Adsorption of Welan Gum on Montmorillonite and Its Influencing Factors
Abstract
:1. Introduction
2. Experimental Section
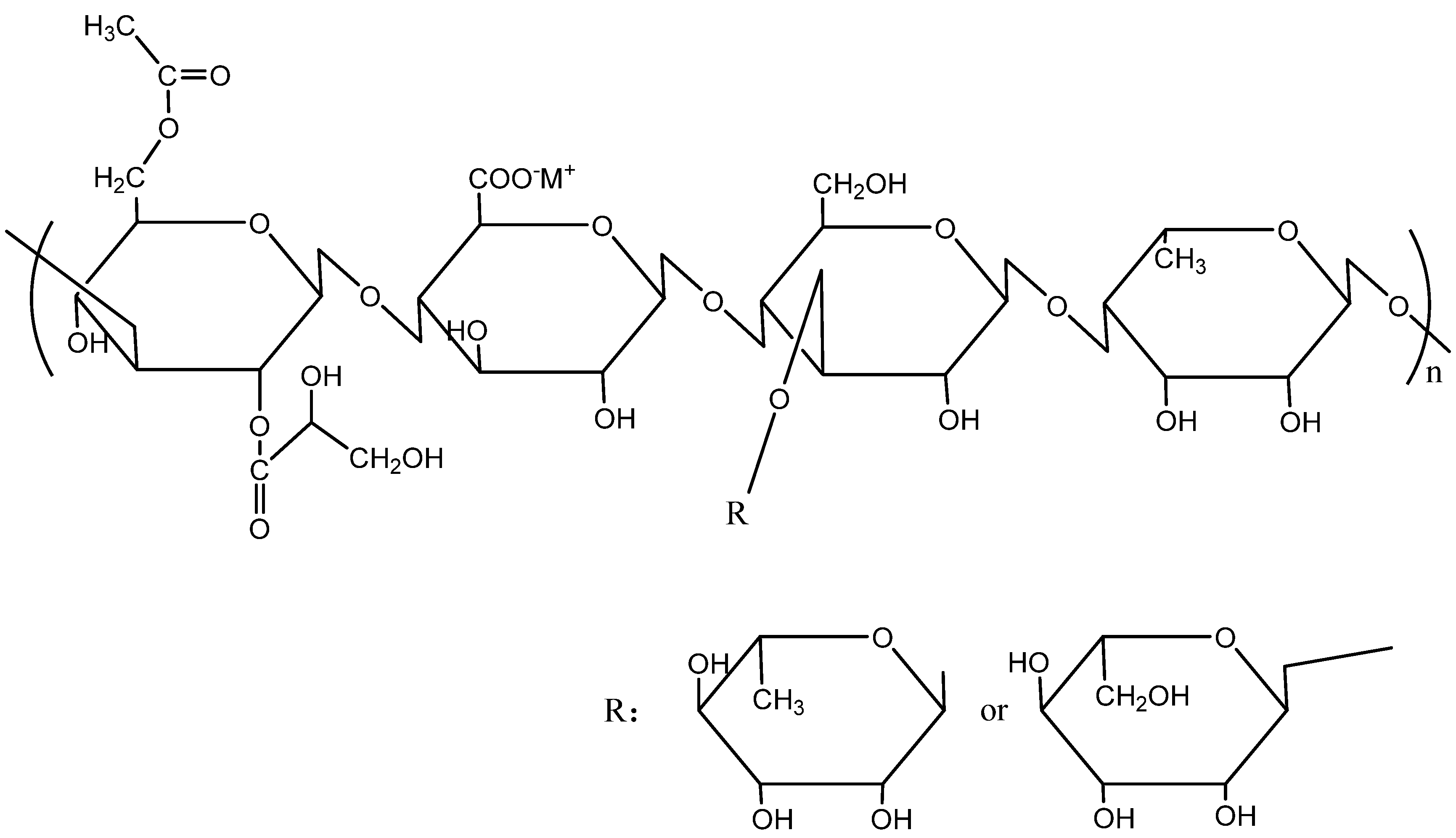
2.1. Materials
2.2. Methods
2.2.1. Determination of the Standard Curve of Absorbance–Welan Gum Concentration
2.2.2. Determination of Adsorption Capacity
2.2.3. Evaluation of the Effect of Temperature, pH Value, and Inorganic Salt on Adsorption Performance
- (1)
- Determination of the effect of temperature:
- (2)
- Determination of the effect of pH value:
- (3)
- Determination of the effect of inorganic salt:
3. Results and Discussion
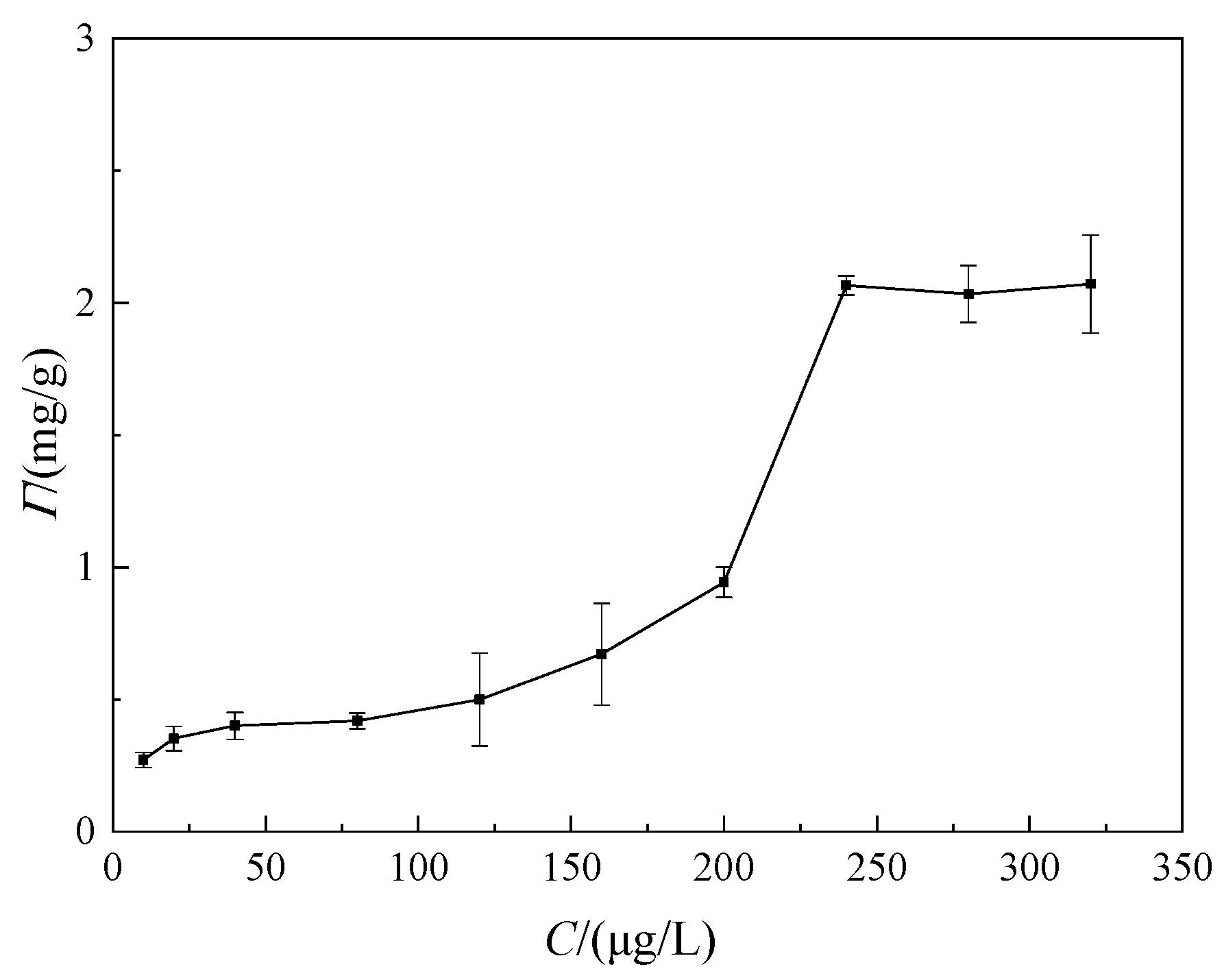
3.1. Adsorption Behavior
- (1)
- Standard curve of absorbance–welan concentration:
- (2)
- Adsorption equilibrium state:
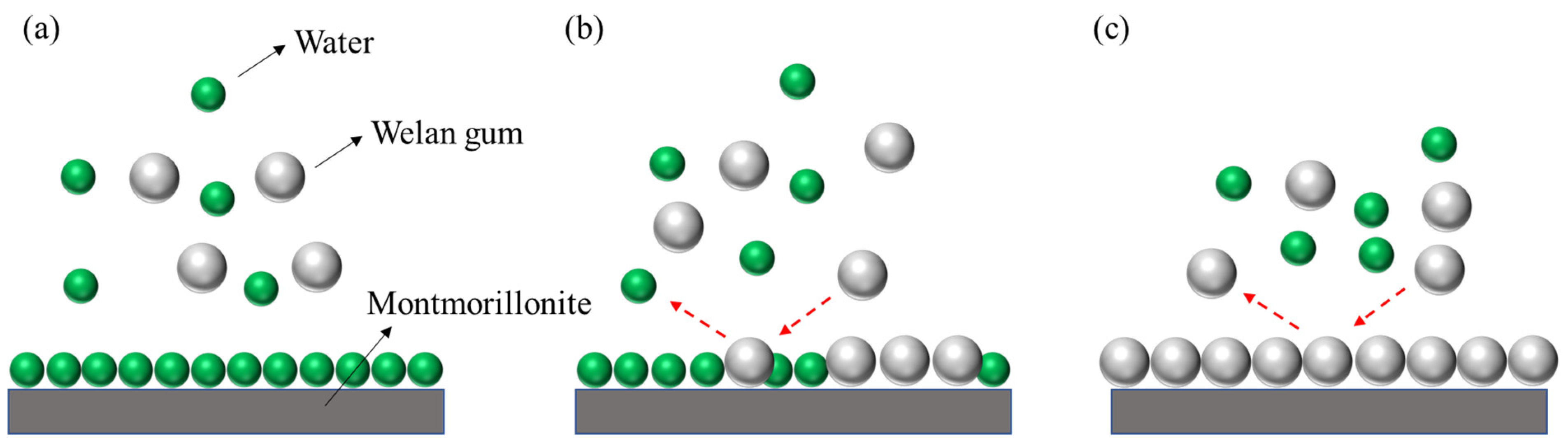
3.2. Adsorption Mechanism
3.2.1. FT-IR
3.2.2. XRD
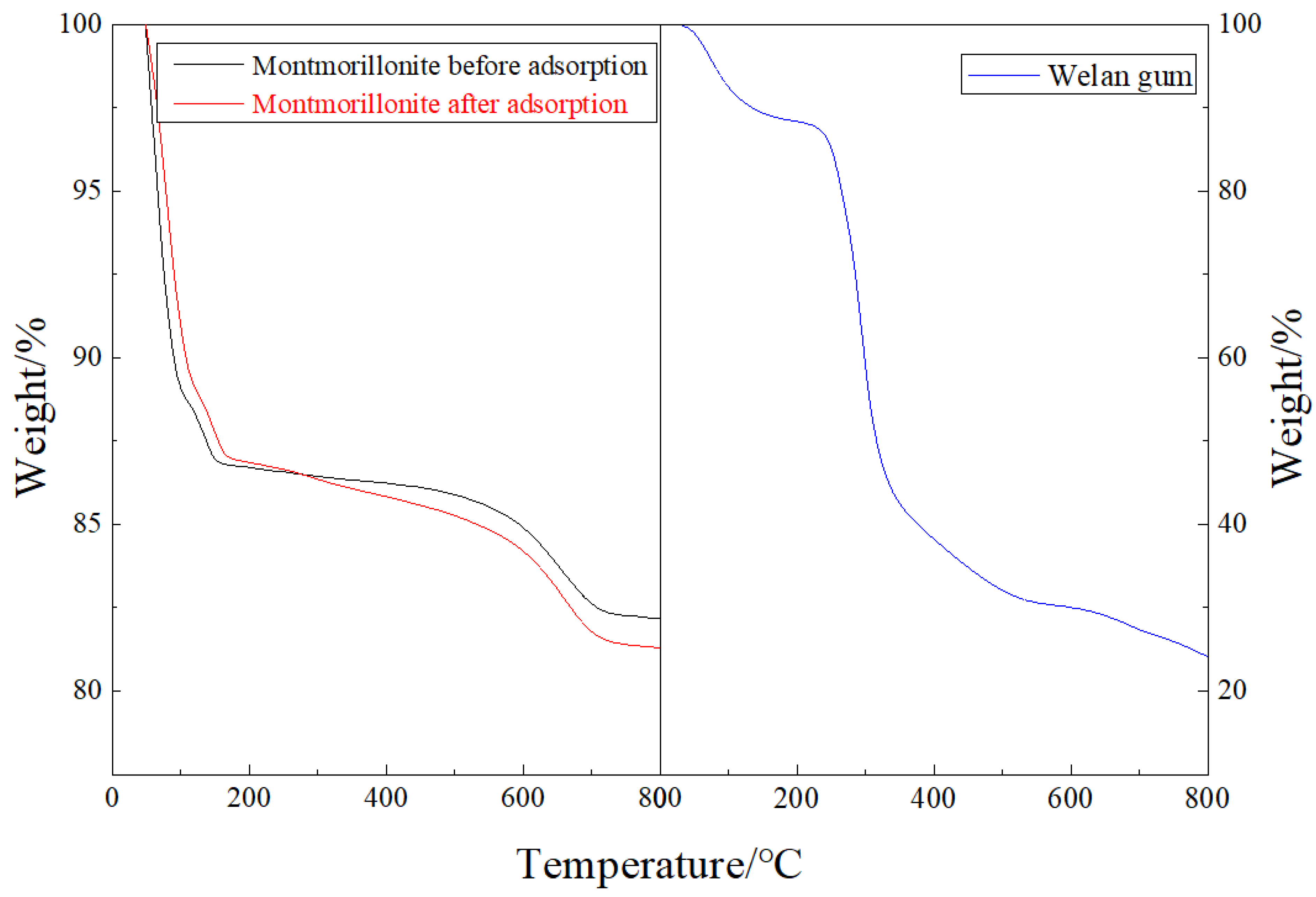
3.2.3. TGA
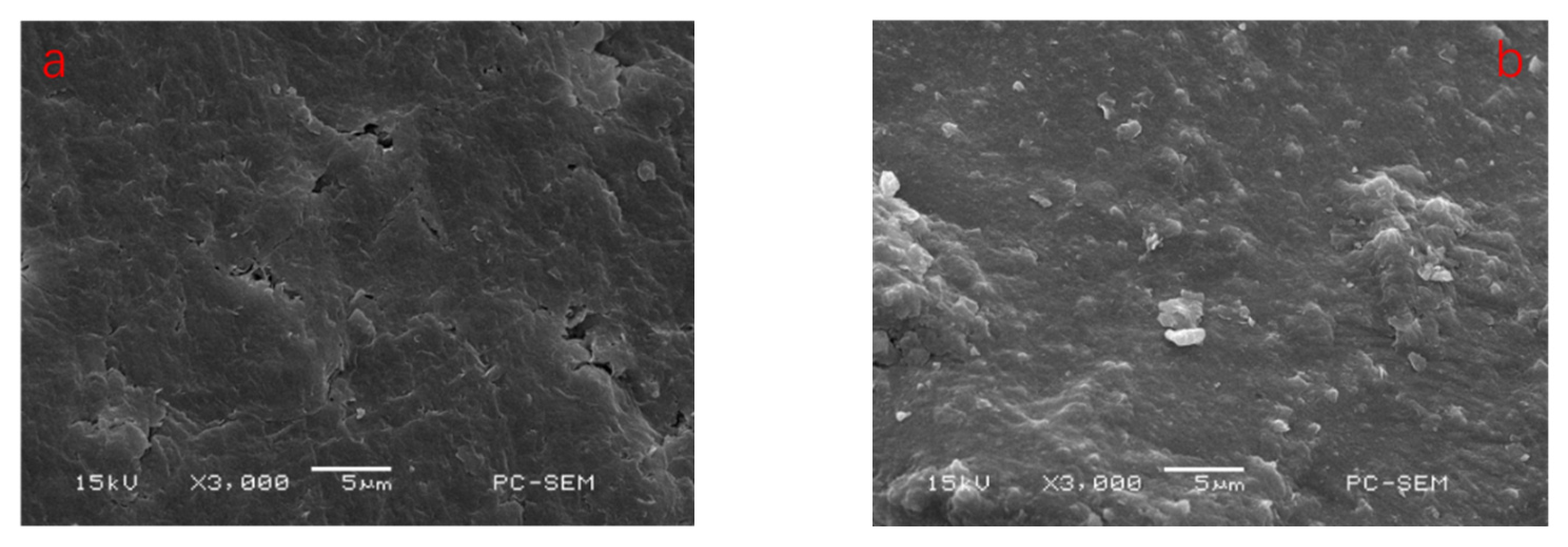
3.2.4. SEM
3.3. Influencing Factors for Adsorption Behavior
3.3.1. Effect of Temperature
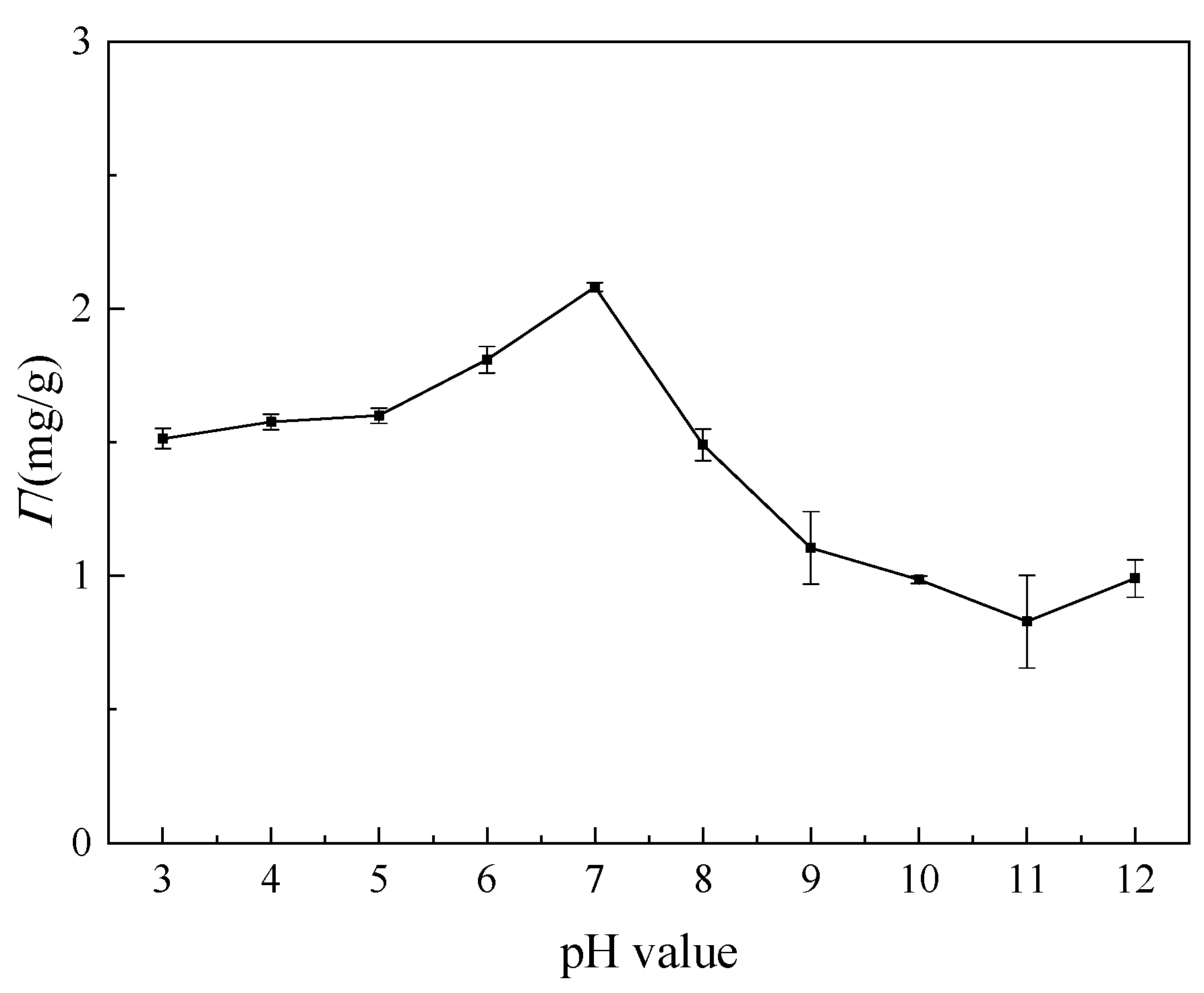
3.3.2. Effect of pH Value
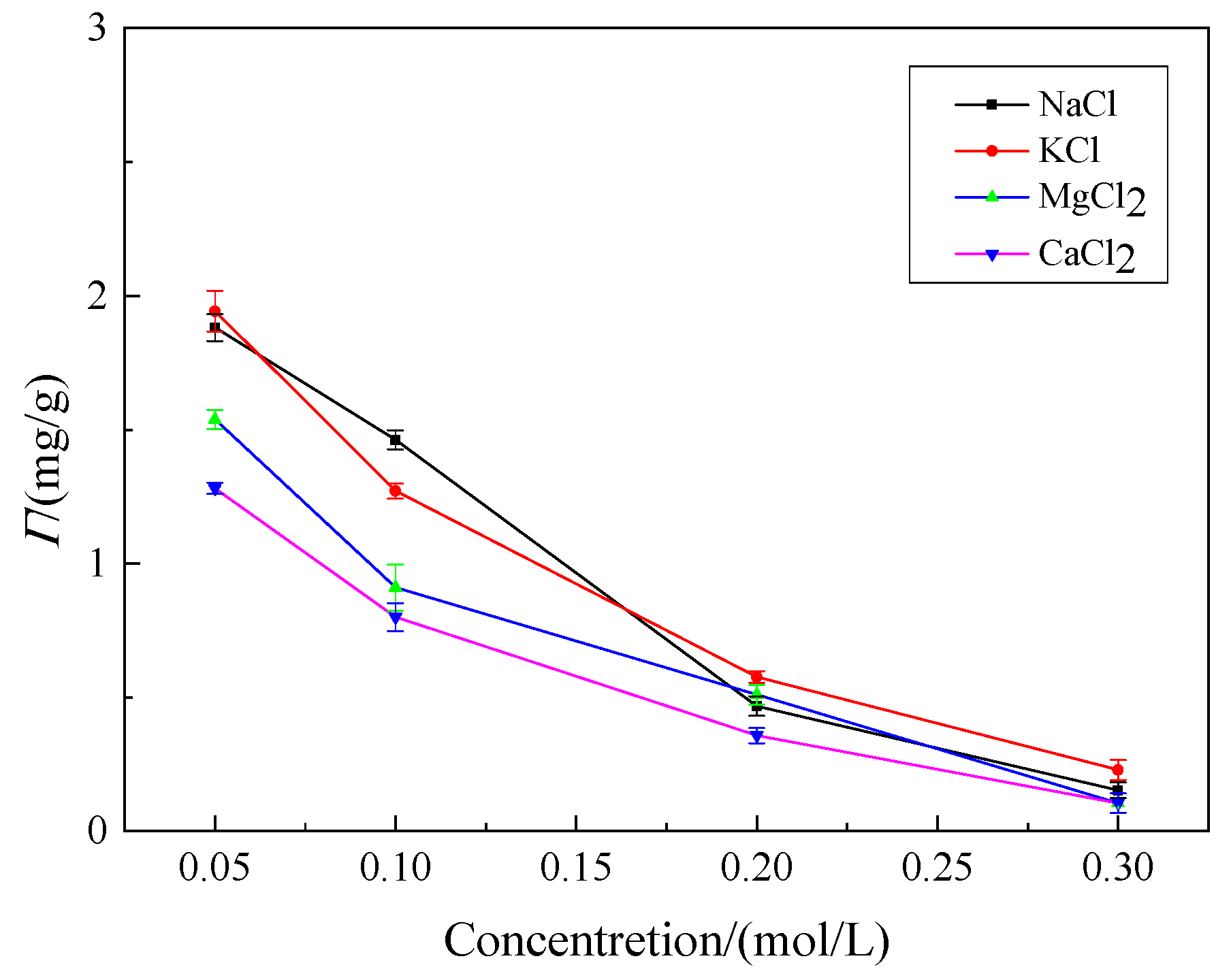
3.3.3. Effect of Inorganic Salt
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tamsilian, Y.; Agirre, A.; Fernandez, M.; Sheng, J.J.; Tomovska, R. High-molar mass acrylamide-co-diacetoneacrylamide graft copolymers as viscosity enhancer for polymer flooding oil recovery. Polym. Test. 2020, 82, 106332. [Google Scholar] [CrossRef]
