Conductive PPy@cellulosic Paper Hybrid Electrodes with a Redox Active Dopant for High Capacitance and Cycling Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
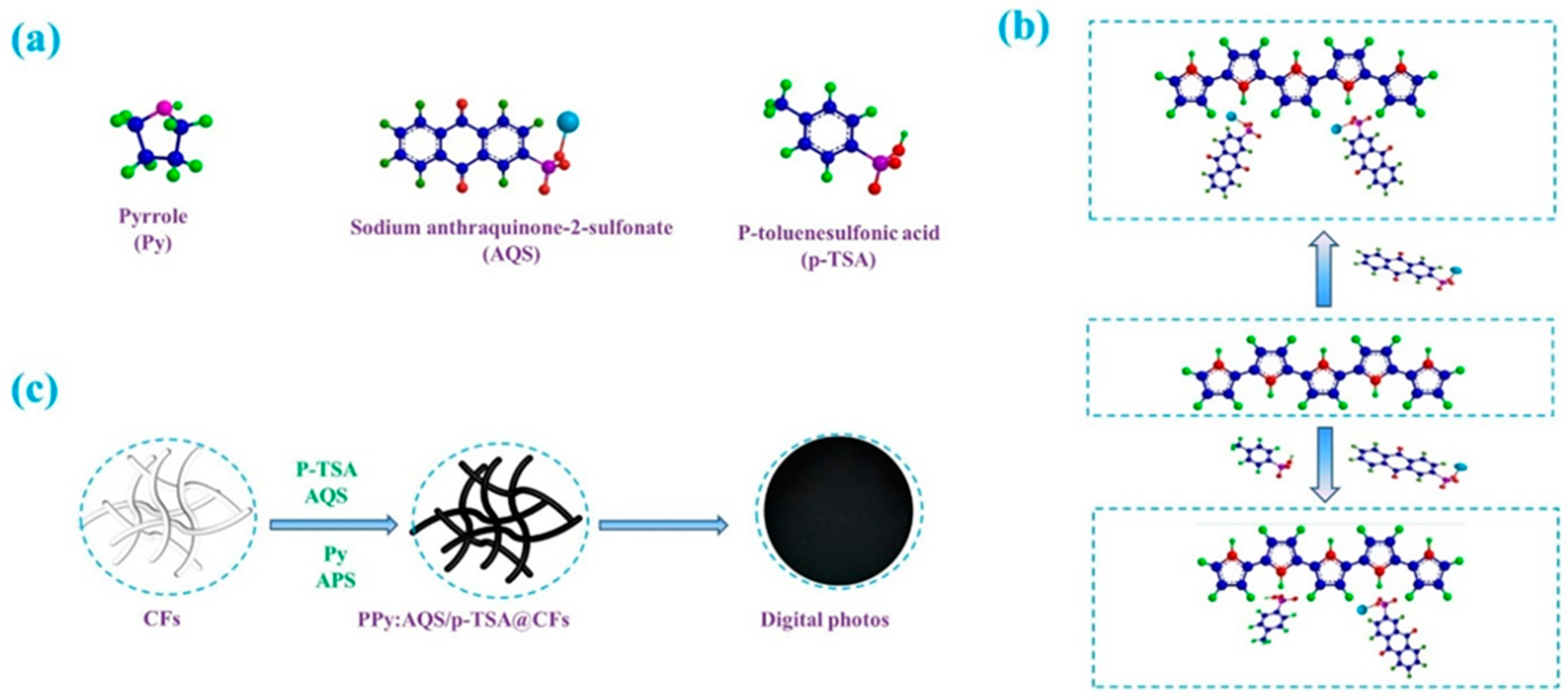
2.2. Preparation of PPy:AQS/p-TSA@CFs Composites
2.3. Characterizations
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Fabrication Process
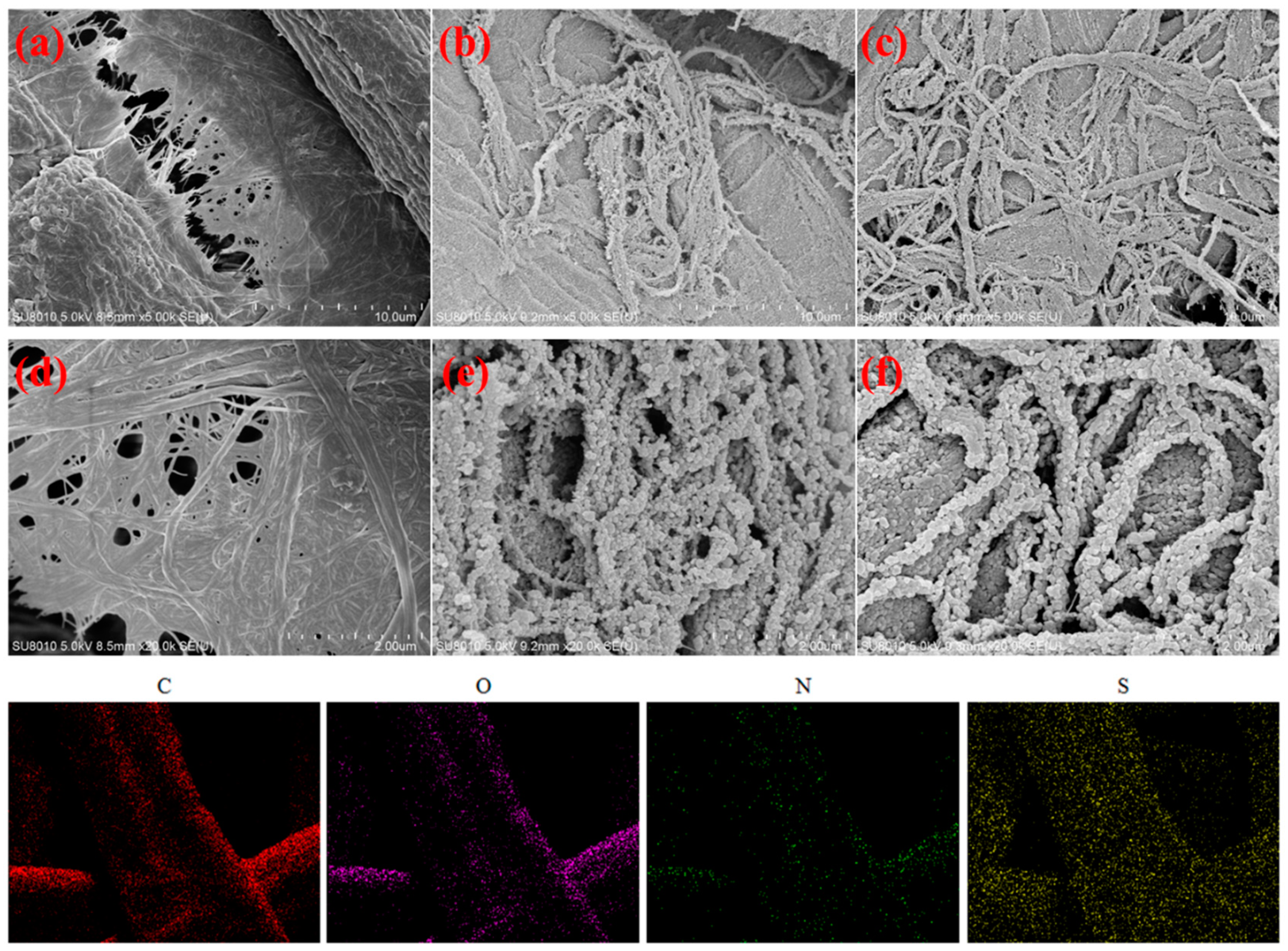
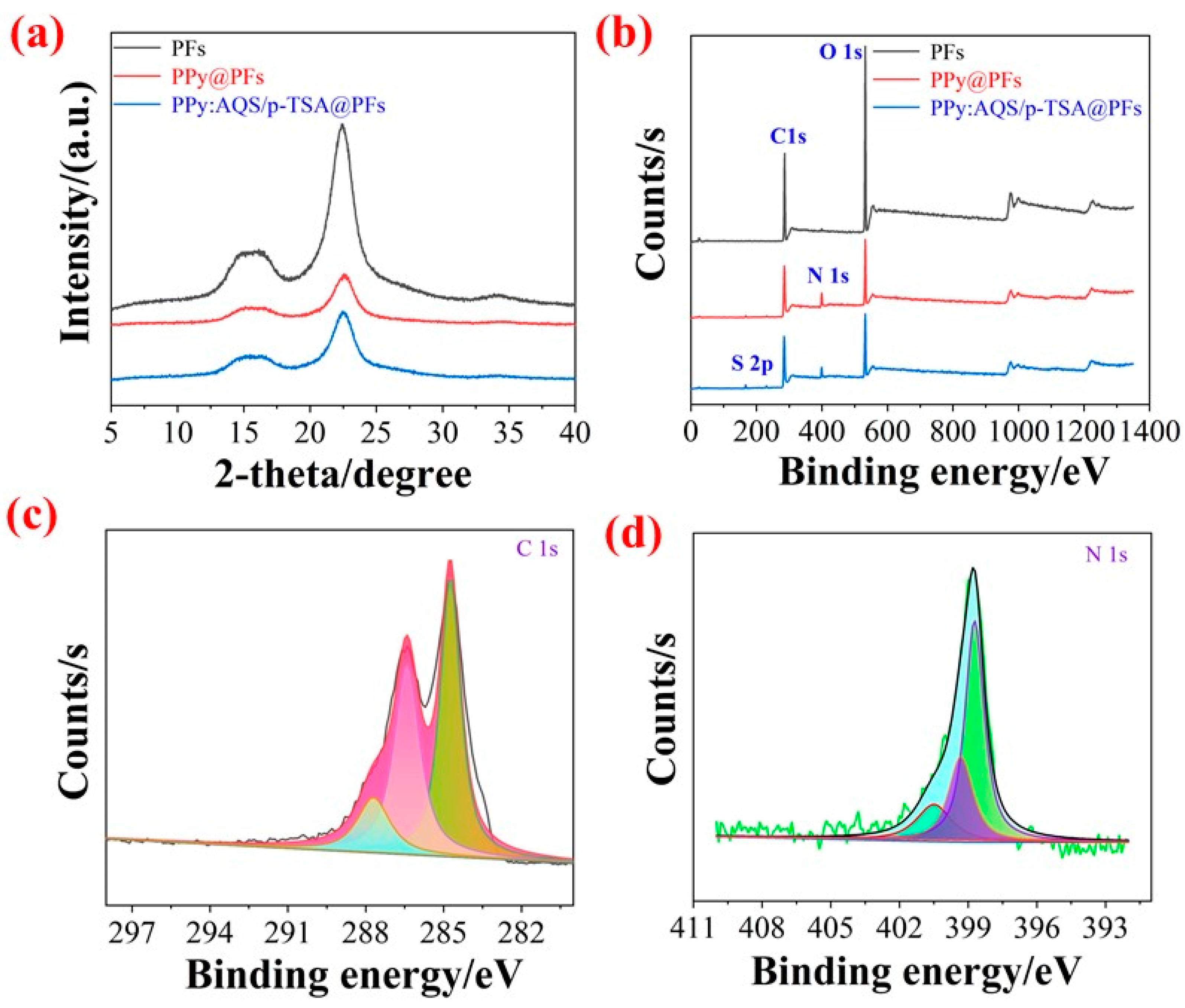
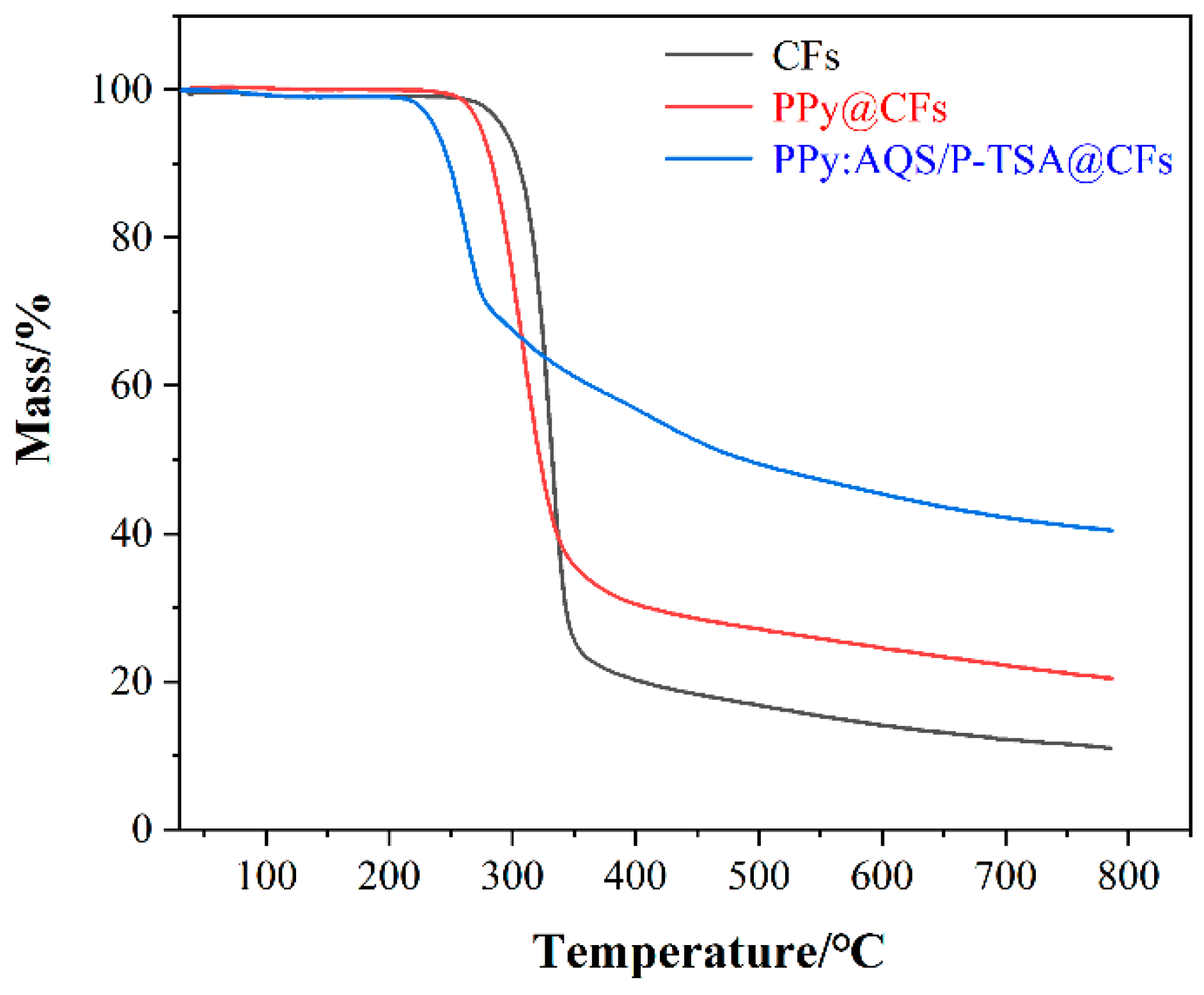
3.2. Characterization Analysis
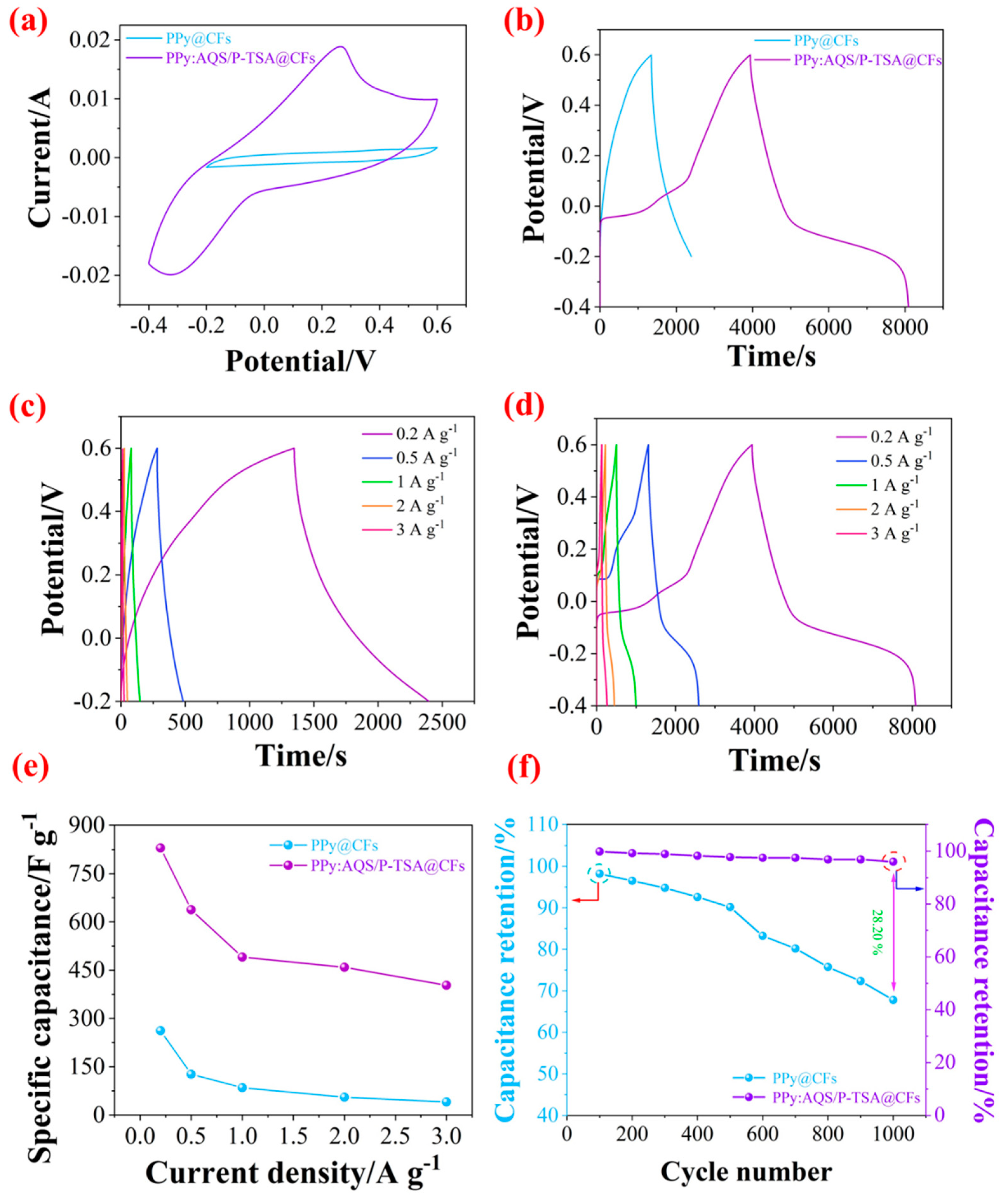
3.3. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lv, B.C.; Liu, Y.; Wu, W.D.; Xie, Y.; Zhu, J.L.; Cao, Y.; Ma, W.Y.; Yang, N.; Chu, W.D.; Jia, Y.; et al. Local large temperature difference and ultra-wideband photothermoelectric response of the silver nanostructure film/carbon nanotube film heterostructure. Nat. Commun. 2020, 13, 1835. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.Q.; Jiang, Z.Q.; Zhang, B.A.; Tian, X.N.; Song, C.S.; Wang, L.K.; Maiyalagan, T.; Hao, X.G.; Jiang, Z.J. N-doped carbon nanotubes derived from graphene oxide with embedment of FeCo nanoparticles as bifunctional air electrode for rechargeable liquid and flexible all-solid-state zinc-air batteries. Adv. Sci. 2021, 8, 2004572. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, Y.; Fu, Z.Y.; Su, B.L. Hierarchically structured porous materials: Synthesis strategies and applications in energy storage. Natl. Sci. Rev. 2020, 7, 1667–1701. