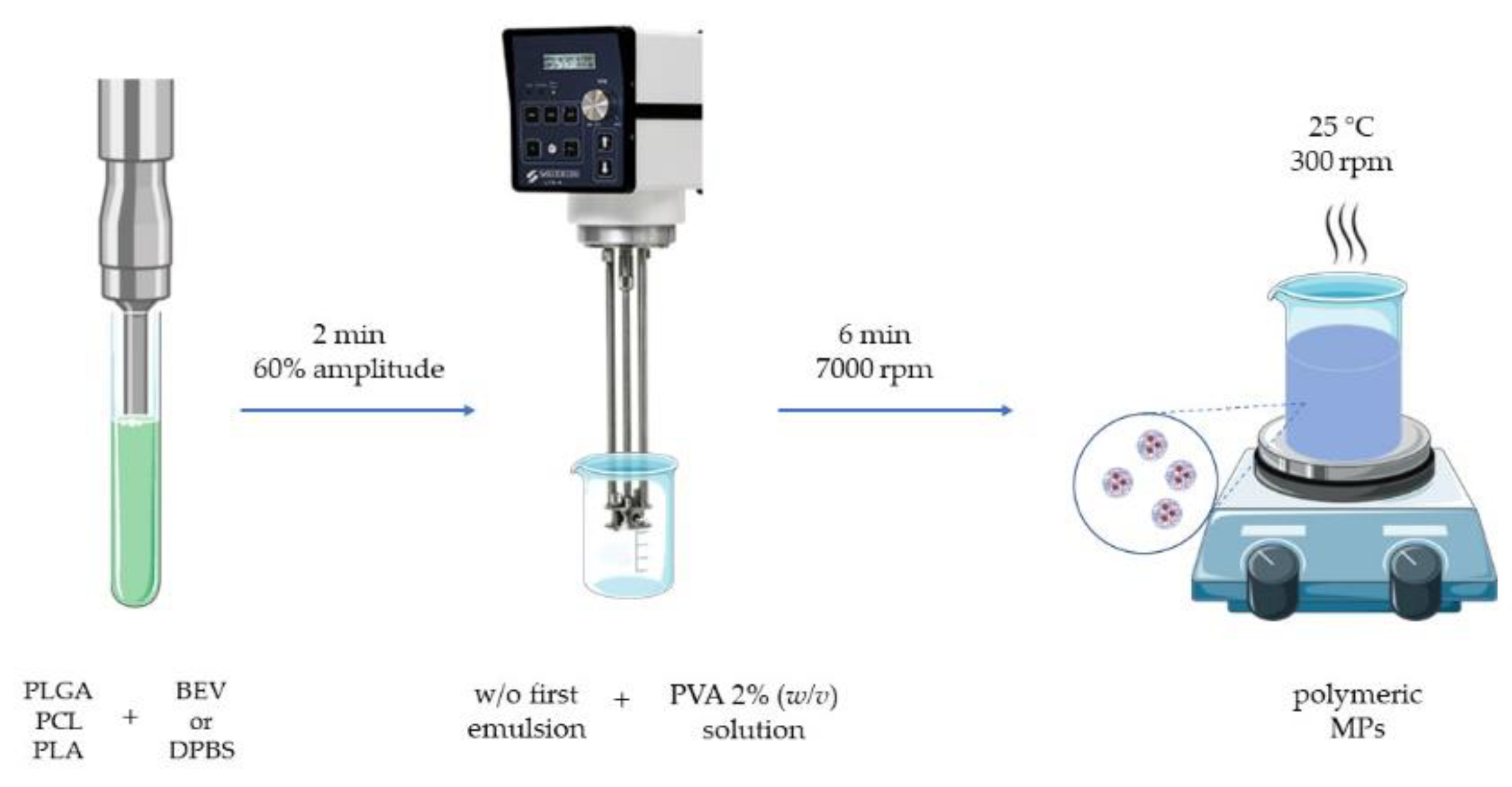
2.2. Polymeric Microparticle Production
2.3. Microparticle Characterization
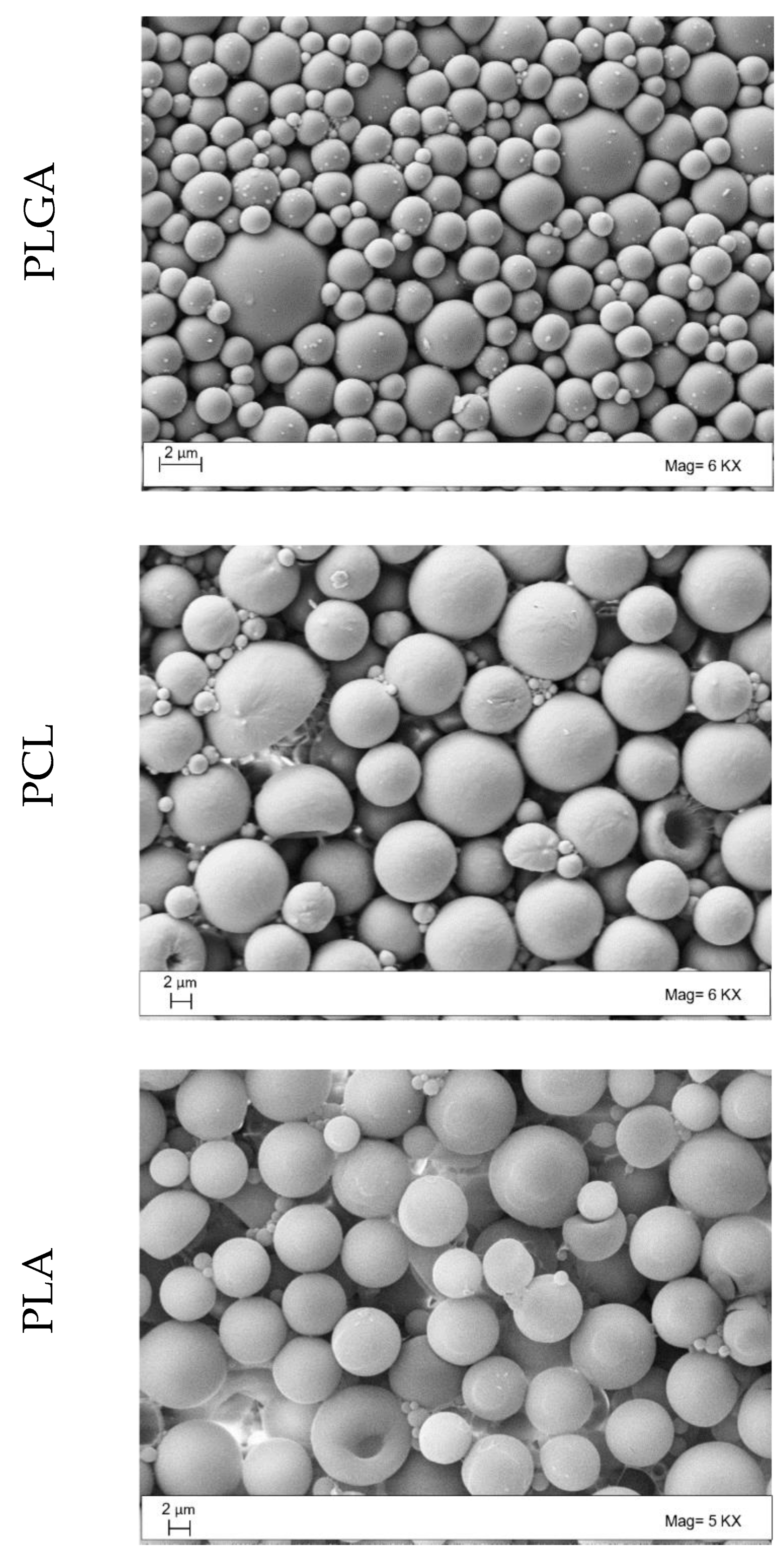
2.3.1. Morphology Studies
2.3.2. Entrapment Efficiency
2.4. In Vitro Release Studies
2.5. Microparticle Biological Validation
2.6. Statistical Analysis
3. Results
3.1. Microparticle Production and Characterization
3.2. Drug Release
3.3. Suspension Stability
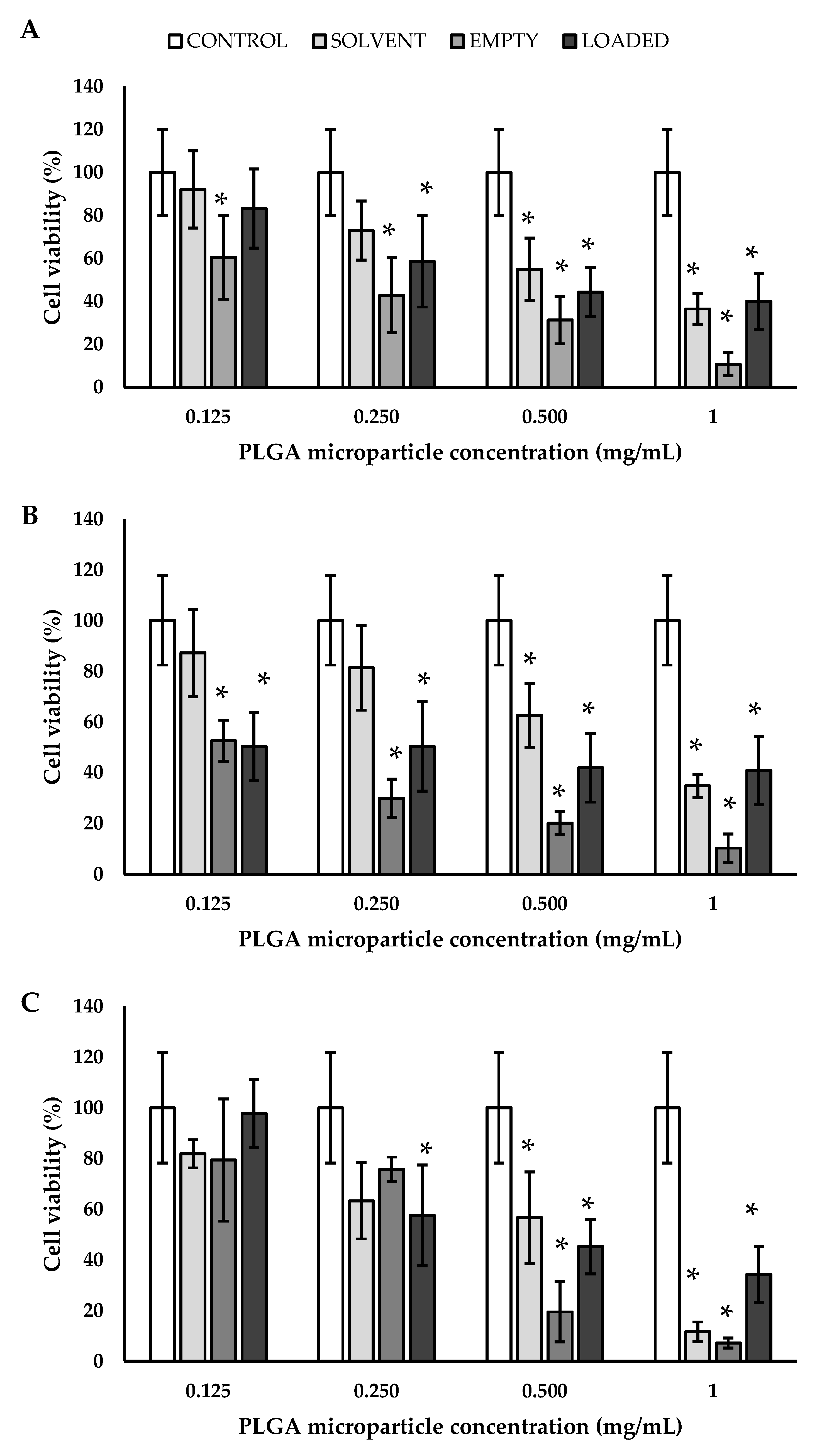
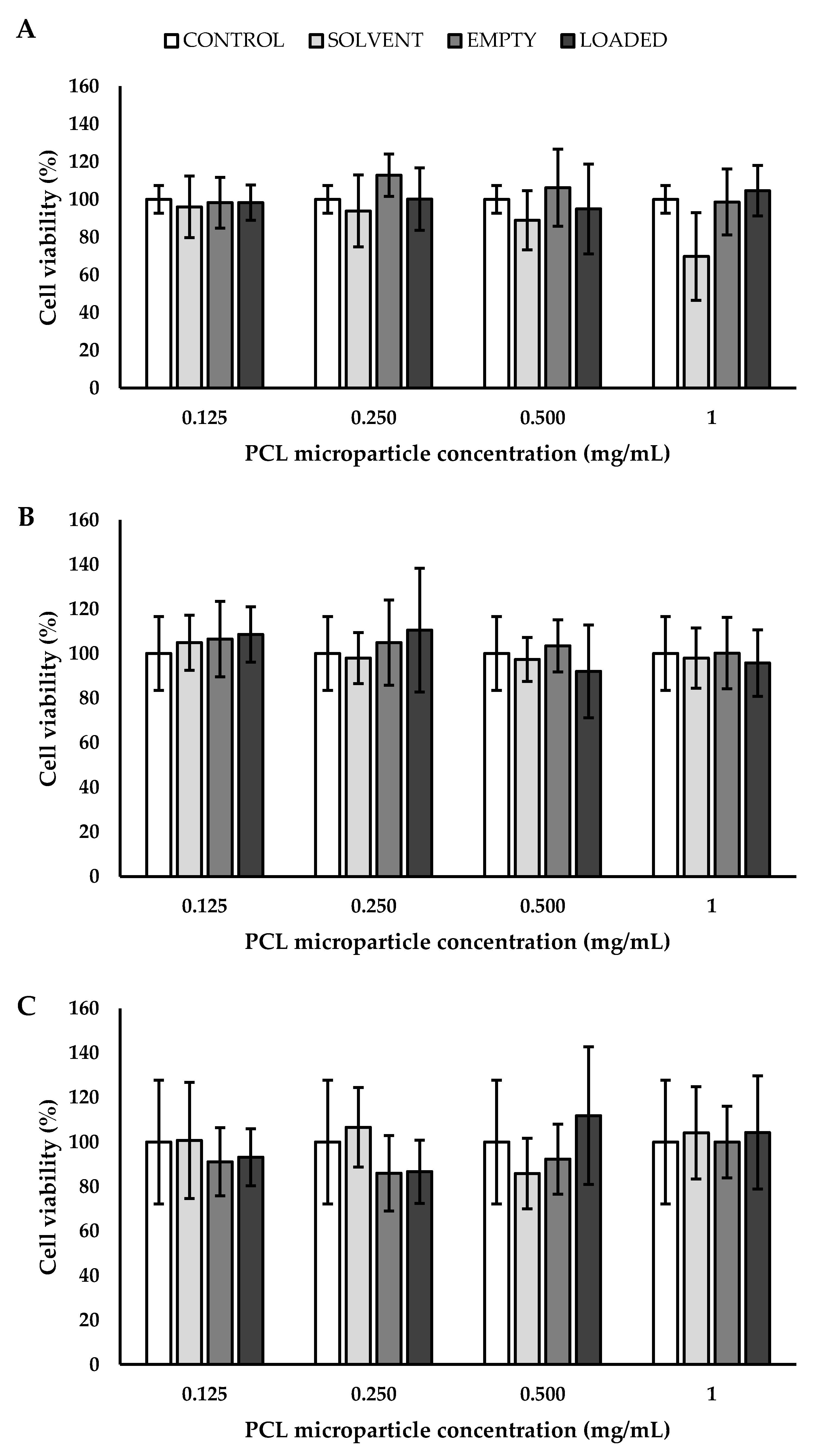
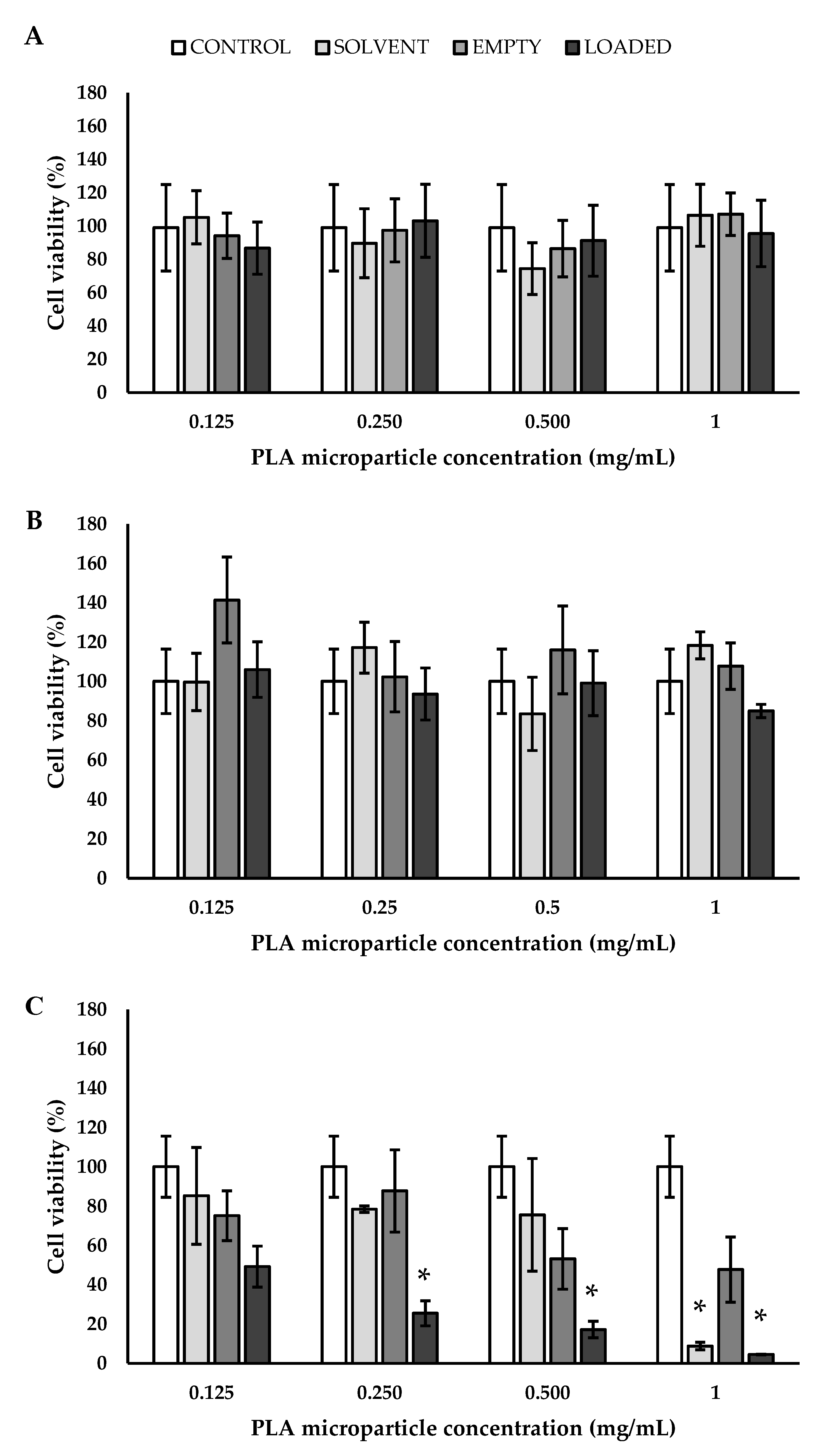
3.4. Biological Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stefanadis, C.; Toutouzas, K.; Stefanadi, E.; Kolodgie, F.; Virmani, R.; Kipshidze, N. First experimental application of bevacizumab-eluting PC coated stent for inhibition of vasa vasorum of atherosclerotic plaque: Angiographic results in a rabbit atheromatic model. Hellenic. J. Cardiol. 2006, 47, 7–10. [Google Scholar] [PubMed]
- Li, Y.; Zhu, Y.; Deng, Y.; Liu, Y.; Mao, Y.; Wang, J.; Sun, J. The therapeutic effect of bevacizumab on plaque neovascularization in a rabbit model of atherosclerosis during contrast-enhanced ultrasonography. Sci. Rep. 2016, 6, 30417. