Immobilization of Horseradish Peroxidase on Magnetite-Alginate Beads to Enable Effective Strong Binding and Enzyme Recycling during Anthraquinone Dyes’ Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Magnetic Particles
2.3. Preparation of Mag-Alginate Beads (MABs)
2.4. Characterization of MABs
2.5. Immobilization of HRP on MABs
2.6. Activity Assay of Free and Immobilized HRP by Different Methods
2.7. Optimum pH, Optimum Temperature and Thermal Stability
2.8. Dye Removal Efficiency and Stability of HRP-MABs
2.9. Reusability of the Immobilized Enzyme
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterization of MABs and HRP-MABs
3.1.1. Beads Characterization
3.1.2. Mechanical Properties and Magnetization
3.1.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.2. Covalent Immobilization of HRP
3.2.1. Effect of Immobilization Conditions on Enzymatic Activity and Immobilization Efficiency
3.2.2. Characterization of the HRP-MABs
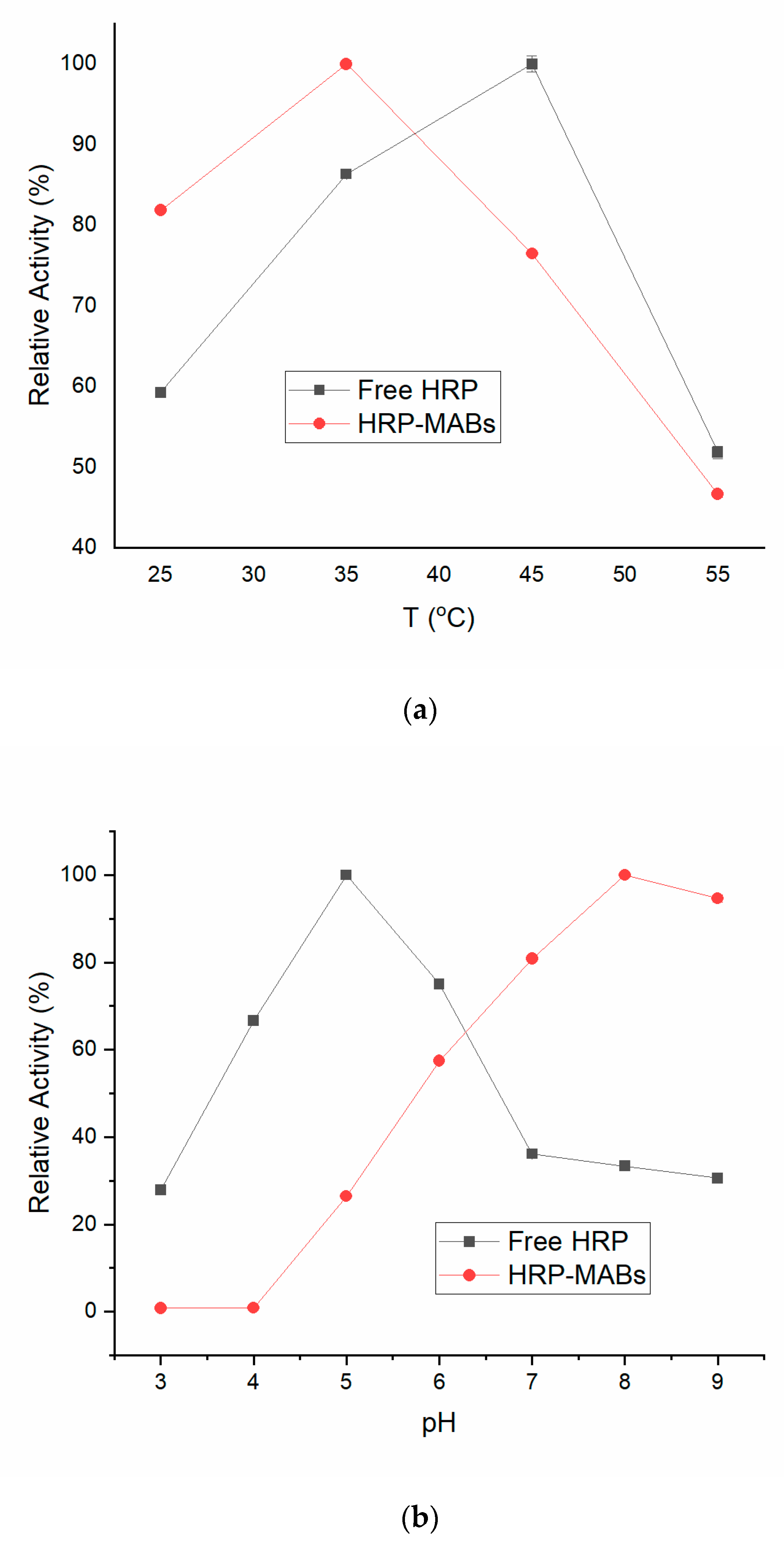
Effect of Temperature and pH on Free HRP and HRP-MABs
3.3. AV109 and AB225 Decolorization
3.3.1. Optimization of Process Parameters for AV109 and AB225 Decolorization
3.3.2. Reusability of the HRP-MABs and Storage Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, Y.; Viraraghavan, T. Fungal Decolorization of Dye Wastewaters: A Review. Bioresour. Technol. 2001, 79, 251–262. [Google Scholar] [CrossRef]
- Pereira, L.; Alves, M. Dyes-Environmental Impact and Remediation. In Environmental Protection Strategies for Sustainable Development; Malik, A., Grohmann, E., Eds.; Springer Nature BV: Dordrecht, The Netherlands, 2012; pp. 111–162. [Google Scholar]
- Pan, H.; Feng, J.; He, G.-X.; Cerniglia, C.E.; Chen, H. Evaluation of Impact of Exposure of Sudan Azo Dyes and their Metabolites on Human Intestinal Bacteria. Anaerobe 2012, 18, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Sun, G.; Xu, X. Sunflower Stalks as Adsorbents for Color Removal from Textile Wastewater. Ind. Eng. Chem. Res. 1997, 36, 808–812. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Solecka, M.; Zylla, R. Biodegradation, Decolourisation and Detoxification of Textile Wastewater enhanced by Advanced Oxidation Processes. J. Biotechnol. 2001, 89, 175–184. [Google Scholar] [CrossRef]
- Sabur, M.A.; Khan, A.A.; Safiullah, S. Treatment of Textile Wastewater by Coagulation Precipitation Method. J. Sci. Res. 2012, 4, 623–633. [Google Scholar] [CrossRef]
