Development of Synthesis and Application of High Molecular Weight Poly(Methyl Methacrylate)
Abstract
:1. Introduction
2. Synthesis of High Molecular Weight PMMA
2.1. Free Radical Polymerization
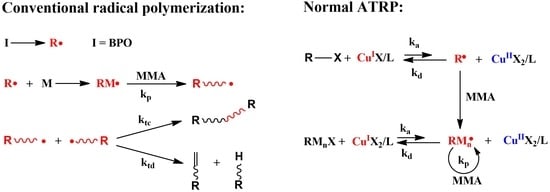
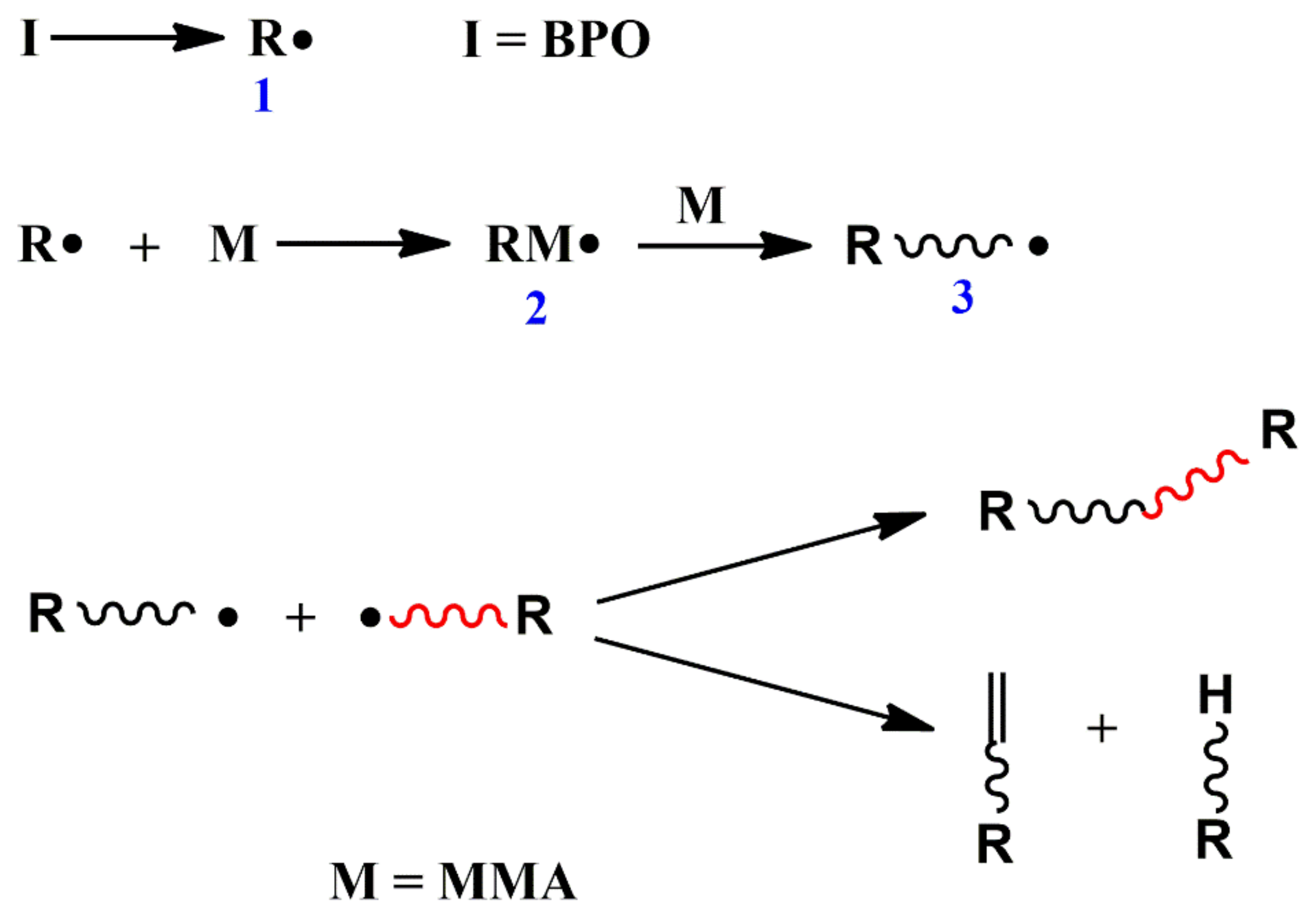
2.1.1. Conventional Radical Polymerization
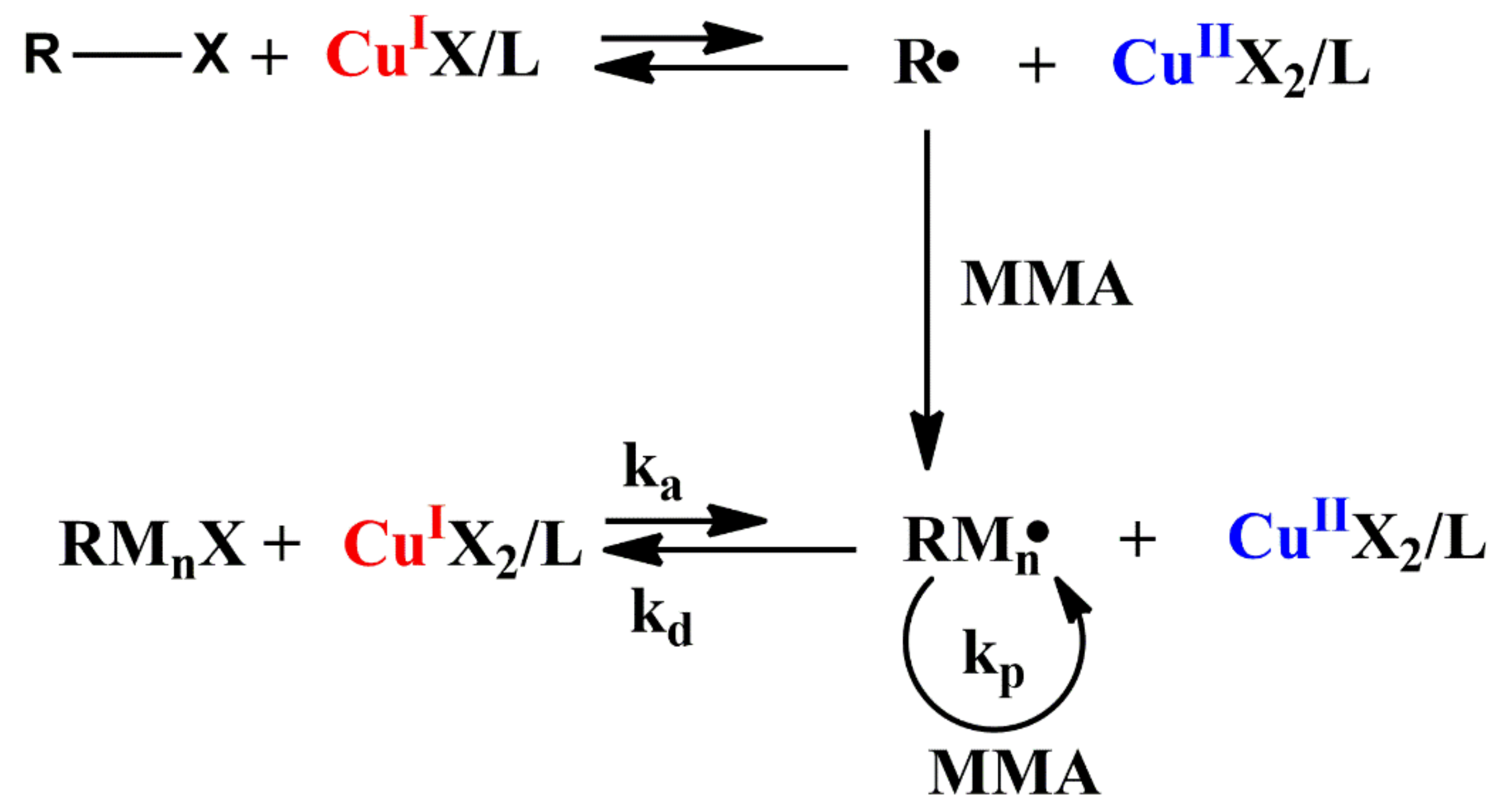
2.1.2. Atom Transfer Radical Polymerization (ATRP)
2.2. Coordination Polymerization
2.3. Other Polymerizations
3. Applications of High Molecular Weight PMMA
3.1. Application of High Molecular Weight PMMA in Medical Field
3.1.1. PMMA Bone Cements
3.1.2. PMMA Denture
3.2. Applications of High Molecular Weight PMMA in Optical and Electricity Area
3.3. Applications of High Molecular Weight PMMA in Other Areas
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khezri, K.; Fazli, Y. ATRP of Methyl methacrylate in the presence of HMDS-modified silica aerogel: ARGET approach. J. Inorg. Organomet. Polym. Mater. 2019, 29, 608–616. [Google Scholar] [CrossRef]
- Otozawa, N.; Hamajima, R.; Yoshioka, M.; Kato, R.; Tanaka, A.; Fukuma, H.; Terao, T.; Manabe, K.; Fujii, S.; Nakamura, Y.; et al. Preparation of polymethyl methacrylate with well-controlled stereoregularity by anionic polymerization in an ionic liquid Solvent. J. Polym. Sci. 2020, 58, 1960–1964. [Google Scholar] [CrossRef]
- Sheng, Y.; Huang, H.; Yu, M.; Zhang, X.; Cheng, L.; Liu, Z.; Liu, W.; Huang, Q.; Yi, J.; Yang, W. Synthesis of 4-arms hydroxy-functionalized PMMA-b-PE through combining free radical polymerization with coordination polymerization. J. Ind. Eng. Chem. 2014, 20, 419–425. [Google Scholar] [CrossRef]
- Archana, R.; Baldia, M.; Jeeva, J.B.; Joseph, M. Strength analysis of cranioplasty PMMA flap material. Mater. Today Proc. 2019, 15, 167–172. [Google Scholar] [CrossRef]
- Kaufmann, T.J.; Jensen, M.E.; Ford, G.; Gill, L.L.; Marx, W.F.; Kallmes, D.F. Cardiovascular effects of polymethylmethacrylate use in percutaneous vertebroplasty. Am. J. Neuroradiol. 2002, 23, 601–604. [Google Scholar]
- Rzayev, J.; Penelle, J. HP-RAFT: A free-radical polymerization technique for obtaining living polymers of ultrahigh molecular weights. Angew. Chem. Int. Ed. 2004, 43, 1691–1694. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y.J.; Dong, Q.Z.; Hu, C.P. Free radical polymerizations of some vinyl monomers in carbon dioxide fluid. J. Appl. Polym. Sci. 2008, 110, 468–474. [Google Scholar] [CrossRef]
- Genix, A.C.; Bocharova, V.; Kisliuk, A.; Carroll, B.; Zhao, S.; Oberdisse, J.; Sokolov, A.P. Enhancing the mechanical properties of glassy nanocomposites by tuning polymer molecular weight. Appl. Mater. Interfaces 2018, 10, 33601–33610. [Google Scholar] [CrossRef]
- Liu, D.; Chen, H.; Yin, P.; Hao, Z.; Fan, L. Use of Gd powder as catalyst for single electron transfer-living radical polymerization: Applications for synthesis of high molecular weight polymethyl methacrylate. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4809–4813. [Google Scholar] [CrossRef] [Green Version]
- Jayabalan, M. Thermal modification of poly(vinyl chloride) and formation of dehydrochlorinated poly (vinyl chloride)-poly(methyl Methacrylate) polymer blend in the process of foaming: Identification and application. J. Appl. Polym. Sci. 1982, 27, 43–52. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Mitra, S.; Ranjith, P.; Gopalakrishnan, S.; Parthipan, P.; Al-Farhood, B. High heat resistant blends of poly(methyl methacrylate) and styrenic copolymers via post reactor modification. J. Appl. Polym. Sci. 2018, 135, 46220. [Google Scholar] [CrossRef]