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Guo, X.T.; Yan, Y.; Xue, H.G.; Pang, H. FeOx-based materials for electrochemical energy storage. Adv. Sci. 2018, 5, 1700986. [Google Scholar] [CrossRef]
- Xiong, G.P.; He, P.G.; Lyu, Z.P.; Chen, T.F.; Huang, B.Y.; Chen, L.; Fisher, T.S. Bioinspired leaves-on-branchlet hybrid carbon nanostructure for supercapacitors. Nat. Commun. 2018, 9, 790. [Google Scholar] [CrossRef] [Green Version]
- Xiao, K.F.; Yang, T.M.; Liang, J.X.; Rawal, A.; Liu, H.B.; Fang, R.P.; Amal, R.; Xu, H.Y.; Wang, D.W. Nanofluidic voidless electrode for electrochemical capacitance enhancement in gel electrolyte. Nat. Commun. 2021, 12, 5515. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Sundaram, M.M.; Henry, D.J.; Gao, X.P. Alginate Biopolymer Effect on the Electrodeposition of Manganese Dioxide on Electrodes for Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 7040–7051. [Google Scholar] [CrossRef]
- Šedajová, V.; Bakandritsos, A.; Błoński, P.; Medveď, M.; Langer, R.; Zaoralová, D.; Ugolotti, J.; Dzíbelová, J.; Jakubec, P.; Kupka, V.; et al. Nitrogen doped graphene with diamond-like bonds achieves unprecedented energy density at high power in a symmetric sustainable supercapacitor. Energy Environ. Sci. 2022, 15, 740–748. [Google Scholar] [CrossRef]