- Babu, S.A.; Raja, S.; Sibi, S.; Neeraja, P. Electrochemical Oxidation of Textile Polluted Water and its Reuse. J. Ind. Pollut. Control 2012, 28, 73–82. [Google Scholar]
- Mijin, D.; Radišić, M.M.; Šekuljica, N.; Grgur, B.N. Electrochemical Decolorization of C.I. Acid Orange 3 in the Presence of Sodium Chloride at Iridium Oxide Electrode. Chem. Pap. 2017, 71, 2173–2184. [Google Scholar] [CrossRef]
- Lau, W.-J.; Ismail, A. Polymeric Nanofiltration Membranes for Textile Dye Wastewater Treatment: Preparation, Performance Evaluation, Transport Modelling, and Fouling Control—A Review. Desalination 2009, 245, 321–348. [Google Scholar] [CrossRef]
- Domingues, F.S.; Geraldino, H.C.L.; Freitas, T.K.F.D.S.; de Almeida, C.A.; de Figueiredo, F.F.; Garcia, J.C. Photocatalytic Degradation of Real Textile Wastewater Using Carbon Black-Nb2O5 Composite Catalyst under UV/Vis Irradiation. Environ. Technol. 2019, 42, 2335–2349. [Google Scholar] [CrossRef]
- Işik, M.; Sponza, D.T. Anaerobic/Aerobic Treatment of a Simulated Textile Wastewater. Sep. Purif. Technol. 2008, 60, 64–72. [Google Scholar] [CrossRef]
- Šekuljica, N.; Jovanovic, J.; Tanasković, S.M.J.; Ognjanović, N.D.; Gazikalović, I.V.; Knežević-Jugović, Z.D.; Mijin, D. Immobilization of Horseradish Peroxidase onto Purolite® A109 and its Anthraquinone Dye Biodegradation and Detoxification Potential. Biotechnol. Prog. 2020, 36, e2991. [Google Scholar] [CrossRef] [PubMed]
- Nunavath, H.; Banoth, C.; Talluri, V.R.; Bhukya, B. An Analysis of Horseradish Peroxidase Enzyme for Effluent Treatment. Bioinformation 2016, 12, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Fang, L.; Wang, J.; Liu, W. Enzymatic decolorization of Orange II: Optimization by response surface methodology and pathway. Environ. Prog. Sustain. Energy 2012, 32, 294–301. [Google Scholar] [CrossRef]
- de Souza, S.M.A.G.U.; Forgiarini, E.; de Souza, A.A.U. Toxicity of Textile Dyes and their Degradation by the Enzyme Horseradish Peroxidase (HRP). J. Hazard. Mater. 2007, 147, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.; Durani, S.; Wangikar, P.P. Horseradish Peroxidase Catalyzed Degradation of Industrially Important Dyes. Biotechnol. Bioeng. 2001, 72, 562–567. [Google Scholar] [CrossRef]