- Firozjaii, A.M.; Saghafi, H.R. Review on chemical enhanced oil recovery using polymer flooding: Fundamentals, experimental and numerical simulation. Petroleum 2020, 6, 115–122. [Google Scholar] [CrossRef]
- Maghsoudian, A.; Tamsilian, Y.; Kord, S.; Soulgani, B.S.; Esfandiarian, A.; Shajirat, M. Styrene intermolecular associating incorporated-polyacrylamide flooding of crude oil in carbonated coated micromodel system at high temperature, high salinity condition: Rheology, wettability alteration, recovery mechanisms. J. Mol. Liq. 2021, 337, 116206. [Google Scholar] [CrossRef]
- Cao, X.; Ji, Y.; Zhu, Y.; Zhao, F. Research advance and technology outlook of polymer flooding. Reserv. Eval. Dev. 2020, 10, 8–16. [Google Scholar]
- Ma, Q.; Shuler, P.J.; Aften, C.W.; Tang, Y. Theoretical studies of hydrolysis and stability of polyacrylamide polymers. Polym. Degrad. Stab. 2015, 121, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.T. The Synthesis of a Macromolecular Surfactant and the Application of Its Compound System in Low-Permeability Reservoir. Master’s Thesis, China University of Petroleum, Beijing, China, 2019. [Google Scholar]
- Zhang, X.Y.; Liu, H.L.; An, Y. Study on the displacement of polyvinyl alcohol in high temperature and high salinity reservoir. Inn. Mong. Petrochem. Ind. 2017, 6, 119–120. [Google Scholar]