- Minakshi, M. Lithium intercalation into amorphous FePO4 cathode in aqueous solutions. Electrochim. Acta 2010, 55, 174–9178. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, J.M.; Carrillo, A.; Mota, M.L.; Ambrosio, R.C.; Aguirre, F.S. Purification and glutaraldehyde activation study on HCl-doped PVA-PANI copolymers with different aniline concentrations. Molecules 2018, 24, 63. [Google Scholar] [CrossRef] [Green Version]
- Kulandaivalu, S.; Suhaimi, N.; Sulaiman, Y. Unveiling high specific energy supercapacitor from layer-by-layer assembled polypyrrole/graphene oxide|polypyrrole/manganese oxide electrode material. Sci. Rep. 2019, 9, 4884. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Chen, S.H.; Tian, D.; Li, Y.X.; Wang, C.; Lu, X.F. Cu2+-doped polypyrrole nanotubes with promoted efficiency for peroxidase mimicking and electrochemical biosensing. Mater. Today Chem. 2020, 18, 100374. [Google Scholar] [CrossRef]
- Wang, Y.X.; Huang, L.H.; Wang, Z.X.; Wang, L.S.; Ma, T. Application of polypyrrole flexible electrode for electrokinetic remediation of Cr(VI)-contaminated soil in a main-auxiliary electrode system. Chem. Eng. J. 2019, 373, 131–139. [Google Scholar] [CrossRef]
- Cao, Y.J.; Wu, N.; Yang, F.; Yang, M.; Zhang, T.T.; Guo, H.; Yang, W. Interpenetrating network structures assembled by “string of candied haws”-like PPY nanotube-interweaved NiCo-MOF-74 polyhedrons for high-performance supercapacitors. Colloids Surf. A 2022, 646, 128954. [Google Scholar] [CrossRef]