- Mohan, S.V.; Prasad, K.K.; Rao, N.C.; Sarma, P. Acid Azo Dye Degradation by Free and Immobilized Horseradish Peroxidase (HRP) Catalyzed Process. Chemosphere 2005, 58, 1097–1105. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Stabilization of Multimeric Enzymes: Strategies to Prevent Subunit Dissociation. Enzym. Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Immobilization as a Strategy for Improving Enzyme Properties-Application to Oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef]
- Aybastıer, Ö.; Demir, C. Optimization of Immobilization Conditions of Thermomyces Lanuginosus Lipase on Styrene–Divinylbenzene Copolymer Using Response Surface Methodology. J. Mol. Catal. B: Enzym. 2010, 63, 170–178. [Google Scholar] [CrossRef]
- Won, K.; Kim, S.; Kim, K.-J.; Park, H.W.; Moon, S.-J. Optimization of Lipase Entrapment in Ca-Alginate Gel Beads. Process Biochem. 2005, 40, 2149–2154. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Hernandez, K.; Fernandez-Lafuente, R. Control of Protein Immobilization: Coupling Immobilization and Site-Directed Mutagenesis to Improve Biocatalyst or Biosensor Performance. Enzym. Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef]
- Majewski, A.P.; Stahlschmidt, U.; Jérôme, V.; Freitag, R.; Müller, A.H.E.; Schmalz, H. PDMAEMA-Grafted Core–Shell–Corona Particles for Nonviral Gene Delivery and Magnetic Cell Separation. Biomacromolecules 2013, 14, 3081–3090. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Potential Applications of Enzymes Immobilized on/in Nano Materials: A Review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Harbi, M.H.; Almulaiky, Y.Q.; Ibrahim, I.H.; El-Shishtawy, R.M. Immobilization of Horseradish Peroxidase on Fe3O4 Magnetic Nanoparticles. Electron. J. Biotechnol. 2017, 27, 84–90. [Google Scholar] [CrossRef]
- Chang, Q.; Tang, H. Immobilization of Horseradish Peroxidase on NH2-Modified Magnetic Fe3O4/SiO2 Particles and its Application in Removal of 2,4-Dichlorophenol. Molecules 2014, 19, 15768–15782. [Google Scholar] [CrossRef]
- Chalkias, N.G.; Kahawong, P.; Giannelis, E.P. Activity Increase of Horseradish Peroxidase in the Presence of Magnetic Particles. J. Am. Chem. Soc. 2008, 130, 2910–2911. [Google Scholar] [CrossRef]