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.N.; Caetano, B.L.; Boni, F.I.; Sousa, F.; Magnani, M.; Sarmento, B.; Ferreira Cury, B.S.; Daflon Gremião, M.P. Alginate-based delivery systems for bevacizumab local therapy: In vitro structural features and release properties. J. Pharm. Sci. 2019, 108, 1559–1568. [Google Scholar] [CrossRef]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-based composites for various biomedical applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef]
- Swider, E.; Koshkina, O.; Tel, J.; Cruz, L.J.; de Vries, I.J.M.; Srinivas, M. Customizing poly(lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018, 73, 38–51. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- De Negri Atanasio, G.; Ferrari, P.F.; Campardelli, R.; Perego, P.; Palombo, D. Innovative nanotools for vascular drug delivery: The atherosclerosis case study. J. Mater. Chem. B 2021, 9, 8558–8568. [Google Scholar] [CrossRef]
- Zhi, K.; Raji, B.; Nookala, A.R.; Khan, M.M.; Nguyen, X.H.; Sakshi, S.; Pourmotabbed, T.; Yallapu, M.M.; Kochat, H.; Tadrous, E.; et al. PLGA nanoparticle-based formulations to cross the blood–brain barrier for drug delivery: From R&D to cGMP. Pharmaceutics 2021, 13, 500. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Zhu, Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater. Sci. Eng. C 2018, 92, 1041–1060. [Google Scholar] [CrossRef]
