The Influence of Polycaprolactone on Structural Changes of Dusts from Molding Sands with Resin-Based Binder before and after the Biodegradation Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- Furfuryl resin, without the presence of nitrogen; free formaldehyde in the range of 0.05–0.15%; the amount of furfuryl alcohol 78%;
- Hardener—an aqueous solution of paratoluenesulfonic acid;
- Polycaprolactone (PCL)—a biodegradable additive and plasticizer with the formula –(O(CH2)5CO)-n; molecular weight 50,000 Da and melting point 58–60 °C; a biodegradable polymer in solid form with an end of hydroxyl group from Polysciences Inc., Warrington, PA, USA.
2.2. Methods
3. Results
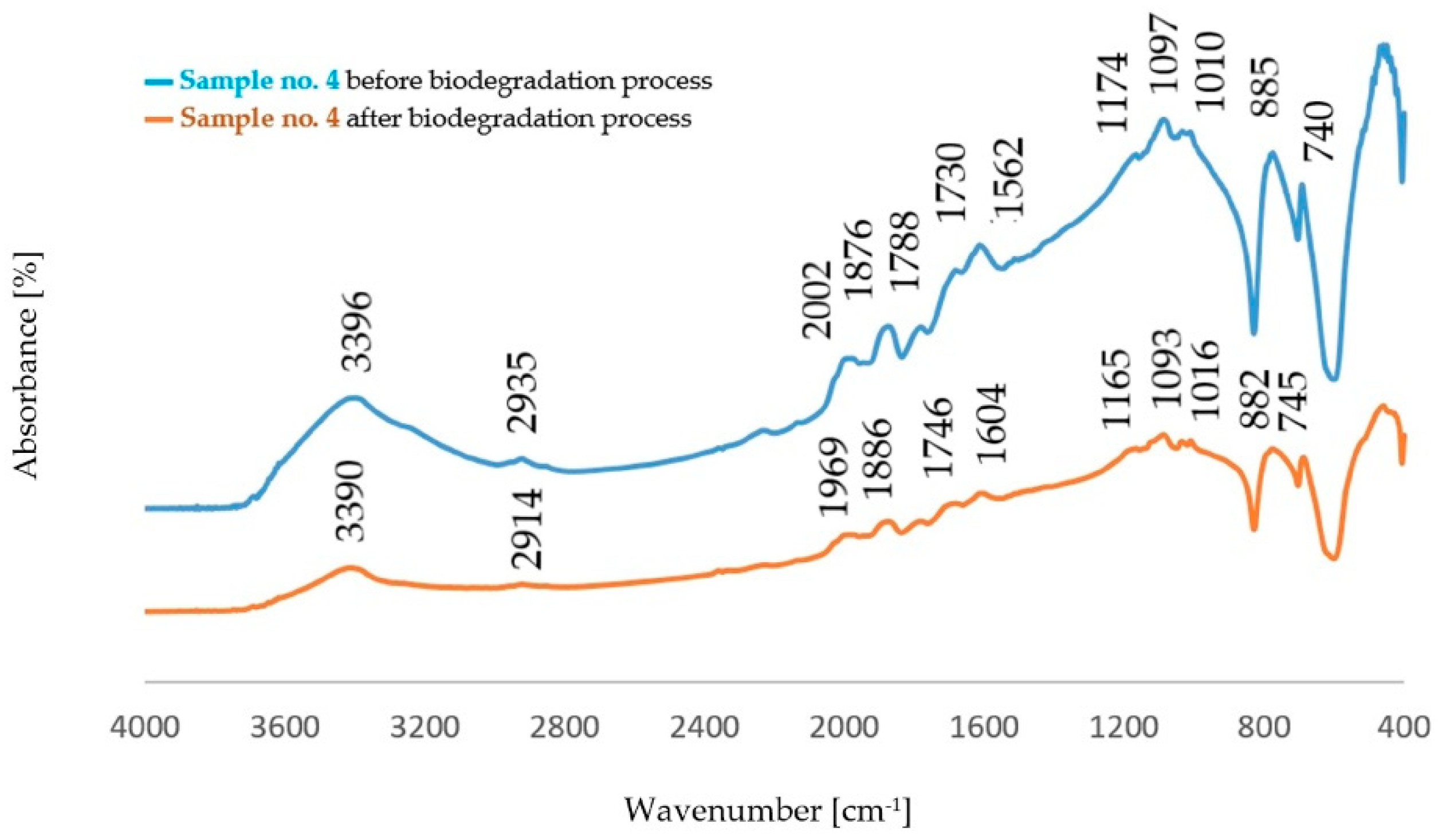
3.1. Structure Analysis by FTIR Spectroscopy
3.1.1. Determination of the Structure of Dust Samples before the Biodegradation Process
3.1.2. Determination of the Structure of Dust Samples after the Biodegradation Process
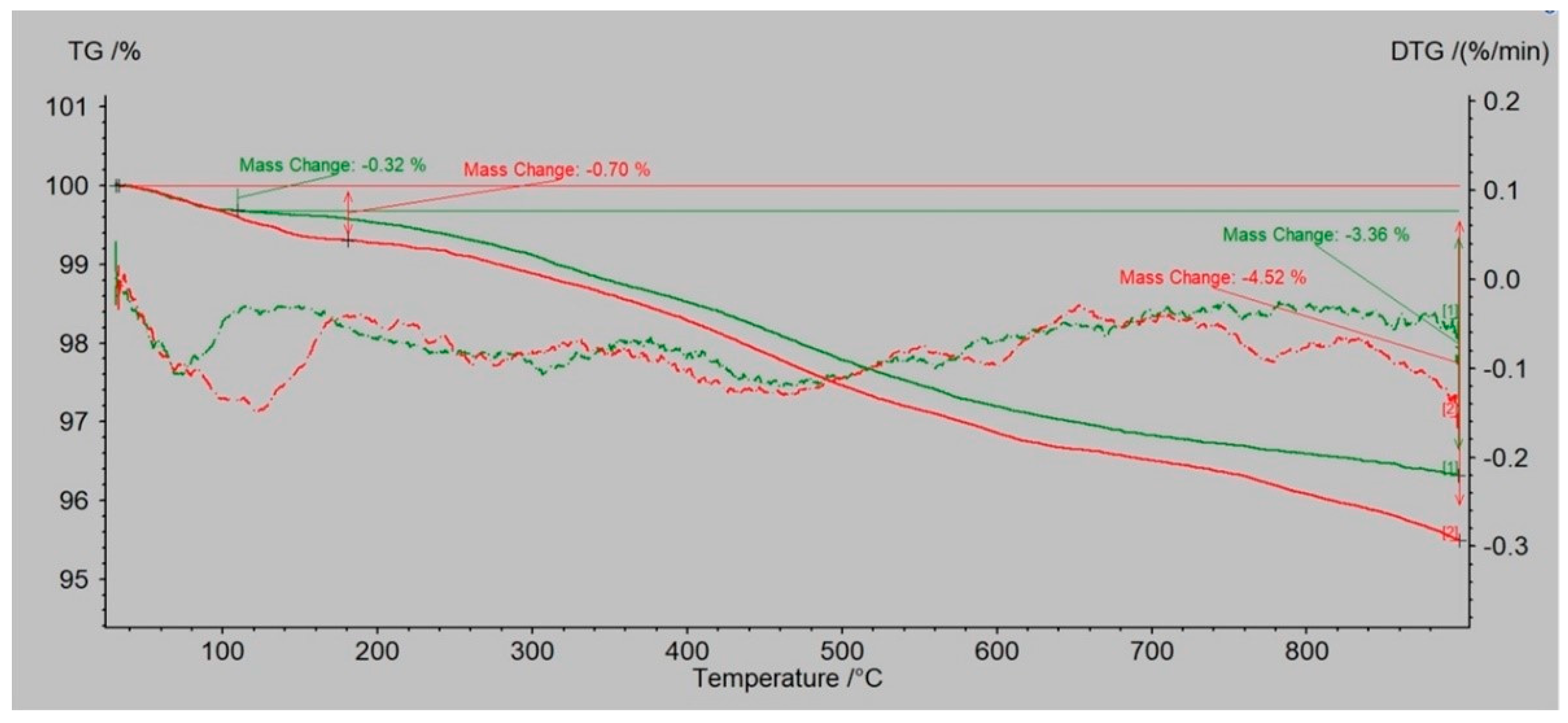
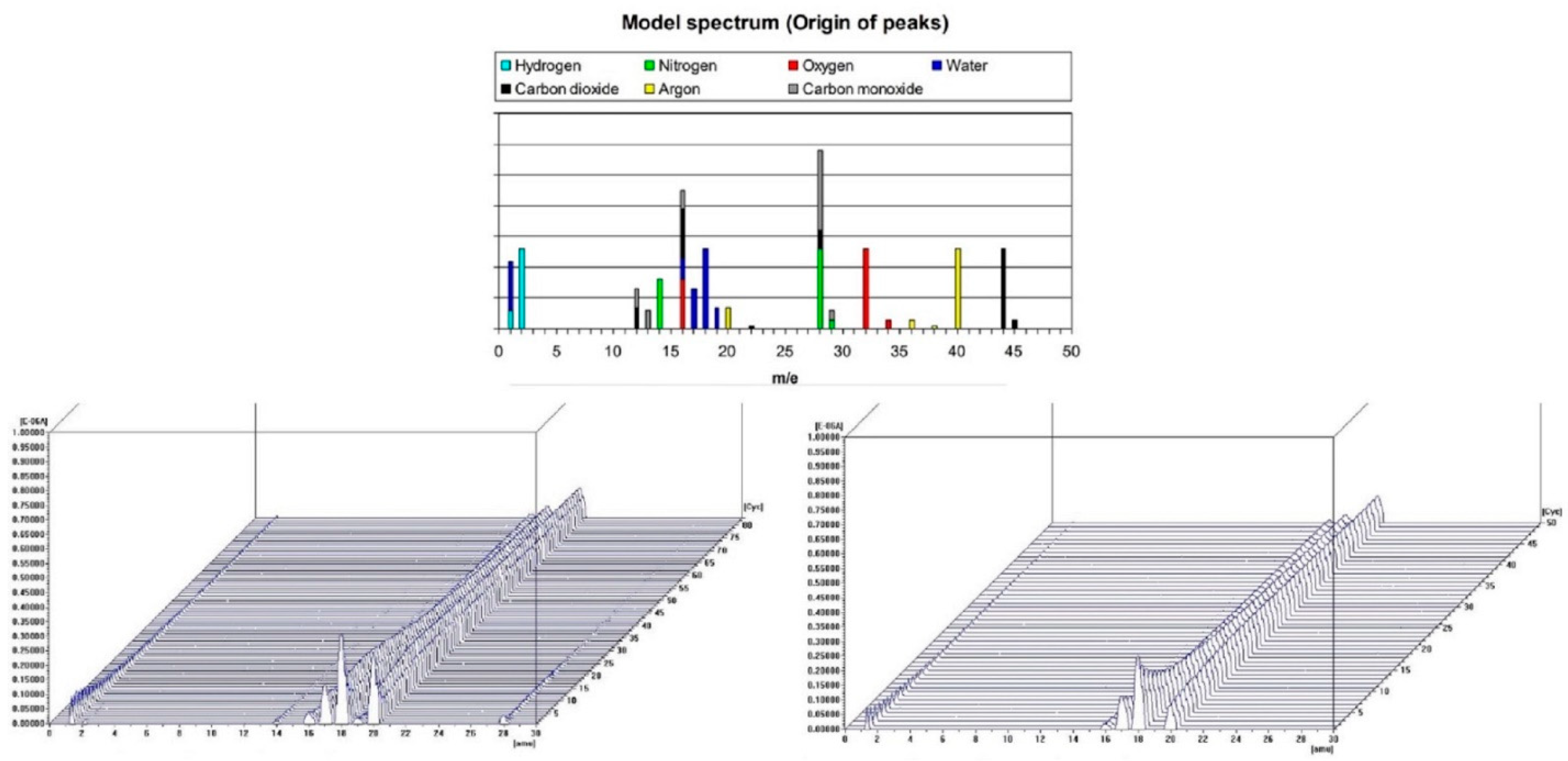
3.2. The Changes in the Mass by TG/DTA-QMS Analysis
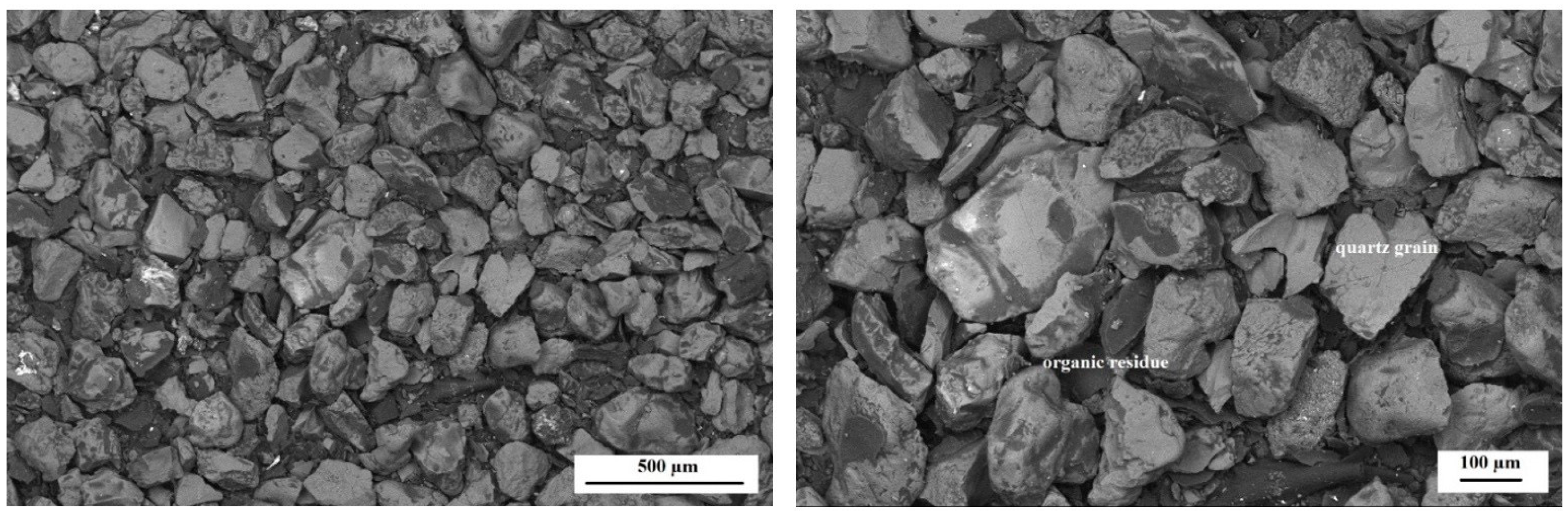
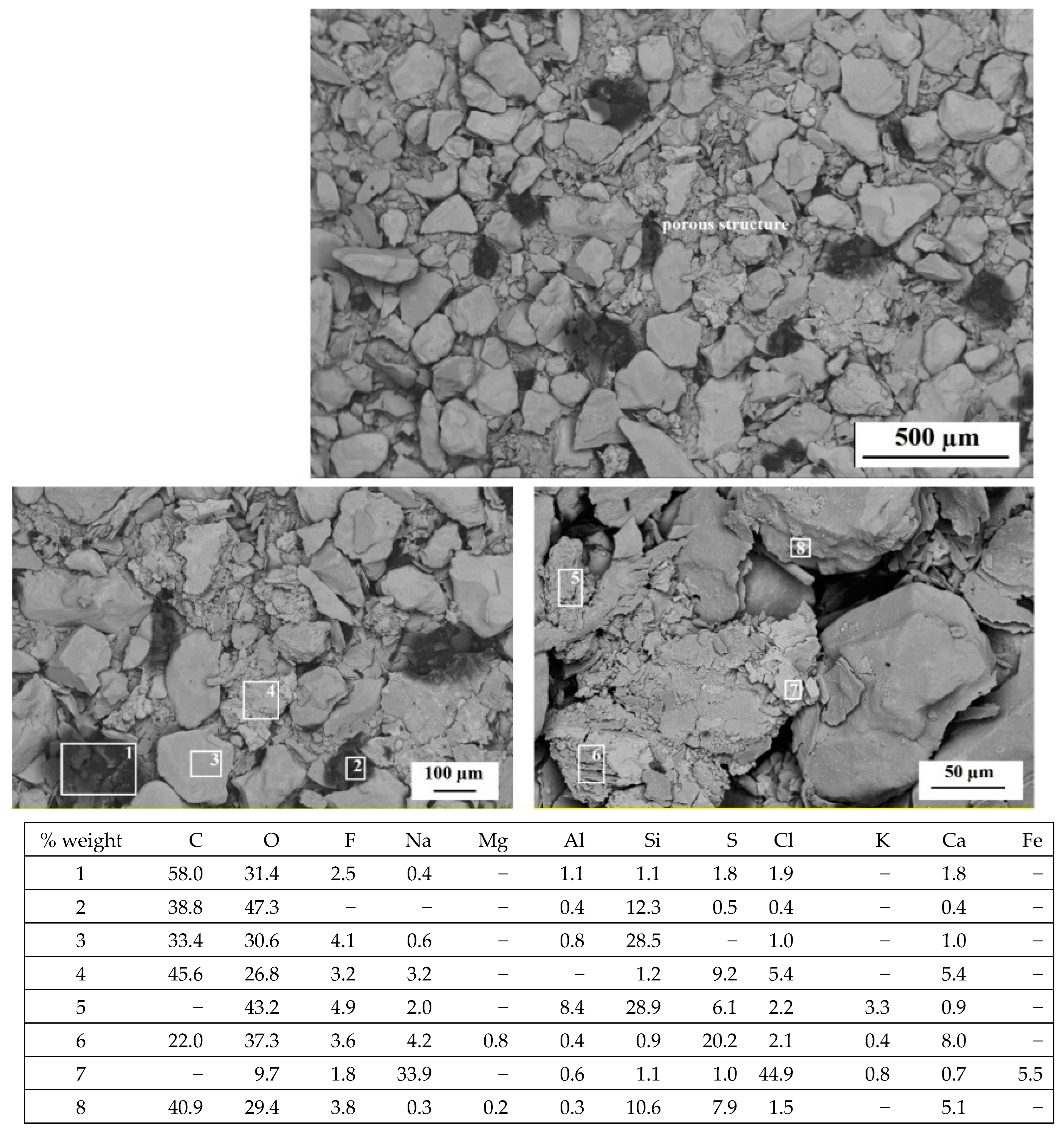
3.3. Microscopic Observations (SEM, EDS)
3.4. COD Measurement