- Harkins, T.T.; Grissom, C.B. Magnetic Field Effects on B12 Ethanolamine Ammonia Lyase: Evidence for a Radical Mechanism. Science 1994, 263, 958–960. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, C.; Zhang, Z.; Wu, W.; Wang, X.; Yu, Z. Synthesis, Functionalization, and Nanomedical Applications of Functional Magnetic Nanoparticles. Chin. Chem. Lett. 2018, 29, 1601–1608. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Xu, D.; Pan, K. Immobilization of Horseradish Peroxidase on Electrospun Magnetic Nanofibers for Phenol Removal. Ecotoxicol. Environ. Saf. 2018, 170, 716–721. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, X. Immobilization of Horseradish Peroxidase on Fe3O4/Nanotubes Composites for Biocatalysis-Degradation of Phenol. Compos. Interfaces 2018, 26, 379–396. [Google Scholar] [CrossRef]
- Zdarta, J.; Antecka, K.; Jędrzak, A.; Synoradzki, K.; Luczak, M.; Jesionowski, T. Biopolymers Conjugated with Magnetite as Support Materials for Trypsin Immobilization and Protein Digestion. Colloids Surfaces B: Biointerfaces 2018, 169, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Lilhare, S.; Mathew, S.; Singh, A.; Carabineiro, S. Calcium Alginate Beads with Entrapped Iron Oxide Magnetic Nanoparticles Functionalized with Methionine—A Versatile Adsorbent for Arsenic Removal. Nanomaterials 2021, 11, 1345. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fan, L.; Xu, Y.; Xia, J. Iron Oxide Nanoparticles with Different Polymer Coatings for Photothermal Therapy. J. Nanoparticle Res. 2017, 19, 333. [Google Scholar] [CrossRef]
- Ahmadpoor, F.; Masood, A.; Feliu, N.; Parak, W.J.; Shojaosadati, S.A. The Effect of Surface Coating of Iron Oxide Nanoparticles on Magnetic Resonance Imaging Relaxivity. Front. Nanotechnol. 2021, 3, 1–12. [Google Scholar] [CrossRef]
- Abushrida, A.; Elhuni, I.; Taresco, V.; Marciani, L.; Stolnik, S.; Garnett, M.C. A Simple and Efficient Method for Polymer Coating of Iron Oxide Nanoparticles. J. Drug Deliv. Sci. Technol. 2019, 55, 101460. [Google Scholar] [CrossRef]
- Leng, G. Inequalities for polars of mixed projection bodies. Sci. China Math. 2004, 41, 175–186. [Google Scholar] [CrossRef]
- Jonović, M.; Žuža, M.; Đorđević, V.; Šekuljica, N.; Milivojević, M.; Jugović, B.; Bugarski, B.; Knežević-Jugović, Z. Immobilized Alcalase on Micron- and Submicron-Sized Alginate Beads as a Potential Biocatalyst for Hydrolysis of Food Proteins. Catalysts 2021, 11, 305. [Google Scholar] [CrossRef]
- Wasak, A.; Drozd, R.; Struk, Ł.; Grygorcewicz, B. Entrapment of DyP-type Peroxidase from Pseudomonas Fluorescens Pf-5 into Ca-Alginate Magnetic Beads. Biotechnol. Appl. Biochem. 2017, 65, 238–245. [Google Scholar] [CrossRef]