- Gu, D.; Zhang, L.; Chen, S.; Song, K.; Pan, D.; Yang, B.; Liu, S. Heat treatment to improve the wear resistance of PTFE/PMMA composites. RSC Adv. 2019, 9, 22289–22294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharjee, M.; Patra, B.N. [Cp2TiCl2] catalyzed polymerization in water: Polymerization of methylmethacrylate to a high molecular weight polymer. Polymer 2004, 45, 3111–3114. [Google Scholar] [CrossRef]
- Wang, J.S.; Matyjaszewski, K. Controlled/”living” radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Johnson, D.R.; Osada, Y.; Bell, A.T.; Shen, M. Studies of the mechanism and kinetics of plasma-initiated polymerization of methyl methacrylate. Macromolecules 1981, 14, 118–124. [Google Scholar] [CrossRef]
- Lyoo, W.S.; Noh, S.K.; Yeum, J.H.; Kang, G.C.; Ghim, H.D.; Lee, J.; Ji, B.C. Preparation of high molecular weight poly(methyl methacrylate) with high yield by room temperature suspension polymerization of methyl methacrylate. Fibers Polym. 2004, 5, 75–81. [Google Scholar] [CrossRef]
- Hsiao, Y.L.; Maury, E.E.; DeSimone, J.M. Dispersion polymerization of methyl methacrylate stabilized with poly(1,1-dihydroperfluorooctyl acrylate) in supercritical carbon dioxide. Macromolecules 1995, 28, 8159–8166. [Google Scholar] [CrossRef]
- Hsiao, Y.L.; DeSimone, J.M. Dispersion polymerization of methyl methacrylate in supercritical carbon dioxide: Influence of helium concentration on particle size and particle size distribution. J. Polym. Sci. A Polym. Chem. 1997, 35, 2009–2013. [Google Scholar] [CrossRef]
- Lepilleur, C.; Beckman, E.J. Dispersion polymerization of methyl methacrylate in supercritical CO2. Macromolecules 1997, 30, 745–756. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156164. [Google Scholar] [CrossRef]
- Seddon, K.R. Ionic liquids for clean technology. J. Chem. Tech. Biotechnol. 1997, 68, 351–356. [Google Scholar] [CrossRef]
- Scott, M.P.; Rahman, M.; Brazel, C.S. Application of ionic liquids as low-volatility plasticizers for PMMA. Eur. Polym. J. 2003, 39, 1947–1953. [Google Scholar] [CrossRef]
- Scott, M.P.; Brazel, C.S.; Benton, M.G.; Mays, J.W.; Holbreyc, J.D.; Rogers, R.D. Application of ionic liquids as plasticizers for poly(methyl methacrylate). Chem. Commun. 2002, 38, 1370–1371. [Google Scholar] [CrossRef] [PubMed]
- Yeon, S.H.; Kim, K.S.; Choi, S.; Cha, J.H.; Lee, H.; Oh, J.; Lee, B.B. Poly(vinylidenefluoride)-hexafluoropropylene gel electrolytes based on N-(2-hydroxyethyl)-N-methyl morpholinium ionic liquids. Korean J. Chem. Eng. 2006, 23, 940–947. [Google Scholar] [CrossRef]
- Tomás-Alonso, F.; Rubio, A.M.; Giménez, A.; De los Ríos, A.P. Influence of ionic liquid composition on the stability of polyvinyl chloride-based ionic liquid inclusion membranes in aqueous solution. AIChE J. 2017, 63, 770–780. [Google Scholar] [CrossRef]
- Dai, S.; Ju, Y.H.; Gao, H.J.; Lin, J.S.; Pennycook, S.J.; Barnes, C.E. Preparation of silica aerogel using ionic liquids as solvents. Chem. Commun. 2000, 36, 243–244. [Google Scholar] [CrossRef]
- Carlin, R.T.; Fuller, J. Ionic liquid-polymer gel catalytic membrane. Chem. Commun. 1997, 33, 1345–1346. [Google Scholar] [CrossRef]
- Vygodskii, Y.S.; Mel’nik, O.A.; Lozinskaya, E.I.; Shaplov, A.S.; Malyshkina, I.A.; Gavrilova, N.D.; Lyssenko, K.A.; Antipin, M.Y.; Golovanov, D.G.; Korlyukov, A.A.; et al. The influence of ionic liquids nature on free radical polymerization of vinyl monomers and ionic conductivity of the obtained polymeric materials. Polym. Adv. Technol. 2007, 18, 50–63. [Google Scholar] [CrossRef]
- Carotenuto, G.; Her, Y.S.; Matijević, E. Preparation and characterization of nanocomposite thin films for optical devices. Ind. Eng. Chem. Res. 1996, 35, 2929–2932. [Google Scholar] [CrossRef]
- Wang, Y.; Herron, N. Photoconductivity of CdS nanocluster-doped polymers. Chem. Phys. Lett. 1992, 27, 71–75. [Google Scholar] [CrossRef]