- Sharma, T.; Joshi, A.; Jian, A.; Chaturvedi, K.R. Enhanced oil recovery and CO2 sequestration potential of Bi-polymer polyvinylpyrrolidone-polyvinyl alcohol. J. Pet. Sci. Eng. 2022, 211, 110167. [Google Scholar] [CrossRef]
- Zoubi, W.A.; Kamil, M.P.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent advances in hybrid organic-inorganic materials with spatial architecture for state-of-the-art applications. Prog. Mater. Sci. 2020, 112, 100663. [Google Scholar] [CrossRef]
- Gou, S.; Zhou, L.; Ye, Z.; Yin, T.; Jiang, W.; Ma, Y.; Yang, C. Synthesis and properties of AM/AA/AMPS/NPAB as oil-replacing agent. Spec. Petrochem. 2014, 31, 19–24. [Google Scholar]
- Zhang, P.; Bai, S.; Chen, S.; Li, D.; Jia, Z.; Zhou, C. Preparation, solution characteristics and displacement performances of a novel acrylamide copolymer for enhanced oil recovery (EOR). Polym. Bull. 2018, 75, 1001–1011. [Google Scholar] [CrossRef]
- Lian, P.; Tong, D.; Li, L. Development of Crosslinked Polymer Flooding in High Temperature and High Salt Reservoirs. Adv. Fine Petrochem. 2010, 11, 4–7. [Google Scholar]
- Li, S.; Braun, O.; Lauber, L.; Leblanc, T.; Su, X.; Feng, Y. Enhancing oil recovery from high–temperature and high–salinity reservoirs with smart thermos viscosifying polymers: A laboratory study. Fuel 2021, 288, 119777. [Google Scholar] [CrossRef]