- Lim, M.P.A.; Lee, W.L.; Widjaja, E.; Loo, S.C.J. One-step fabrication of core–shell structured alginate–PLGA/PLLA microparticles as a novel drug delivery system for water soluble drugs. Biomater. Sci. 2013, 1, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.-Z. Biocompatibility and toxicity of nanoparticles and nanotubes. J. Nanomater. 2012, 2012, 1–19. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Yun, Y.; Park, K. PLA micro- and nano-particles. Adv. Drug Deliv. Rev. 2016, 107, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, M.; Zarei-Ghanavati, S.; Soroush, D.; Rouhbakhsh, M.; Jaafari, M.R.; Malaekeh-Nikouei, B. Preparation, characterization, and in vivo evaluation of nanoliposomes-encapsulated bevacizumab (Avastin) for intravitreal administration. Retina 2009, 29, 699–703. [Google Scholar] [CrossRef]
- Alves, A.d.C.S.; Bruinsmann, F.A.; Guterres, S.S.; Pohlmann, A.R. Organic nanocarriers for bevacizumab delivery: An overview of development, characterization and applications. Molecules 2021, 26, 4127. [Google Scholar] [CrossRef] [PubMed]
- Jenjob, R.; Phakkeeree, T.; Seidi, F.; Theerasilp, M.; Crespy, D. Emulsion techniques for the production of pharmacological nanoparticles. Macromol. Biosci. 2019, 19. [Google Scholar] [CrossRef]
- Gharieh, A.; Khoee, S.; Mahdavian, A.R. Emulsion and miniemulsion techniques in preparation of polymer nanoparticles with versatile characteristics. Adv. Colloid Interface Sci. 2019, 269, 152–186. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.; Fonte, P.; Cruz, A.; Kennedy, P.J.; Pinto, I.M.; Sarmento, B. Polyester-based nanoparticles for the encapsulation of monoclonal antibodies. In Recombinant Glycoprotein Production; Picanço-Castro, V., Swiech, K., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1674, pp. 239–253. [Google Scholar]
- Ye, Z.; Ji, Y.-L.; Ma, X.; Wen, J.-G.; Wei, W.; Huang, S.-M. Pharmacokinetics and distributions of bevacizumab by intravitreal injection of bevacizumab-PLGA microspheres in rabbits. Int. J. Ophthalmol. 2015, 8, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Jacobs, K.M.; Ohr, M.P.; Swindle-Reilly, K.E. Chitosan–polycaprolactone core–shell microparticles for sustained delivery of bevacizumab. Mol. Pharmaceutics 2020, 17, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Yandrapu, S.K.; Upadhyay, A.K.; Petrash, J.M.; Kompella, U.B. Nanoparticles