- Celebi, M.; Altikatoglu, M.; Akdeste, Z.M.; Yildirim, H. Determination of Decolorization Properties of Reactive Blue 19 dye Using Horseradish Peroxidase Enzyme. Turk. J. Biochem. 2013, 38, 200–206. [Google Scholar] [CrossRef]
- Radovanović, M.; Jugović, B.; Gvozdenović, M.; Jokić, B.; Grgur, B.; Bugarski, B.; Knežević-Jugović, Z. Immobilization of α-Amylase via Adsorption on Magnetic Particles Coated with Polyaniline. Starch-Stärke 2015, 68, 427–435. [Google Scholar] [CrossRef]
- Neri, D.F.; Balcão, V.M.; Dourado, F.O.; Oliveira, J.M.; Carvalho, L.B.; Teixeira, J.A. Immobilized β-Galactosidase onto Magnetic Particles Coated with Polyaniline: Support Characterization and Galactooligosaccharides Production. J. Mol. Catal. B: Enzym. 2011, 70, 74–80. [Google Scholar] [CrossRef]
- Knezevic, Z.; Bobic, S.; Milutinovic, A.; Obradovic, B.; Mojovic, L.; Bugarski, B. Alginate-Immobilized Lipase by Electrostatic Extrusion for the Purpose of Palm Oil Hydrolysis in Lecithin/Isooctane System. Process Biochem. 2002, 38, 313–318. [Google Scholar] [CrossRef]
- Sim, B.; Chae, H.S.; Choi, H.J. Fabrication of Polyaniline Coated Iron Oxide Hybrid Particles and their Dual Stimuli-Response under Electric and Magnetic Fields. Express Polym. Lett. 2015, 9, 736–743. [Google Scholar] [CrossRef]
- Kloster, G.A.; Muraca, D.; Londoño, O.M.; Pirota, K.R.; Mosiewicki, M.A.; Marcovich, N.E. Alginate Based Nanocomposites with Magnetic Properties. Compos. Part A: Appl. Sci. Manuf. 2020, 135, 105936. [Google Scholar] [CrossRef]
- Tee, B.L.; Kaletunç, G. Immobilization of a Thermostable α-Amylase by Covalent Binding to an Alginate Matrix Increases High Temperature Usability. Biotechnol. Prog. 2009, 25, 436–445. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Prodanovic, O.; Prokopijević, M.; Spasojević, D.; Stojanović, Z.; Radotić, K.; Knežević-Jugović, Z.D.; Prodanović, R. Improved Covalent Immobilization of Horseradish Peroxidase on Macroporous Glycidyl Methacrylate-Based Copolymers. Appl. Biochem. Biotechnol. 2012, 168, 1288–1301. [Google Scholar] [CrossRef]
- Cvetić, T.; Sabovljević, A.; Pristov, J.B.; Sabovljević, M. Effects of Day Length on Photosynthetic Pigments and Antioxidative Metabolism of in vitro Cultured Moss Atrichum undulatum (Hedw.) P. Beauv. (Bryophyta). Botanica Serbica 2009, 33, 83–88. [Google Scholar]