- Hailstone, R.K. Computer simulation studies of silver cluster formation on AgBr microcrystals. J. Phys. Chem. 1995, 99, 4414–4428. [Google Scholar] [CrossRef]
- Lira-Cantú, M.; Gómez-Romero, P. Electrochemical and chemical syntheses of the hybrid organic-inorganic electroactive material formed by phosphomolybdate and polyaniline. Application as cation-insertion electrodes. Chem. Mater. 1998, 10, 698–704. [Google Scholar] [CrossRef] [Green Version]
- Yeum, J.H.; Deng, Y. Synthesis of high molecular weight poly(methyl methacrylate) microspheres by suspension polymerization in the presence of silver nanoparticles. Colloid Polym. Sci. 2005, 283, 1172–1179. [Google Scholar] [CrossRef]
- Jiang, W.; Yang, W.; Zeng, X.; Fu, S. Structure and properties of poly(methyl methacrylate) particles prepared by a modified microemulsion polymerization. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 733–741. [Google Scholar] [CrossRef]
- Norakankorn, C.; Pan, Q.; Rempel, G.L.; Kiatkamjornwong, S. Synthesis of poly(methyl methacrylate) nanoparticles initiated by 2,2′-azoisobutyronitrile via differential microemulsion polymerization. Macromol. Rapid Commun. 2007, 28, 1029–1033. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; MacManus-Driscoll, J.L. ZnO—nanostructures, defects, and devices. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Asiri, A.M.; Khan, S.B.; Alamry, K.A.; Yagci, Y. Semiconductor nanoparticles for photoinitiation of free radical polymerization in aqueous and organic media. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1500–1507. [Google Scholar] [CrossRef]
- Strandwitz, N.C.; Khan, A.; Boettcher, S.W.; Mikhailovsky, A.A.; Hawker, C.J.; Nguyen, T.Q.; Stucky, G.D. One- and two-photon induced polymerization of methylmethacrylate using colloidal CdS semiconductor quantum dots. J. Am. Chem. Soc. 2008, 130, 8280–8288. [Google Scholar] [CrossRef]
- Stroyuk, A.L.; Granchak, V.M.; Korzhak, A.V.; Kuchmii, S.Y. Photoinitiation of buthylmethacrylate polymerization by colloidal semiconductor nanoparticles. J. Photochem. Photobiol. A Chem. 2004, 162, 339–351. [Google Scholar] [CrossRef]
- Balta, D.K.; Arsu, N.; Yagci, Y.; Sundaresan, A.K.; Jockusch, S.; Turro, N.J. Mechanism of photoinitiated free radical polymerization by thioxanthone—anthracene in the presence of air. Macromolecules 2011, 44, 2531–2535. [Google Scholar] [CrossRef]
- Pynaert, R.; Buguet, J.; Croutxe-Barghorn, C.; Moireau, P.; Allonas, X. Effect of reactive oxygen species on the kinetics of free radical photopolymerization. Polym. Chem. 2013, 4, 2475–2479. [Google Scholar] [CrossRef]
- Dule, M.; Biswas, M.; Biswas, Y.; Mandal, T.K. Redox-active poly(ionic liquid)-engineered Ag nanoparticle-decorated ZnO nanoflower heterostructure: A reusable composite catalyst for photopolymerization into high-molecular-weight polymers. Polymer 2017, 133, 223–231. [Google Scholar] [CrossRef]
- Xue, L.; Agarwal, U.S.; Lemstra, P.J. High molecular weight PMMA by ATRP. Macromolecules 2002, 35, 8650–8652. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Queffelec, J.; Gaynor, S.G.; Matyjaszewski, K. Optimization of atom transfer radical polymerization using Cu(I)/tris(2-(dimethylamino)ethyl)amine as a catalyst. Macromolecules 2000, 33, 8629–8639. [Google Scholar] [CrossRef]
- Mao, B.W.; Gan, L.H.; Gan, Y.Y. Ultra high molar mass poly[2-(dimethylamino)ethyl methacrylate] via atom transfer radical polymerization. Polymer 2006, 47, 3017–3020. [Google Scholar] [CrossRef]
- Matyjaszewski, K. The importance of exchange reactions in controlled/living radical polymerization in the presence of alkoxyamines and transition metals. Macromol. Symp. 1996, 111, 47–61. [Google Scholar] [CrossRef]