4. Discussion
5. Conclusions
- It is possible to use biodegradable polymer as a component of foundry resin-based binder.
- There were no significant changes in the dust samples’ structures before and after the biodegradation process. An increased intensity of the band originating from stretching vibrations of the OH− groups (νO-H) was found. This effect may confirm the partial degradation of the structure, mainly PCL.
- Dust samples after the biodegradation process had higher weight loss than the same samples before biodegradation. Dust samples from molding sand with PCL addition had higher weight loss than dust samples from molding sand without PCL addition.
- Morphology studies showed the presence of irregular, porous structures mainly in the dust samples from molding sand MS no. 2 after the biodegradation process.
- It was found that increasing the regeneration time reduces the amount of organic matter in the tested samples, which has a significant impact on the decrease in the COD value along with the duration of the process.
- The conducted research and the obtained results indicate the directions of further research and work in the selection of components of molding sand with biodegradable properties, which should be carried out in terms of determining the minimum and maximum amount of polycaprolactone as an addition of a biodegradable component to the molding sand. It would be also essential to carry out a biodegradation process in longer time - between 6 and 12 months. Important for determining the properties of biodegradable materials is the biodegradation process in which the inoculum should be activated sludge used in sewage treatment plants or under the influence of microorganisms that break down aromatic hydrocarbons, e.g., bacteria such as Aeromonas, Pseudomonas, Flavobacterium and Bacillus.