- Šekuljica, N.; Prlainović, N.; Stefanović, A.B.; Žuža, M.G.; Čičkarić, D.Z.; Mijin, D.; Knežević-Jugović, Z.D. Decolorization of Anthraquinonic Dyes from Textile Effluent Using Horseradish Peroxidase: Optimization and Kinetic Study. Sci. World J. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Šekuljica, N.; Prlainović, N.; Jovanovic, J.; Stefanović, A.B.; Djokic, V.; Mijin, D.; Knežević-Jugović, Z.D. Immobilization of Horseradish Peroxidase onto Kaolin. Bioprocess Biosyst. Eng. 2016, 39, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Asgher, M. Dye Decolorization and Detoxification Potential of Ca-Alginate Beads Immobilized Manganese Peroxidase. BMC Biotechnol. 2015, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Asgher, M. Sandal Reactive Dyes Decolorization and Cytotoxicity Reduction Using Manganese Peroxidase Immobilized onto Polyvinyl Alcohol-Alginate Beads. Chem. Central J. 2015, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.; Sharma, S. Investigation of Swelling/Degradation Behaviour of Alginate BEADS crosslinked with Ca2+ and Ba2+ Ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Larosa, C.; Salerno, M.; de Lima, J.S.; Meri, R.M.; da Silva, M.F.; de Carvalho, L.B.; Converti, A. Characterisation of Bare and Tannase-Loaded Calcium Alginate Beads by Microscopic, Thermogravimetric, FTIR and XRD Analyses. Int. J. Biol. Macromol. 2018, 115, 900–906. [Google Scholar] [CrossRef]
- Hammouda, S.B.; Adhoum, N.; Monser, L. Synthesis of Magnetic Alginate Beads Based on Fe3O4 Nanoparticles for the Removal of 3-Methylindole from Aqueous Solution Using Fenton Process. J. Hazard. Mater. 2015, 294, 128–136. [Google Scholar] [CrossRef]
- Czichy, C.; Spangenberg, J.; Günther, S.; Gelinsky, M.; Odenbach, S. Determination of the Young’s Modulus for Alginate-based Hydrogel with Magnetite-Particles Depending on Storage Conditions and Particle Concentration. J. Magn. Magn. Mater. 2020, 501, 166395. [Google Scholar] [CrossRef]
- Manuja, A.; Kumar, S.; Dilbaghi, N.; Bhanjana, G.; Chopra, M.; Kaur, H.; Kumar, R.; Manuja, B.K.; Singh, S.K.; Yadav, S.C. Quinapyramine Sulfate-Loaded Sodium Alginate Nanoparticles Show Enhanced Trypanocidal Activity. Nanomedicine 2014, 9, 1625–1634. [Google Scholar] [CrossRef]