- Xue, L.; Agarwal, U.S.; Zhang, M.; Staal, B.B.P.; Mu1ller, A.H.E.; Bailly, C.M.E.; Lemstra, P.J. Synthesis and direct topology visualization of high-molecular-weight star PMMA. Macromolecules 2005, 38, 2093–2100. [Google Scholar] [CrossRef]
- Min, K.; Gao, H.; Matyjaszewski, K. Use of ascorbic acid as reducing agent for synthesis of well-defined polymers by ARGET ATRP. Macromolecules 2007, 40, 1789–1791. [Google Scholar] [CrossRef]
- Nicolaÿ, R.; Kwak, Y.; Matyjaszewski, K. A green route to well-defined high-molecular-weight (Co)polymers using ARGET ATRP with alkyl pseudohalides and copper catalysis. Angew. Chem. 2010, 122, 551–554. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, T.; Lin, K.C.; Li, S.; Yan, J.; Olszewski, M.; Sobieski, J.; Pietrasik, J.; Bockstaller, M.R.; Matyjaszewski, K. Synthesis of ultra-high molecular weight SiO2-g-PMMA particle brushes. J. Inorg. Organomet. Polym. Mater. 2000, 30, 174–181. [Google Scholar] [CrossRef]
- Jeon, H.J.; Youk, J.H.; Ahn, S.H.; Choi, J.H.; Cho, K.S. Synthesis of high molecular weight 3-arm star PMMA by ARGET ATRP. Macromol. Res. 2009, 17, 240–244. [Google Scholar] [CrossRef]
- Arita, T.; Kayama, Y.; Ohno, K.; Tsujii, Y.; Fukuda, T. High-pressure atom transfer radical polymerization of methyl methacrylate for well-defined ultrahigh molecular-weight polymers. Polymer 2008, 49, 2426–2429. [Google Scholar] [CrossRef]
- Olaj, O.F.; Kornherr, A.; Zifferer, G. On the (im)possibility of evaluating correct individual rate constants of chain propagation kp and chain termination kt by combining kp2/kt and kp/kt data for chain-length dependent termination. Macromol. Theory Simul. 2000, 9, 131–140. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Jurczak, J.; Pietrasik, J.; Jakubowski, W.; Mueller, L.; Matyjaszewski, K. High molecular weight polymethacrylates by AGET ATRP under high pressure. Macromolecules 2008, 41, 1067–1069. [Google Scholar] [CrossRef]
- Sun, J.; Pan, Z.; Zhong, Y.; Hu, W.; Yang, S. Polymerization of methyl methacrylate by single component catalyst of lanthanocene chloride O(C2H4C5H3CH3)2LnCl (Ln = Y, Nd, Sm). Eur. Polym. J. 2000, 36, 2375–2380. [Google Scholar]
- Sun, J.; Pan, Z.; Hu, W.; Yang, S. Polymerization of methyl methacrylate with ethylene bridged heterodinuclear metallocene of samarium and titanium. Eur. Polym. J. 2002, 38, 545–549. [Google Scholar]
- Jones, R.D.; Summerville, D.A.; Basolo, F. Synthetic oxygen carriers related to biological systems. Chem. Rev. 1979, 79, 139–179. [Google Scholar] [CrossRef]
- Yousaf, M.; Chatha, A.A.; Jabbar, A.; Ahmad, H.B.; Zahoor, A.F.; Khosa, M.K.; Alam, S.S. Role of tridentate schiff base and methoxyethylindenyl derivatives of lanthanocene complexes for the synthesis of high molecular weight polymethyl methacrylate (PMMA). Turk. J. Chem. 2007, 31, 471–477. [Google Scholar]
- Kim, D.; Song, Y.; Kim, S.; Lee, H.J.; Lee, H. N,N’, X-bidentate versus N,N’, X-tridentate N-substituted 2-iminomethylpyridine- and 2-iminomethylquinoline-coordinated palladium(II)complexes. J. Coord. Chem. 2014, 67, 2312–2329. [Google Scholar] [CrossRef]
- Song, Y.; Kim, D.; Lee, H.J.; Lee, H. Cd(II) and Zn(II) complexes containing N,N’-bidentate N-(pyridin-2-ylmethylene)cyclopentanamine: Synthesis, characterisation and methyl methacrylate polymerisation. Bull. Korean Chem. Soc. 2014, 35, 2929–2934. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.; Lee, H.; Nayab, S. Polymerizations of methyl methacrylate and rac-lactide by Zinc(II) precatalyst containing N-substituted 2-iminomethylpyridine and 2-iminomethylquinoline. J. Coord. Chem. 2017, 70, 3837–3858. [Google Scholar] [CrossRef]