- The next step of research would be a structural and molecular weight analysis of the polymer.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holtzer, M.; Dańko, R.; Kmita, A. Influence of a Reclaimed Sand Addition to Moulding Sand with Furan Resin on Its Impact on the Environment. Water Air Soil Pollut. 2016, 227, 16. [Google Scholar] [CrossRef] [Green Version]
- Łatas, W.; Dańko, R.; Czapla, P. Application of 3-D Drucker–Prager Material Model to Determine Optimal Operating Parameters of Centrifugal Regeneration Device. Materials 2020, 13, 2134. [Google Scholar] [CrossRef] [PubMed]
- Major-Gabryś, K.; Hosadyna-Kondracka, M.; Skrzyński, M.; Stachurek, I. The influence of biomaterial in the binder composition on the quality of reclaim from furan no-bake sands. Arch. Civ. Eng. 2022. Decision letter ACE-00021-2022-01: 21 March 2022. [Google Scholar]
- Brenner, V. Biodegradace persistentních xenobiotik. Biodegradace VI 2003, 2003, 45–47. [Google Scholar]
- Bastian, K.C.; Alleman, J.E. Environmental Bioassay Evaluation of Foundry Waste Residuals; Joint Transportation Research Program Technical Report Series; Purdue University: West Lafayette, IN, USA, 1996. [Google Scholar]
- Sobków, D.; Barton, J.; Czaja, K.; Sudoł, M.; Mazoń, B. Research on the resistance of materials to environmental factors. Badania odporności materiałów na działanie czynników środowiska naturalnego. Chemik 2014, 68, 347–354. [Google Scholar]
- Eastman, J. Protein-Based Binder Update: Performance Put to the Test. Mod. Cast. 2000, 90, 32–34. [Google Scholar]
- Kramářová, D.; Brandštetr, J.; Rusín, K.; Henzlová, P. Biogenní polymerní materiály jako pojiva slévárenských forem a jader. Slévárenství 2003, 60, 71–73. [Google Scholar]
- Grabowska, B.; Holtzer, M.; Dańko, R.; Górny, M.; Bobrowski, A.; Olejnik, E. New BioCo Binders Containing Biopolymers for Foundry Industry. Metalurgija 2013, 52, 47–50. [Google Scholar]
- Major-Gabryś, K. Environmentally friendly foundry molding and core sand. Odlewnicze masy formierskie i rdzeniowe przyjazne dla środowiska. Katowice-Gliwice. Wydaw. Arch. Foundry Eng. 2016, 69, 114–120. (In Polish) [Google Scholar]
- Major-Gabryś, K.; Grabarczyk, A.; Dobosz, S.M.; Jakubski, J. New bicomponent binders for foundry moulding sands, XXI. In Proceedings of the Mezinárodní Konferenci “Spolupráce 2015”, Jeseníky, Czech Republic, 22–24 April 2015; pp. 1–5. [Google Scholar]
- Major-Gabryś, K.; Grabarczyk, A.; Dobosz, S.M.; Drożyński, D. Effect of a biodegradable addition as a binary binder component for casting moulding and cores sands on the properties of the binder and the sands. Trans. Foundry Res. Inst. 2016, 56, 319–328. [Google Scholar]
- Major-Gabryś, K.; Bobrowski, A.; Grabarczyk, A.; Dobosz, S.M. The thermal and structural analysis of new bicomponent binders for moulding sands consisting of furfuryl resin and polycaprolactone (PCL). Arch. Metall. Mater. 2017, 62, 369–372. [Google Scholar] [CrossRef] [Green Version]
- Major-Gabryś, K.; Grabarczyk, A.; Dobosz, S.M. The compositions: Biodegradable materia—Synthetic resins as moulding sands binders. Arch. Foundry Eng. 2016, 16, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Major-Gabryś, K. Biodegradable materials as binders for IVth generation moulding sands. China Foundry 2015, 12, 375–381. [Google Scholar]
- Major-Gabryś, K. Biodegradable materials as foundry moulding sands binders. Metalurgija 2015, 54, 591–593. [Google Scholar]
- Choi, E.-J.; Park, J.-K. Study on biodegradability of PCL/SAN blend using composting method. Polym. Degrad. Stab. 1996, 52, 321–326. [Google Scholar] [CrossRef]
- Scott, G. Environmentally Degradable Polyolefins: When, Why and How, Expert Group Meeting on Environmentally Degradable Plastics, Present Status and Perspectives; ICS-UNIDO: Trieste, Italy, 2001; pp. 37–48. [Google Scholar]
- Scott, G. Green Polymers. Polym. Degrad. Stab. 2000, 68, 1–7. [Google Scholar] [CrossRef]
- Wiles, D.M.; Scott, G. Polyolefins with Controlled Environmental Biodegradability. Polym. Degrad. Stab. 2006, 91, 1581–1592. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological Degradation of Plastics: A Comprehensive Review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Griffith, L.G. Polymeric biomaterials. Acta Mater. 2000, 48, 263–277. [Google Scholar] [CrossRef]
- Iwamoto, A.; Tokiwa, Y. Enzymatic degradation of plastics containing polycaprolactone. Polym. Degrad. Stab. 1994, 45, 205–213. [Google Scholar] [CrossRef]
- Eastmond, G.C. Poly(ε-caprolactone) Blends. Adv. Polym. Sci. 2000, 149, 59–222. [Google Scholar]
- Test Report No. 2279/I/2020 and 2279/II/2020 of 11 August 2020 issued by the Central Laboratory of MPWiK S.A. in Cracow.