- Yeom, C.K.; Lee, K.-H. Characterization of Sodium Alginate Membrane Crosslinked with Glutaraldehyde in Pervaporation Separation. J. Appl. Polym. Sci. 1998, 67, 209–219. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M. Green Synthesized Gold Nanoparticles as a Probe for the Detection of Fe3+ Ions in Water. J. Clust. Sci. 2013, 25, 969–978. [Google Scholar] [CrossRef]
- Navarrete, J.T.L.; Hernandez, V.; Ramírez, F.J. Ir and Raman Spectra of L-Aspartic Acid and Isotopic Derivatives. Biopolymers 1994, 34, 1065–1077. [Google Scholar] [CrossRef]
- Dennis, G.; Harrison, W.; Agnes, K.; Erastus, G. Effect of Biological Control Antagonists Adsorbed on Chitosan Immobilized Silica Nanocomposite on Ralstonia Solanacearum and Growth of Tomato Seedlings. Adv. Res. 2016, 6, 1–23. [Google Scholar] [CrossRef]
- Cárdenas-Jirón, G.; Leal, D.; Matsuhiro, B.; Osorio-Román, I.O. Vibrational Spectroscopy and Density Functional Theory Calculations of Poly-D -Mannuronate and Heteropolymeric Fractions from Sodium Alginate. J. Raman Spectrosc. 2011, 42, 870–878. [Google Scholar] [CrossRef]
- Chandía, N.; Matsuhiro, B.; Vásquez, A. Alginic Acids in Lessonia Trabeculata: Characterization by Formic Acid Hydrolysis and FT-IR Spectroscopy. Carbohydr. Polym. 2001, 46, 81–87. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and Characterization of Calcium Alginate Nanoparticles, Sodium Homopolymannuronate Salt and its Calcium Nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Obireddy, S.R.; Chintha, M.; Kashayi, C.R.; Venkata, K.R.K.S.; Subbarao, S.M.C. Gelatin-Coated Dual Cross-Linked Sodium Alginate/Magnetite Nanoparticle Microbeads for Controlled Release of Doxorubicin. ChemistrySelect 2020, 5, 10276–10284. [Google Scholar] [CrossRef]
- Barbosa, G.S.D.S.; Oliveira, M.E.P.S.; Dos Santos, A.B.S.; Sánchez, O.C.; Soares, C.M.F.; Fricks, A.T. Immobilization of Low-Cost Alternative Vegetable Peroxidase (Raphanus sativus L. peroxidase): Choice of Support/Technique and Characterization. Molecules 2020, 25, 3668. [Google Scholar] [CrossRef]
- Kayal, S.; Ramanujan, R. Doxorubicin Loaded PVA Coated Iron Oxide Nanoparticles for Targeted Drug Delivery. Mater. Sci. Eng. C 2010, 30, 484–490. [Google Scholar] [CrossRef]
- Barbosa, E.F.; Molina, F.J.; Lopes, F.M.; García-Ruíz, P.A.; Caramori, S.S.; Fernandes, K.F. Immobilization of Peroxidase onto Magnetite Modified Polyaniline. Sci. World J. 2012, 2012, 1–5. [Google Scholar] [CrossRef][Green Version]