- Lansalot-Matras, C.; Bonnette, F.; Mignard, E.; Lavastre, O. N-tripodal ligands to generate copper catalysts for the syndiotactic polymerization of methyl-methacrylate: Crystal structures of copper complexes. J. Organomet. Chem. 2008, 693, 393–398. [Google Scholar] [CrossRef]
- Yang, M.; Park, W.J.; Yoon, K.B.; Jeong, J.H.; Lee, H. Synthesis, Characterization, and MMA polymerization activity of tetrahedral Co (II)complex bearing N, N-bis(1-pyrazolyl)methyl ligand based on aniline moiety. Inorg. Chem. Commun. 2011, 14, 189–193. [Google Scholar] [CrossRef]
- Shin, S.; Nayab, S.; Lee, H. Polymerizations of methyl methacrylate and rac-lactide by 4-coordinate cobalt(II) complexes supported by N’-substituted N,N’,N-bis((1H-pyrazol-1-yl)methyl)amine derivatives. Polyhedron 2018, 141, 309–321. [Google Scholar] [CrossRef]
- Buchowicz, W.; Kozioł, A.; Jerzykiewicz, L.B.; Lis, T.; Pasynkiewicz, S.; Pecherzewska, A.; Pietrzykowski, A. N-heterocyclic carbene complexes of cyclopentadienylnickel(II): Synthesis, structure and catalytic activity in styrene polymerization. J. Mol. Catal. A Chem. 2006, 257, 118–123. [Google Scholar] [CrossRef]
- Buchowicz, W.; Wojtczak, W.; Pietrzykowski, A.; Lupa, A.; Jerzykiewicz, L.B.; Makal, A.; Woźniak, K. Synthesis, structure, and polymerization activity of cyclopentadienylnickel(II) N-heterocyclic carbene complexes: Selective cross-metathesis in metal coordination spheres. Eur. J. Inorg. Chem. 2010, 2010, 648–656. [Google Scholar] [CrossRef]
- Ihara, E.; Fujimura, T.; Yasuda, H.; Maruo, T.; Kanehisa, N.; Kai, Y. High polymerization of methyl methacrylate with organonickel/MMAO systems. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 4764–4775. [Google Scholar] [CrossRef]
- Buchowicz, W.; Conder, J.; Hryciuk, D.; Zachara, J. Nickel-mediated polymerization of methyl methacrylate. J. Mol. Catal. A Chem. 2014, 381, 16–20. [Google Scholar] [CrossRef]
- Kitaura, T.; Kitayama, T. Anionic polymerization of methyl methacrylate with the aid of lithium trimethylsilanolate (Me3SiOLi)—superior control of isotacticity and molecular weight. Macromol. Rapid Commun. 2007, 28, 1889–1893. [Google Scholar] [CrossRef]
- Osada, Y.; Bell, A.T.; Shen, M. Plasma-initiated polymerization of methyl methacrylate. J. Polym. Sci. Polym. Lett. Ed. 1978, 16, 309–311. [Google Scholar] [CrossRef]
- Gavens, L.M.; Wu, B.J.; Hess, D.W.; Bell, A.T.; Soong, D.S. Ultrahigh molecular weight poly(methyl methacrylate) as an electron-beam resist. J. Vac. Sci. Technol. B 1983, 1, 481–486. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, X.; Zhu, J.; Cheng, Z. Plasma-initiated controlled/living radical polymerization of methyl methacrylate in the presence of 2-cyanoprop-2-yl 1-dithionaphthalate (CPDN). Macromol. Rapid Commun. 2004, 25, 818–824. [Google Scholar] [CrossRef]
- Yuan, M.; Xu, L.; Cui, X.; Lv, J.; Zhang, P.; Tang, H. Facile synthesis of ultrahigh molecular weight poly(methyl methacrylate) by organic halides in the presence of palladium nanoparticles. Polymers 2020, 12, 2747. [Google Scholar] [CrossRef]
- Zhang, Y.; Miyake, G.M.; Chen, E.Y.X. Alane-based classical and frustrated lewis pairs in polymer synthesis: Rapid polymerization of MMA and naturally renewable methylene butyrolactones into high-molecular-weight polymers. Angew. Chem. Int. Ed. 2010, 49, 10158–10162. [Google Scholar] [CrossRef]
- Demirel, G.; Akovali, G. Some studies on plasma-initiated polymerization of (MMA) and on plasma polymerized (PMMA). J. Polym. Sci. Polym. Chem. Ed. 1985, 23, 2377–2383. [Google Scholar] [CrossRef]
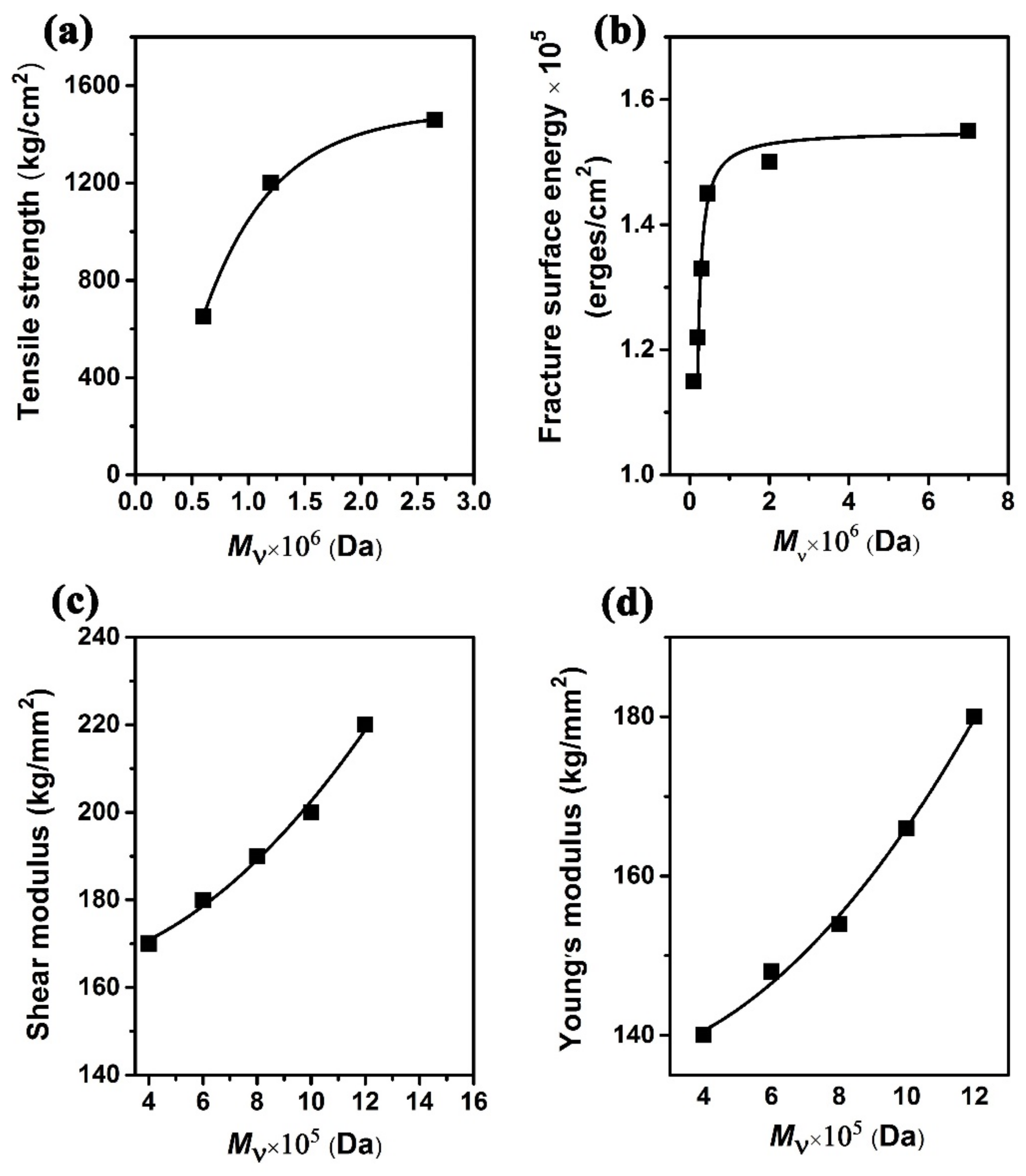
- Martin, J.R.; Johnson, J.F.; Cooper, A.R. Mechanical properties of polymers: The influence of molecular weight and molecular weight distribution. J. Macromol. Sci. Rev. Macromol. Chem. 1972, 8, 57–199. [Google Scholar] [CrossRef]