- Cai, S.W. Study on Rheological Properties and Synergistic Effect of HPAM and Xanthan Gum Solution. Master’s Thesis, Southwest Petroleum University, Chengdu, China, 2014. [Google Scholar]
- Ming, H.; Lu, Y.J.; Zhai, W.; Wang, L.W.; Huang, C.H. The properties and analysis of application prospect of xanthan gum fracturing fluid. Spec. Petrochem. 2016, 33, 66–69. [Google Scholar]
- Xu, L.; Qiu, Z.; Gong, H.; Zhu, C.; Sang, Q.; Li, Y.; Dong, M. Synergy of microbial polysaccharides and branched-preformed particle gel on thickening and enhanced oil recovery. Chem. Eng. Sci. 2019, 208, 115138. [Google Scholar] [CrossRef]
- Xu, L.; Xu, G.; Liu, T.; Chen, Y.; Gong, H. The comparison of rheological properties of aqueous welan gum and xanthan gum solutions. Carbohydr. Polym. 2013, 92, 516–522. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, H.; Lv, F.; An, J.; Huo, T.; Ma, Y. Properties of novel microbial polysaccharides suitable for the polymer flooding of harsh oil reservoirs. Oil Drill. Prod. Technol. 2019, 41, 393–398. [Google Scholar]
- Jansson, P.E.; Widmalm, G. Welan gum (S-130) contains repeating units with randomly distributed l-mannosyl and l-rhamnosyl terminal groups, as determined by FABMS. Carbohydr. Res. 1994, 256, 327–330. [Google Scholar] [CrossRef]