- Yin, Y.; Prabhakar, M.; Ebbinghaus, P.; Silva, C.C.; Rohwerder, M. Neutral inhibitor molecules entrapped into polypyrrole network for corrosion protection. Chem. Eng. J. 2022, 440, 135739. [Google Scholar] [CrossRef]
- Ma, M.L.; Bi, Y.X.; Jiao, Z.G.; Yue, J.W.; Liao, Z.J.; Wang, Y.; Ma, Y.; Huang, W.B. Facile fabrication of metal–organic framework derived Fe/Fe3O4/FeN/N-doped carbon composites coated with PPy for superior microwave absorption. J. Colloid Interface Sci. 2022, 608, 525–535. [Google Scholar] [CrossRef]
- Alsaiari, N.S.; Katubi, K.M.; Alzahrani, F.M.; Amari, A.; Osman, H.; Rebah, F.B.; Tahoon, M.A. Synthesis, Characterization and Application of Polypyrrole Functionalized Nanocellulose for the Removal of Cr(VI) from Aqueous Solution. Polymers 2021, 13, 3691. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F.; Dong, L.; Li, Z.; Chen, L.; He, X.; Gong, J.; Zhang, J.; Li, Q. Design and Optimization of Flexible Polypyrrole/Bacterial Cellulose Conductive Nanocomposites Using Response Surface Methodology. Polymers 2019, 11, 960. [Google Scholar] [CrossRef] [Green Version]
- Jyothibasu, J.P.; Lee, R.-H. Facile, Scalable, Eco-Friendly Fabrication of High-Performance Flexible All-Solid-State Supercapacitors. Polymers 2018, 10, 1247. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.J.; Tian, J.X.; Rao, W.D.; Guo, B.; Fan, L.L.; Xu, W.L.; Xu, J. Polypyrrole@metal-organic framework (UIO-66)@cotton fabric electrodes for flexible supercapacitors. Cellulose 2019, 26, 3387–3399. [Google Scholar] [CrossRef]