in porous microparticles prepared by supercritical infusion and pressure quench technology for sustained delivery of bevacizumab. Mol. Pharmaceutics 2013, 10, 4676–4686. [Google Scholar] [CrossRef]
- Sousa, F.; Cruz, A.; Fonte, P.; Pinto, I.M.; Neves-Petersen, M.T.; Sarmento, B. A new paradigm for antiangiogenic therapy through controlled release of bevacizumab from PLGA nanoparticles. Sci. Rep. 2017, 7, 3736. [Google Scholar] [CrossRef]
- Bassim, N.; Scott, K.; Giannuzzi, L.A. Recent advances in focused ion beam technology and applications. MRS Bull. 2014, 39, 317–325. [Google Scholar] [CrossRef]
- Abdelkader, D.H.; El-Gizawy, S.A.; Faheem, A.M.; McCarron, P.A.; Osman, M.A. Effect of process variables on formulation, in-vitro characterisation and subcutaneous delivery of insulin PLGA nanoparticles: An optimisation study. J. Drug Deliv. Sci. Technol. 2018, 43, 160–171. [Google Scholar] [CrossRef]
- Pillay, V.; Fassihi, R. In vitro release modulation from crosslinked pellets for site-specific drug delivery to the gastrointestinal tract. J. Control. Release 1999, 59, 229–242. [Google Scholar] [CrossRef]