- Web Library NIST. Available online: https://webbook.nist.gov/ (accessed on 15 May 2022).
- PN-ISO 6060:2006; Water Quality. Determination of the Chemical Oxygen Demand. ISO: Geneva, Switzerland, 2006.
- Srivastava, R.; Srivastava, D. Mechanical, chemical, and curing characteristics of caedano-furfural-based novolac resin for application in green coatings. J. Coat. Technol. Res. 2015, 12, 303–311. [Google Scholar] [CrossRef]
- Koley, R.; Kasilingam, R.; Sahoo, S.; Chattopadhyay, S.; Bhowmicka, A.K. Synthesis. Characterization of Phenol Furfural Resin from Moringa Oleifera Gum and Biophenol and Its Application in Styrene Butadiene Rubber. Ind. Eng. Chem. Res. 2019, 48, 18519–18532. [Google Scholar] [CrossRef]
- Kai, Y.; Mingli, J.; Xiaomei, Z.; Wanshun, J.; Quan, D.; Ying, L.; Jian, C. Structure, stability, and properties of phenolic fibers modified by phenyl molybdate. Polym. Polym. Compos. 2021, 29, 574–582. [Google Scholar] [CrossRef]
- Benkaddour, A.; Jradi, K.; Robert, S.; Daneault, C. Grafting of Polycaprolactone on Oxidized Nanocelluloses by Click Chemistry. J. Nanomater. 2013, 3, 141–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanal, T.; Hazer, B.; Kocaak, I. Synthesis of comb-type amphiphilic graft copolymers derived from chlorinated poly(ɛ-caprolactone) via click reaction. Polym. Bull. 2017, 74, 977–995. [Google Scholar] [CrossRef]
- Thamizhlarasan, A.; Meenarathi, B.; Anbarasan, R. Effect of macro and molecular initiators on the structure-property relationship of poly(ε-caprolactone). J. Thermoplast. Compos. Mater. 2021. [Google Scholar] [CrossRef]
- Dadras, M.; Jalali-arani, A.; González, F.B.; Urreaga, J.M. Biodegradable Nanocomposites Developed from PLA/PCL Blends and Silk Fibroin Nanoparticles: Study on the Microstructure, Thermal Behavior, Crystallinity and Performance. J. Polym. Environ. 2020, 28, 1252–1264. [Google Scholar] [CrossRef]
- Lyu, J.S.; Lee, J.S.; Han, J. Development of a biodegradable polycaprolactone film incorporated with an antimicrobial agent via an extrusion process. Sci. Rep. 2019, 9, 20236. [Google Scholar] [CrossRef]
- Permyakova, E.S.; Kiryukhantsev-Korneev, P.V.; Gudz, K.Y.; Konopatsky, A.S.; Polčak, J.; Zhitnyak, I.Y.; Gloushankova, N.A.; Shtansky, D.V.; Manakhov, A.M. Comparison of Different Approaches to Surface Functionalization of Biodegradable Polycaprolactone Scaffolds. Nanomaterials 2019, 9, 1769. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://wodociagi.krakow.pl/admin/files/Files/Raporty_roczne/WMK-Raport_roczny_2020.pdf (accessed on 12 May 2022).