- Lan, J.; Huang, X.; Hu, M.; Li, Y.; Qu, Y.; Gao, P.; Wu, D. High Efficient Degradation of Dyes with Lignin Peroxidase Coupled with Glucose Oxidase. J. Biotechnol. 2006, 123, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Jamal, F.; Qidwai, T.; Pandey, P.K.; Singh, R.; Singh, S. Azo and Anthraquinone Dye Decolorization in Relation to its Molecular Structure using Soluble Trichosanthes Dioica Peroxidase Supplemented with Redox Mediator. Catal. Commun. 2011, 12, 1218–1223. [Google Scholar] [CrossRef]

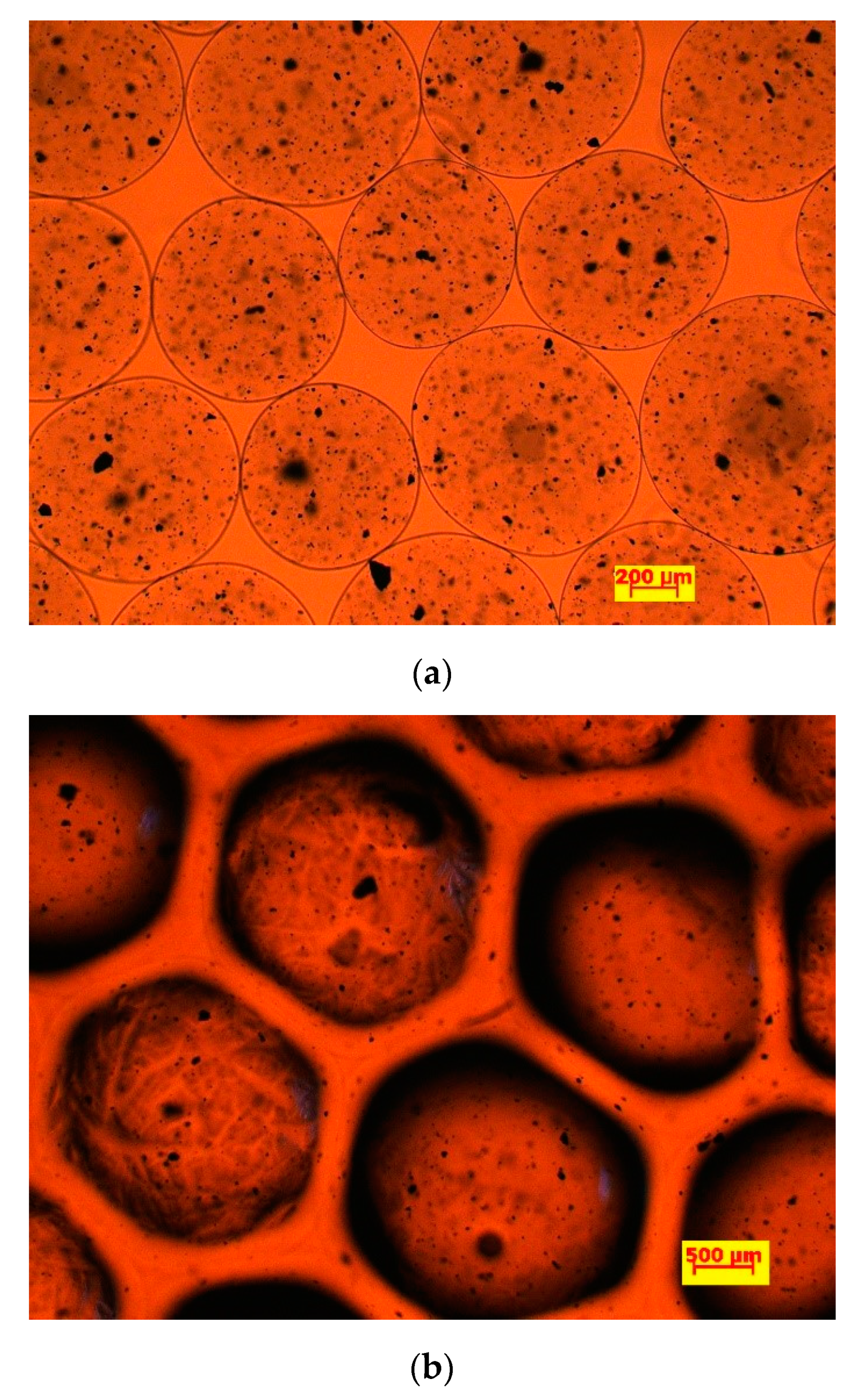




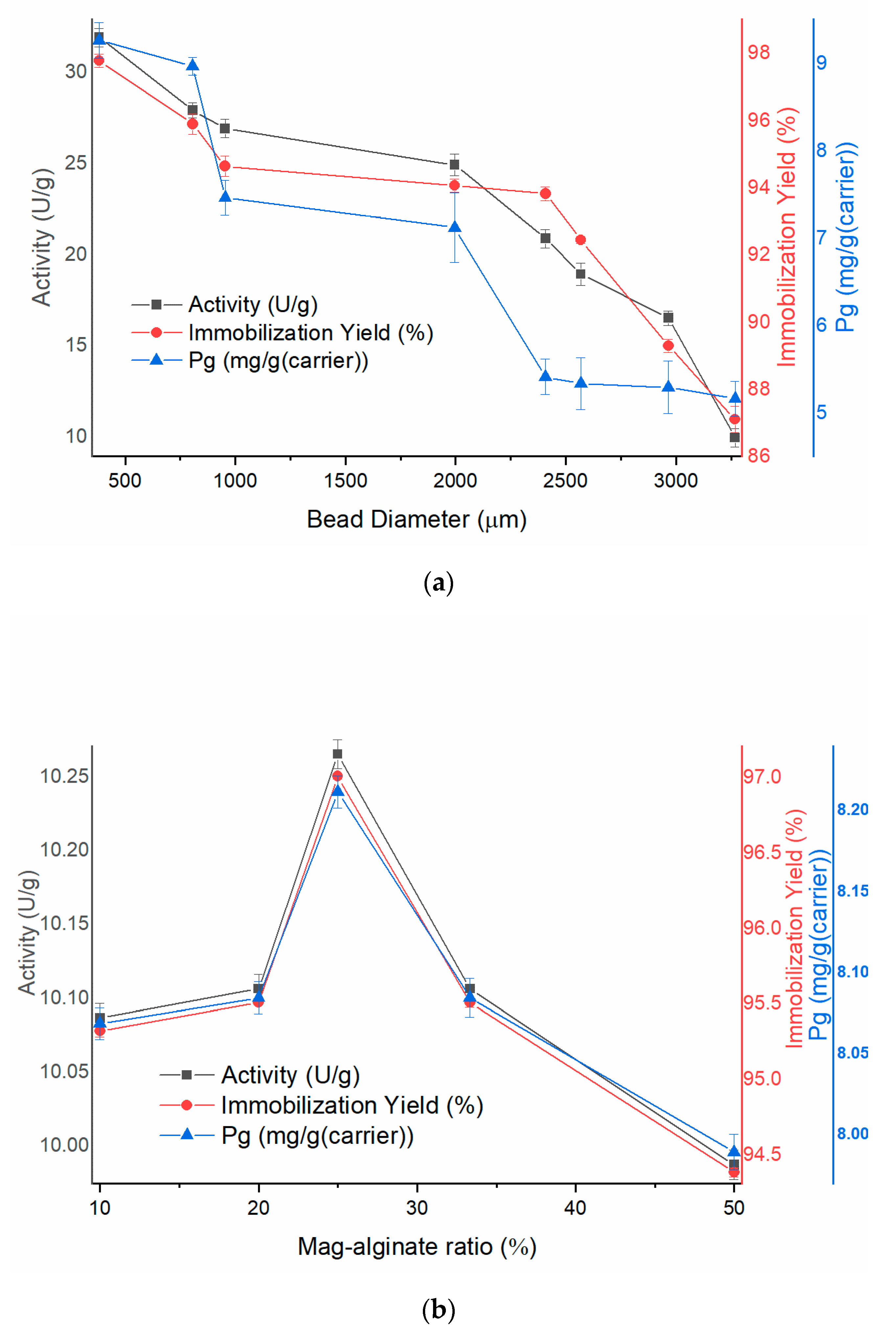






| Nozzle Size | Before Immobilization d (µm) | After Immobilization d (µm) |
|---|---|---|
| 18G | 3268.0 ± 32.2 | 3309.1 ± 60.3 |
| 20G | 2965.9 ± 39.6 | 3003.2 ± 40.1 |
| 22G | 2568.3 ± 53.5 | 2662.1 ± 60.2 |
| 25G | 2408.1 ± 40.7 | 2513.1 ± 54.8 |
| 18Gee | 1995.8 ± 80.0 | 2095.3 ± 74.3 |
| 20Gee | 953.1 ± 60.3 | 1112.3 ± 23.7 |
| 22Gee | 806.6 ± 82.1 | 1030.9 ± 73.8 |
| 25Gee | 382.2 ± 91.2 | 472.3 ± 51.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonović, M.; Jugović, B.; Žuža, M.; Đorđević, V.; Milašinović, N.; Bugarski, B.; Knežević-Jugović, Z. Immobilization of Horseradish Peroxidase on Magnetite-Alginate Beads to Enable Effective Strong Binding and Enzyme Recycling during Anthraquinone Dyes’ Degradation. Polymers 2022, 14, 2614. https://doi.org/10.3390/polym14132614
Jonović M, Jugović B, Žuža M, Đorđević V, Milašinović N, Bugarski B, Knežević-Jugović Z. Immobilization of Horseradish Peroxidase on Magnetite-Alginate Beads to Enable Effective Strong Binding and Enzyme Recycling during Anthraquinone Dyes’ Degradation. Polymers. 2022; 14(13):2614. https://doi.org/10.3390/polym14132614
Chicago/Turabian StyleJonović, Marko, Branimir Jugović, Milena Žuža, Verica Đorđević, Nikola Milašinović, Branko Bugarski, and Zorica Knežević-Jugović. 2022. "Immobilization of Horseradish Peroxidase on Magnetite-Alginate Beads to Enable Effective Strong Binding and Enzyme Recycling during Anthraquinone Dyes’ Degradation" Polymers 14, no. 13: 2614. https://doi.org/10.3390/polym14132614
APA StyleJonović, M., Jugović, B., Žuža, M., Đorđević, V., Milašinović, N., Bugarski, B., & Knežević-Jugović, Z. (2022). Immobilization of Horseradish Peroxidase on Magnetite-Alginate Beads to Enable Effective Strong Binding and Enzyme Recycling during Anthraquinone Dyes’ Degradation. Polymers, 14(13), 2614. https://doi.org/10.3390/polym14132614