- Liu, Q.Z.; Wang, B.; Chen, J.H.; Li, F.; Liu, K.; Wang, Y.D.; Li, M.F.; Lu, Z.T.; Wang, W.W.; Wang, D. Facile synthesis of three-dimensional (3D) interconnecting polypyrrole (PPy) nanowires/nanofibrous textile composite electrode for high performance supercapacitors. Compos. Part A 2017, 101, 30–40. [Google Scholar] [CrossRef]
- Chang, Z.Y.; Li, S.Y.; Sun, L.J.; Ding, C.Y.; An, X.H.; Qian, X.R. Paper-based electrode comprising zirconium phenylphosphonate modified cellulose fibers and porous polyaniline. Cellulose 2019, 26, 6739–6754. [Google Scholar] [CrossRef]
- Jyothibasu, J.P.; Kuo, D.W.; Lee, R.H. Flexible and freestanding electrodes based on polypyrrole/carbon nanotube/cellulose composites for supercapacitor application. Cellulose 2019, 26, 4495–4513. [Google Scholar] [CrossRef]
- Jyothibasu, J.P.; Wang, R.H.; Ong, K.; Ong, J.H.L.; Lee, R.H. Cellulose/carbon nanotube/MnO2 composite electrodes with high mass loadings for symmetric supercapacitors. Cellulose 2021, 28, 3549–3567. [Google Scholar] [CrossRef]
- Zhang, M.M.; Nautiyal, A.; Du, H.S.; Li, J.H.; Liu, Z.Q.; Zhang, X.Y.; Wang, R.G. Polypyrrole film based flexible supercapacitor: Mechanistic insight into influence of acid dopants on electrochemical performance. Electrochim. Acta 2020, 357, 136877. [Google Scholar] [CrossRef]