- Ji, S.X.; Li, H.; Zhu, H.; Wang, J.Q.; Hai, X.; Li, J.; Li, K.H.; Guo, Z.R.; Xue, H. Rheology and displacement efficiency of biopolymer welan gum. In Proceedings of the 17th Chinese Conference on Colloid and Interface Chemistry, Wuxi, China, 29 July–1 August 2019. [Google Scholar]
- Li, Q.W.; Zhou, Y.; Ke, C.Z.; Meng, J.K.; Bai, Y.X.; Liu, X.L.; Li, S.B. Research Progress in Microbial Production of Welan Gum. Sci. Technol. Food Ind. 2019, 40, 337–342, 348. [Google Scholar]
- Wang, R.; Pu, W.; Dang, S.; Jiang, F.; Zhao, S. Synthesis and characterization of a graft-modified copolymer for enhanced oil recovery. J. Pet. Sci. Eng. 2020, 184, 106473. [Google Scholar] [CrossRef]
- Fu, L.; Liao, K.; Ge, J.; Huang, W.; Chen, L.; Sun, X.; Zhang, J. Study on the damage and control method of fracturing fluid to tight reservoir matrix. J. Nat. Gas Sci. Eng. 2020, 82, 103464. [Google Scholar] [CrossRef]
- Fu, L.; Liao, K.; Ge, J.; He, Y.; Huang, W.; Du, E. Preparation and inhibition mechanism of bis-quaternary ammonium salt as shale inhibitor used in shale hydrocarbon production. J. Mol. Liq. 2020, 309, 113244. [Google Scholar] [CrossRef]
- Wang, J.; Alsofi, A.M.; Boqmi, A.M. A core-scale study of polymer retention in carbonates at different wettability and residual oil conditions. J. Pet. Sci. Eng. 2021, 197, 108099. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Fu, D.; Jiao, C. The Effect of the Content of Clay on Polymer Retention and Entrapment. Inn. Mong. Petrochem. Ind. 2008, 34, 140–142. [Google Scholar]