- Gallagher, K.M.; Corrigan, O.I. Mechanistic aspects of the release of levamisole hydrochloride from biodegradable polymers. J. Control. Release 2000, 69, 261–272. [Google Scholar] [CrossRef]
- De Negri Atanasio, G.; Ferrari, P.F.; Campardelli, R.; Perego, P.; Palombo, D. Poly (lactic-co-glycolic acid) nanoparticles and nanoliposomes for protein delivery in targeted therapy: A comparative in vitro study. Polymers 2020, 12, 2566. [Google Scholar] [CrossRef] [PubMed]
- Giannuzzi, L.A. Introduction to Focused ion Beams: Instrumentation, Theory, Techniques and Practice; Springer: New York, NY, USA, 2005. [Google Scholar]
- Yoo, J.; Won, Y.-Y. Phenomenology of the initial burst release of drugs from PLGA microparticles. ACS Biomater. Sci. Eng. 2020, 6, 6053–6062. [Google Scholar] [CrossRef]
- Pagels, R.F.; Prud’homme, R.K. Polymeric nanoparticles and microparticles for the delivery of peptides, biologics, and soluble therapeutics. J. Control. Release 2015, 219, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.D.; Mandal, A.; Patel, S.; Mitra, A.K. Extended release microparticle-in-gel formulation of octreotide: Effect of polymer type on acylation of peptide during in vitro release. Int. J. Pharm. 