- Mao, H.; Dong, Y.L.; Qian, X.R.; An, X.H. Enhancement of bonding strength of polypyrrole/cellulose fiber (PPy/CF) hybrid through lignosulfonate doping. Cellulose 2017, 24, 2255–2263. [Google Scholar] [CrossRef]
- Sharma, P.; Sundaram, M.M.; Watcharatharapong, T.; Jungthawan, S.; Ahuja, R. Tuning the nanoparticle interfacial properties and stability of the core-shell structure in Zn-doped NiMoO4@AWO4. ACS Appl. Mater. Interfaces 2021, 13, 56116–56130. [Google Scholar] [CrossRef]
- Gopakumar, D.A.; Pai, A.R.; Pottathara, Y.B.; Pasquini, D.; Carlos de Morais, L.; Luke, M.; Kalarikkal, N.; Grohens, Y.; Thomas, S. Cellulose nanofiber-based polyaniline flexible papers as sustainable microwave absorbers in the X-band. ACS Appl. Mater. Interfaces. 2018, 10, 20032–20043. [Google Scholar] [CrossRef]
- Wang, H.; Biswas, S.K.; Zhu, S.; Lu, Y.; Yue, Y.; Han, J.; Xu, X.; Wu, Q.; Xiao, H. Self-healable electro-conductive hydrogels based on core-shell structured nanocellulose/carbon nanotubes hybrids for use as flexible supercapacitors. Nanomaterials 2020, 10, 112. [Google Scholar] [CrossRef] [Green Version]
- Raghunathan, S.P.; Narayanan, S.; Poulose, A.C.; Joseph, R. Flexible regenerated cellulose/polypyrrole composite films withenhanced dielectric properties. Carbohydr. Polym. 2017, 157, 1024–1032. [Google Scholar] [CrossRef]