- Kusy, R.P.; Greenberg, A.R. Influence of molecualr weight on the dynamic mechanical properties of poly(methyl methacrylate). J. Therm. Anal. 1980, 18, 117–126. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, J.H.; Lee, H.Y.; Lee, S.H.; Keem, S.H. Predictive factors for subsequent vertebral fracture after percutaneous vertebroplasty. J. Neurosurg. Spine 2008, 9, 129–136. [Google Scholar] [CrossRef]
- Zhang, W.; Liao, S.S.; Cui, F.Z. Hierarchical self-assembly of nano-fibrils in mineralized collagen. Chem. Mater. 2003, 15, 3221–3226. [Google Scholar] [CrossRef]
- Yu, X.; Xu, L.; Cui, F.Z.; Qu, Y.; Lian, X.J.; Wang, X.M.; Wang, Y. Clinical evaluation of mineralized collagen as a bone graft substitute for anterior cervical intersomatic fusion. J. Biomater. Tissue Eng. 2012, 2, 170–176. [Google Scholar] [CrossRef]
- Soha, S.; Pal, S. Mechanical properties of bone cement: A review. J. Biomed. Mater. Res. 1984, 18, 435–462. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.D. PMMA Cements; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Heo, S.J.; Shin, H.J.; Park, S.A.; Lee, Y.J.; Yoon, T.R.; Seo, H.Y.; Ahn, K.C.; Kim, S.E.; Shin, J.W. Evaluation of bonding stress for the newly suggested bone cement: Comparison with currently used PMMA through animal studies. Key Eng. Mater. 2007, 342, 373–376. [Google Scholar] [CrossRef]
- Lewis, G. Alternative acrylic bone cement formulations for cemented arthroplasties: Present status, key issues, and future prospects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 84, 301–319. [Google Scholar] [CrossRef]
- Liu, H.; Webster, T.J. Nanomedicine for implants: A review of studies and necessary experimental tools. Biomaterials 2007, 28, 354–369. [Google Scholar] [CrossRef]
- Khandaker, M.; Li, Y.; Morris, T. Micro and nano MgO particles for the improvement of fracture toughness of bone-cement interfaces. J. Biomech. 2013, 46, 1035–1039. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Chan, A.; Tan, X.; Yang, H.; Yang, L. Fabrication and characterizations of metallic Mg containing PMMA-based partially degradable composite bone cements. Acta Metall. Sin. 2019, 32, 808–816. [Google Scholar] [CrossRef] [Green Version]
- Mori, A.D.; Gregorio, E.D.; Kao, A.P.; Tozzi, G.; Barbu, E.; Sanghani-Kerai, A.; Draheim, R.R.; Roldo, M. Antibacterial PMMA composite cements with tunable thermal and mechanical properties. ACS Omega 2019, 4, 19664–19675. [Google Scholar] [CrossRef] [Green Version]
- Wolf-Brandstetter, C.; Roessler, S.; Storch, S.; Hempel, U.; Gbureck, U.; Nies, B.; Bierbaum, S.; Scharnweber, D. Physicochemical and cell biological characterization of PMMA bone cements modified with additives to increase bioactivity. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 599–609. [Google Scholar] [CrossRef]
- Kusy, R.P.; Turner, D.T. Influence of the molecular weight of poly(methyl methacrylate) on fracture surface energy in notched tension. Polymer 1976, 17, 161–166. [Google Scholar] [CrossRef]
- Huggett, R.; Bates, J.F.; Packham, D.E. The effect of the curing cycle upon the molecular weight and properties of denture base materials. Dent. Mater. 1987, 3, 107–112. [Google Scholar] [CrossRef]
- Nejatian, T.; Pezeshki, S.; Yaqin Syed, A.U. Acrylic denture base materials. In Advanced Dental Biomaterials; Khurshid, Z., Najeeb, S., Zafar, M.S., Sefat, F., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 79–104. [Google Scholar]
- Habib, S.R.; Vohra, F.A. Replacing existing dentures by copy-denture technique for geriatric patients: A case report. JPDA 2013, 22, 265–270. [Google Scholar]