- Sorbie, K. Polymer-Improved Oil Recovery; Springer: Dordrecht, The Netherlands, 1991. [Google Scholar]
- Alfazazi, U.; Thomas, N.C.; Alameri, W.; Al-Shalabi, E.W. Experimental investigation of polymer injectivity and retention under harsh carbonate reservoir conditions. J. Pet. Sci. Eng. 2020, 192, 107262. [Google Scholar] [CrossRef]
- Quezada, G.R.; Rozas, R.E.; Toledo, P.G. Polyacrylamide adsorption on (101) quartz surfaces in saltwater for a range of pH values by molecular dynamics simulations. Miner. Eng. 2021, 162, 106741. [Google Scholar] [CrossRef]
- Chiappa, L.; Menneiia, A.; Lockhart, T.P.; Burrafato, G. Polymer adsorption at the brine/rock interface: The role of electrostatic interactions and wettability. J. Pet. Sci. Eng. 1999, 24, 113–122. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, Y.; Zhang, C. Study of Adsorption Behavior of Hydroxypropyl Guar Gum on Kaolin. Chin. J. Anal. Chem. 2019, 47, 93–98. [Google Scholar]
- Yin, Z.; Wang, Y.; San, J. Adsorption behavior of hydroxypropyl guar gum onto montmorillonite and reducing adsorption in the reservoir. Appl. Clay Sci. 2018, 166, 123–130. [Google Scholar] [CrossRef]
- Fu, L.; Jiang, L.; Liao, K.; An, J.; Huang, W.; Sun, X.; Li, T.; He, Y. Adsorption behavior of welan gum on quartz sand in reservoir. J. Pet. Sci. Eng. 2021, 205, 108850. [Google Scholar] [CrossRef]