2015, 496, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Sumpter, B.G.; Noid, D.W.; Barnes, M.D. Recent developments in the formation, characterization, and simulation of micron and nano-scale droplets of amorphous polymer blends and semi-crystalline polymers. Polymer 2003, 44, 4389–4403. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.D.; Lee, P.I. Probing the mechanisms of drug release from amorphous solid dispersions in medium-soluble and medium-insoluble carriers. J. Control. Release 2015, 211, 85–93. [Google Scholar] [CrossRef] [PubMed]





| MPs | DMD ± SD (μm) | EE ± SD (%) | PMD ± SD (μm) |
|---|---|---|---|
| PLGA E | 2.127 ± 0.006 a | - | 2.077 ± 0.006 a |
| PCL E | 4.090 ± 0.001 b | - | 4.700 ± 0.010 b |
| PLA E | 6.120 ± 0.066 c | - | 5.117 ± 0.006 c |
| PLGA BEV | 3.423 ± 0.025 a | 77.15 ± 0.36 a | 2.720 ± 0.017 a |
| PCL BEV | 4.437 ± 0.081 b | 76.54 ± 5.44 a | 4.600 ± 0.020 b |
| PLA BEV | 6.447 ± 0.064 c | 51.00 ± 0.28 b | 6.767 ± 0.006 c |
| MPs | Temperature (°C) | PMD ± SD (μm) |
|---|---|---|
| PLGA | 4 | 5.08 ± 2.43 a |
| PCL | 3.28 ± 2.23 b | |
| PLA | 5.06 ± 1.20 a | |
| PLGA | 25 | 4.32 ± 2.30 a |
| PCL | 3.69 ± 2.74 c | |
| PLA | 5.08 ± 1.21 b | |
| PLGA | 37 | 2.72 ± 1.61 a |
| PCL | 4.49 ± 3.29 b | |