- Zafar, M.S. Prosthodontic applications of polymethyl methacrylate (PMMA): An update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.A. PMMA-eggshell composite preparation and studying mechanical properties as a dental material. J. Eng. Sustain. Dev. 2020, 24, 30–47. [Google Scholar] [CrossRef]
- Raszewski, Z.; Nowakowska-Toporowska, A.; Wezgowiec, J.; Nowakowska, D.; Wieckiewicz, W. Influence of silanized silica and silanized feldspar addition on the mechanical behavior of polymethyl methacrylate resin denture teeth. J. Prosthet. Dent. 2020, 123, 647–653. [Google Scholar] [CrossRef]
- Gad, M.M.; Rahoma, A.; Al-Thobity, A.M.; ArRejaie, A.S. Influence of incorporation of ZrO2 nanoparticles on the repair strength of polymethyl methacrylate denture bases. Int. J. Nanomed. 2016, 11, 5633–5643. [Google Scholar] [CrossRef] [Green Version]
- Shifman, A. Clinical applications of visible light-cured resin in maxillofacial prosthetics. part I: Denture base and reline material. J. Prosthet. Dent. 1990, 64, 578–582. [Google Scholar] [CrossRef]
- Fellman, S. Visible light-cured denture resin used in making dentures with conventional teeth. J. Prosthet. Dent. 1989, 62, 356–359. [Google Scholar] [CrossRef]
- Polyzois, G.L.; Handley, R.W.; Stafford, G.D. Repair strength of denture base resins using various methods. Eur. J. Prosthodont. Restor. Dent. 1995, 3, 183–186. [Google Scholar]
- Khodair, Z.T.; Saeed, M.H.; Abdul-Allah, M.H. Study of optical properties of (PMMA) doped by methyl red and methyl blue films. Iraqi J. Phys. 2014, 12, 47–51. [Google Scholar]
- Al-Ammar, K.; Hashim, A.; Husaien, M. Synthesis and study of optical properties of (PMMA-CrCl2) composites. Chem. Mater. Eng. 2013, 1, 85–87. [Google Scholar] [CrossRef]
- Gupta, A.K.; Bafna, M.; Vijay, Y.K. Study of optical properties of potassium permanganate (KMnO4) doped poly(methylmethacrylate) (PMMA) composite films. Bull. Mater. Sci. 2018, 41, 160. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Chen, Z.; Zheng, Y.; Newman, C.; Quinn, J.R.; Dötz, F.; Kastler, M.; Facchetti, A. A high-mobility electron-transporting polymer for printed transistors. Nature 2009, 457, 679–686. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Y.; Zhu, D. π-Conjugated molecules with fused rings for organic field-effect transistors: Design, synthesis and applications. Chem. Soc. Rev. 2010, 39, 1489–1502. [Google Scholar] [CrossRef] [Green Version]
- Mao, Z.; Guo, Y.; Chen, H.; Zhang, W.; Yu, G. Tailoring molecular weight of polymeric dielectric to enhance electron and hole mobilities in polymer field-effect transistors. Polymer 2016, 99, 496–502. [Google Scholar] [CrossRef]
- Ren, H.; Cui, N.; Tang, Q.; Tong, Y.; Liu, Y. High-performance, ultrathin, ultraflexible organic thin-film transistor array via solution process. Small 2018, 14, 1801020. [Google Scholar] [CrossRef]
- Panigrahi, D.; Dhar, A. Tuning dielectric-semiconductor interfacial properties in organic phototransistors for ultralow light detection. Org. Electron. 2019, 70, 107–112. [Google Scholar] [CrossRef]
- Bibi, S.; Mohamad, S.; Manan, N.S.A.; Ahmad, J.; Kamboh, M.A.; Khor, S.M.; Yamin, B.M.; Abdul Halim, S.N. Synthesis, characterization, photoluminescence, and electrochemical studies of novel mononuclear Cu (II) and Zn (II) complexes with the 1-benzylimidazolium ligand. J. Mol. Struct. 2017, 1141, 31–38. [Google Scholar] [CrossRef]
- Kara, H.; Oylumluoglu, G.; Coban, M.B. Photoluminescence properties of a new Sm(III) complex/PMMA electrospun composite fibers. J. Clust. Sci. 2020, 31, 701–708. [Google Scholar] [CrossRef]
- Rajeh, A.; Ragab, H.M.; Abutalib, M.M. Co doped ZnO reinforced PEMA/PMMA composite: Structural, thermal, dielectric and electrical properties for electrochemical applications. J. Mol. Struct. 2020, 1217, 128447. [Google Scholar] [CrossRef]