- Bénard, P.; Chahine, R. Modeling of high-pressure adsorption isotherms above the critical temperature on microporous adsorbents: application to methane. Langmuir 1997, 13, 808–813. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Selvendran, R.R.; Morris, V.J.; Eagles, J. Structure of the extracellular polysaccharide produced by the bacterium Alcaligenes (ATCC 31555) species. Carbohydr. Res. 1986, 147, 295–313. [Google Scholar] [CrossRef]
- Özdemir, G.; Yapar, S. Adsorption and desorption behavior of copper ions on Na-montmorillonite: Effect of rhamnolipids and pH. J. Hazard. Mater. 2009, 166, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Lv, G.; Gu, J.; Chen, L. Study on the relationship between charge and structure of montmorillonite. Acta Phys.-Chim. Sin. 2003, 19, 1171–1175. [Google Scholar]






| No. | Polymer Types | Key Findings | Ref. |
|---|---|---|---|
| 1 | Xanthan gum, HPAM | Xanthan has good performance at 80 °C, but weakens when the temperature exceeds 100 °C. | Li et al. [13] |
| 2 | Xanthan gum, HPAM | Xanthan gum and HPAM have synergistic effects. | Cai [14] |
| 3 | Xanthan gum, Guanidine gum | XG is affected little by acid, alkali, and bacterial environments. | Ming et al. [15] |
| 4 | Xanthan gum | It had good salt tolerance, and could be used for enhancing heavy oil recovery. | Xu et al. [16] |