- Babu, K.F.; Subramanian, S.P.S.; Kulandainathan, M.A. Functionalisation of fabrics with conducting polymer for tuning capacitance and fabrication of supercapacitor. Carbohydr. Polym. 2013, 94, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.W.; Wu, B.; Li, D.G. Core-shell structured cellulose nanofibers/graphene@polypyrrole microfibers for all-solid-state wearable supercapacitors with enhanced electrochemical performance. Macromol. Mater. Eng. 2020, 305, 1900854. [Google Scholar] [CrossRef]
- Chen, G.Y.; Chen, T.; Hou, K.; Ma, W.J.; Tebyetekerwa, M.; Cheng, Y.H.; Weng, W.; Zhu, M.F. Robust, hydrophilic graphene/cellulose nanocrystal fiber-based electrode with high capacitive performance and conductivity. Carbon 2018, 127, 218–227. [Google Scholar] [CrossRef]
- Hanif, Z.; Lee, S.; Qasim, G.H.; Ardiningsih, I.; Kim, J.A.; Seon, J.; Han, S.; Hong, S.; Yoon, M.H. Polypyrrole multilayer-laminated cellulose for large-scale repeatable mercury ion removal. J. Mater. Chem. A. 2018, 6, 19266. [Google Scholar] [CrossRef] [Green Version]
- Ruangchuay, L.; Schwank, J.; Sirivat, A. Surface degradation of a-naphthalene sulfonate-doped polypyrrole during XPS characterization. Appl. Surf. Sci. 2002, 199, 128–137. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Shang, Z.; Shen, M.X.; Chowdhury, S.P.; Ignaszak, A.; Sun, S.H.; Ni, Y.H. Cellulose nanofibers/reduced graphene oxide/polypyrrole aerogel electrodes for high-capacitance flexible all-solid-state supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 11175–11185. [Google Scholar] [CrossRef]