- Bakan, H.I.; Jumadi, Y.; Messer, P.F.; Davies, H.A.; Ellis, B. Study of processing parameters for MIM feedstock based on composite PEG-PMMA binder. Powder Metall. 1998, 41, 289–291. [Google Scholar] [CrossRef]
- Fu, Y.J.; Hu, C.C.; Lee, K.R.; Tsai, H.A.; Ruaan, R.C.; Lai, J.Y. The correlation between free volume and gas separation properties in high molecular weight poly(methyl methacrylate) membranes. Eur. Polym. J. 2007, 43, 959–967. [Google Scholar] [CrossRef]
- Ghosal, K.; Chern, R.T.; Freeman, B.D. Effect of basic substituents on gas sorption and permeation in polysulfone. Macromolecules 1996, 29, 4360–4369. [Google Scholar] [CrossRef]
- Wright, C.T.; Paul, D.R. Gas sorption and transport in poly(tertiary-butyl methacrylate). Polymer 1997, 38, 1871–1878. [Google Scholar] [CrossRef]


| Entry | Catalyst | Ligand/Additive | Initiator | Temp. (°C) | Time (h) | Conv. (%) | Mn (g/mol) | Mw/Mn | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | / | cyanoisopropyl dithiobenzoate | AIBN | 65 | 7.0 | 99.0 | 1.25 × 106 | 1.03 | [6] |
| 2 | / | Supercritical CO2 | AIBN | 65 | 10.0 | 100.0 | 1.31 × 105 | 2.54 | [7] |
| 3 | / | PVA | ADMVN | 25 | 96.0 | 83.0 | 3.61 × 106 | 2.40 | [16] |
| 4 | / | Poly(FOA) | AIBN | N/A | 4.0 | 92.0 | 3.16 × 105 | 2.09 | [17] |
| 5 | / | Poly(FOA) | AIBN | 65 | 4.0 | 88.0 | 3.65 × 105 | 2.48 | [18] |
| 6 | / | P(MMA-co-HEMA)-g-PFPO | AIBN | 65 | 10.0 | 90.0 | 3.55 × 105 | 1.70 | [19] |
| 7 | / | SDS | APS | 70.0 | N/A | N/A | 5.30 × 105 | 1.43 | [34] |
| 8 | / | SDS | AIBN | 70 | N/A | 50.0 | 1.00 × 106 | 1.80 | [35] |
| 9 | / | TEA | Fe/ZnO | N/A | N/A | 36.0 | 2.10 × 105 | 2.85 | [37] |
| 10 | / | poly(1-vinyl-3-butylimidazolium ascorbate) | ZnO/Ag | 30 | 9.0 | 82.0 | 1.94 × 105 | 1.38 | [42] |
| 11 | CuCl | dnNbpy | BMPE | 90 | 2.0 | N/A | 3.67 × 105 | 1.20 | [43] |
| 12 | CuBr | Copper powder | CDB | 80 | 125.0 | 43.9 | 1.25 × 106 | 1.21 | [50] |
| 13 | CuBr2 | Tris(2-dimethylaminoethyl)amine | SiO2-Br | 60 | 24.0 | 40.5 | 1.70 × 106 | 1.31 | [51] |
| 14 | CuBr2 | PMDETA | TBIB | 90 | 4.5 | 53.0 | 5.70 × 105 | 1.36 | [52] |
| 15 | CuCl | dnNbpy | EBiB | 60 | 24.0 | N/A | 3.60 × 106 | 1.24 | [53] |
| 16 | CuBr2 | TPMA | EBiB | 20 | 15.0 | 57.0 | 1.88 × 106 | 1.25 | [55] |
| 17 | Cu(OAc)2 | MAO | / | 30 | 2.0 | 31.0 | 3.39 × 105 | N/A | [63] |
| 18 | CoCl2 | N,N-bis{(1-pyrazolyl)methyl}aniline | / | 60 | N/A | N/A | 1.13 × 106 | 1.75 | [64] |
| 19 | Ni(II) complexes | MAO | / | 50 | 3.5 | 20.0 | 1.50 × 106 | N/A | [69] |
| 20 | / | Me3SiOLi | Li-iPrIB | −78 | 1.0 | 100.0 | 8.25 × 105 | 1.16 | [70] |
| 21 | / | / | / | 25 | 16 | 5.0 | 1.41 × 106 | 2.03 | [73] |
| 22 | Palladium nanoparticles | / | EBiB | 70 | 24 | 82.8 | 4.65 × 106 | 1.73 | [74] |
| Entry | Mw (Da) | n-Channel μe,max (cm2 V−1 S−1) | p-Channel μh,max (cm2 V−1 S−1) | n-Channel Ntrap (×1011 cm−2) | p-Channel Ntrap (×1011 cm−2) |
|---|---|---|---|---|---|
| 1 | 1.2 × 105 | 0.30 | 0.01 | 5.46 | 3.64 |
| 2 | 5.5 × 105 | 0.55 | 0.18 | 4.67 | 2.33 |
| 3 | 1.0 × 106 | 0.85 | 0.35 | 1.38 | 1.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, M.; Huang, D.; Zhao, Y. Development of Synthesis and Application of High Molecular Weight Poly(Methyl Methacrylate). Polymers 2022, 14, 2632. https://doi.org/10.3390/polym14132632
Yuan M, Huang D, Zhao Y. Development of Synthesis and Application of High Molecular Weight Poly(Methyl Methacrylate). Polymers. 2022; 14(13):2632. https://doi.org/10.3390/polym14132632
Chicago/Turabian StyleYuan, Ming, Dayun Huang, and Yixuan Zhao. 2022. "Development of Synthesis and Application of High Molecular Weight Poly(Methyl Methacrylate)" Polymers 14, no. 13: 2632. https://doi.org/10.3390/polym14132632