| Sample No. | Designation of Samples | Molding Sand’s Composition |
|---|---|---|
| 1 | Dust after initial regeneration | Molding sand MS no. 1 silica sand 100 p.b.w.; furfuryl resin 1.1 p.b.w. hardener 0.55 p.b.w. |
| 2 | Dust after mechanical regeneration: 5 min | |
| 3 | Dust after mechanical regeneration: 10 min | |
| 4 | Dust after mechanical regeneration: 15 min | |
| 5 | Dust after initial regeneration | Molding sand MS no. 2 silica sand 100 p.b.w.; furfuryl resin 1.045 p.b.w. (95%) PCL 0.055 p.b.w. (5%); hardener 0.55 p.b.w. |
| 6 | Dust after mechanical regeneration: 5 min | |
| 7 | Dust after mechanical regeneration: 10 min | |
| 8 | Dust after mechanical regeneration: 15 min |
| Tested Feature | Unit | Result |
|---|---|---|
| pH | - | 7.6 (in 22.4 °C) |
| specific electrical conductivity | µS/cm | 1568 (in 25 °C) |
| chlorides | mg/L | 369 |
| sulfur | mg/L | 65 |
| nitrates | mg/L | 5.7 |
| Zn | mg/L | 0.07 |
| Pb | mg/L | 0.024 |
| Cu | mg/L | 0.004 |
| Ni | mg/L | 0.0025 |
| chemical oxygen demand | mg/L | 20 |
| dissolved oxygen | mg/L | 7.6 |
| Group | Wavenumber (cm−1) | |
|---|---|---|
| Dust from MS no. 1 | Dust from MS no. 2 | |
| O-H stretch vibration νO-H | 3400–3393 | 3361–3358 |
| C-H | 2935–2930 | 2961–2958 |
| C=O | 1731–1729 | 1768–1762 |
| C=C | 1562–1558 | 1595–1549 |
| OH vibrations from the furan or phenolic ring (OH group), deforming the bond along the plane | 1361–1357 | 1350–1345 |
| vibration from the bond characteristic of phenol-furfuryl resin | 1148–1142 | 1149–1146 |
| C−H deforming vibrations from furan ring | 886–884 | 889–887 |
| skeletal oscillation from bonds found in furfuryl alcohol | 743–740 | 746–742 |
| vibration from the furan ring | 600–599 | 599–597 |
| Group | Wavenumber (cm−1) |
|---|---|
| O-H stretch vibration νO-H | 3361–3358 |
| CH2-O asymmetric stretching vibration νO-H | 2946–2944 |
| CH2—symmetrical stretching vibration νC-H | 2865–2859 |
| CO2—stretching vibrations νC = O from aliphatic esters | 1722–1719 |
| C-H asymmetric stretching vibration νC-H | 1471–1439 |
| CH2—asymmetric stretching vibration νC-H | 1369–1367 |
| C−O; C−C; crystal phase stretching vibrations from crνC=O and crνC-C | 1298–1296 |
| C−O−C asymmetric vibration νC-O-C | 1241–1240 |
| CH2 | 1106–1104 |
| C−H asymmetric stretching vibration νC-H | 731–730 |
| Sample Number | Determined COD Value; (mg O2/dm3) |
|---|---|
| 1 | 691 |
| 2 | 586 |
| 3 | 442 |
| 4 | 446 |
| 5 | 1152 |
| 6 | 691 |
| 7 | 516 |
| 8 | 413 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Major-Gabryś, K.; Stachurek, I.; Hosadyna-Kondracka, M.; Homa, M. The Influence of Polycaprolactone on Structural Changes of Dusts from Molding Sands with Resin-Based Binder before and after the Biodegradation Process. Polymers 2022, 14, 2605. https://doi.org/10.3390/polym14132605
Major-Gabryś K, Stachurek I, Hosadyna-Kondracka M, Homa M. The Influence of Polycaprolactone on Structural Changes of Dusts from Molding Sands with Resin-Based Binder before and after the Biodegradation Process. Polymers. 2022; 14(13):2605. https://doi.org/10.3390/polym14132605
Chicago/Turabian StyleMajor-Gabryś, Katarzyna, Iwona Stachurek, Małgorzata Hosadyna-Kondracka, and Marta Homa. 2022. "The Influence of Polycaprolactone on Structural Changes of Dusts from Molding Sands with Resin-Based Binder before and after the Biodegradation Process" Polymers 14, no. 13: 2605. https://doi.org/10.3390/polym14132605