| PLA | 5.10 ± 1.19 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Negri Atanasio, G.; Ferrari, P.F.; Campardelli, R.; Firpo, G.; Perego, P.; Palombo, D. Bevacizumab-Controlled Delivery from Polymeric Microparticle Systems as Interesting Tools for Pathologic Angiogenesis Diseases. Polymers 2022, 14, 2593. https://doi.org/10.3390/polym14132593
De Negri Atanasio G, Ferrari PF, Campardelli R, Firpo G, Perego P, Palombo D. Bevacizumab-Controlled Delivery from Polymeric Microparticle Systems as Interesting Tools for Pathologic Angiogenesis Diseases. Polymers. 2022; 14(13):2593. https://doi.org/10.3390/polym14132593
Chicago/Turabian StyleDe Negri Atanasio, Giulia, Pier Francesco Ferrari, Roberta Campardelli, Giuseppe Firpo, Patrizia Perego, and Domenico Palombo. 2022. "Bevacizumab-Controlled Delivery from Polymeric Microparticle Systems as Interesting Tools for Pathologic Angiogenesis Diseases" Polymers 14, no. 13: 2593. https://doi.org/10.3390/polym14132593
APA StyleDe Negri Atanasio, G., Ferrari, P. F., Campardelli, R., Firpo, G., Perego, P., & Palombo, D. (2022). Bevacizumab-Controlled Delivery from Polymeric Microparticle Systems as Interesting Tools for Pathologic Angiogenesis Diseases. Polymers, 14(13), 2593. https://doi.org/10.3390/polym14132593