| Samples | AQS mmol | p-TSA mmol | Py mL | n(APS)/n(Py) | Conductivity S m−1 |
|---|---|---|---|---|---|
| PPy:AQS@CFs | 2 | 0 | 0.25 | 1 | 20.12 |
| PPy:AQS/p-TSA-1@CFs | 2 | 1 | 0.25 | 1 | 25.13 |
| PPy:AQS/p-TSA-2@CFs | 2 | 2 | 0.25 | 1 | 34.06 |
| PPy:AQS/p-TSA-3@CFs | 2 | 3 | 0.25 | 1 | 31.01 |
| PPy:AQS/p-TSA-4@CFs | 2 | 4 | 0.25 | 1 | 23.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Qian, X. Conductive PPy@cellulosic Paper Hybrid Electrodes with a Redox Active Dopant for High Capacitance and Cycling Stability. Polymers 2022, 14, 2634. https://doi.org/10.3390/polym14132634
Yang S, Qian X. Conductive PPy@cellulosic Paper Hybrid Electrodes with a Redox Active Dopant for High Capacitance and Cycling Stability. Polymers. 2022; 14(13):2634. https://doi.org/10.3390/polym14132634
Chicago/Turabian StyleYang, Shuaishuai, and Xueren Qian. 2022. "Conductive PPy@cellulosic Paper Hybrid Electrodes with a Redox Active Dopant for High Capacitance and Cycling Stability" Polymers 14, no. 13: 2634. https://doi.org/10.3390/polym14132634
APA StyleYang, S., & Qian, X. (2022). Conductive PPy@cellulosic Paper Hybrid Electrodes with a Redox Active Dopant for High Capacitance and Cycling Stability. Polymers, 14(13), 2634. https://doi.org/10.3390/polym14132634