| 5 | Xanthan gum, welan gum | Welan gum had better viscosity-retention rate (72%) than xanthan gum (50%) at 75 °C. | Xu et al. [17] |
| 6 | Welan gum, dieter gum, xanthan gum, and HPAM | Welan gum has better temperature resistance than xanthan gum, resulting better ability in improving oil recovery. | Zhou et al. [18] |
| 7 | Welan gum, HPAM | Welan gum solution is pseudoplastic fluid with good viscoelasticity, and is affected little by sodium ion and potassium ion. | Ji et al. [20] |
| 8 | Welan gum | It is a good candidate for polymer flooding in reservoirs with high temperatures and high salt content. | Li et al. [21], Wang et al. [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, K.; An, J.; Fu, L.; Zhang, H.; Wei, M.; Bai, J.; He, Y. Adsorption of Welan Gum on Montmorillonite and Its Influencing Factors. Polymers 2022, 14, 2599. https://doi.org/10.3390/polym14132599
Liao K, An J, Fu L, Zhang H, Wei M, Bai J, He Y. Adsorption of Welan Gum on Montmorillonite and Its Influencing Factors. Polymers. 2022; 14(13):2599. https://doi.org/10.3390/polym14132599
Chicago/Turabian StyleLiao, Kaili, Junnan An, Lipei Fu, Houye Zhang, Meng Wei, Jinmei Bai, and Yanfeng He. 2022. "Adsorption of Welan Gum on Montmorillonite and Its Influencing Factors" Polymers 14, no. 13: 2599. https://doi.org/10.3390/polym14132599






