Cytostatic Effects of Polyethyleneimine Surfaces on the Mesenchymal Stromal Cell Cycle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polyelectrolyte Multilayers
2.2. Cell Culture
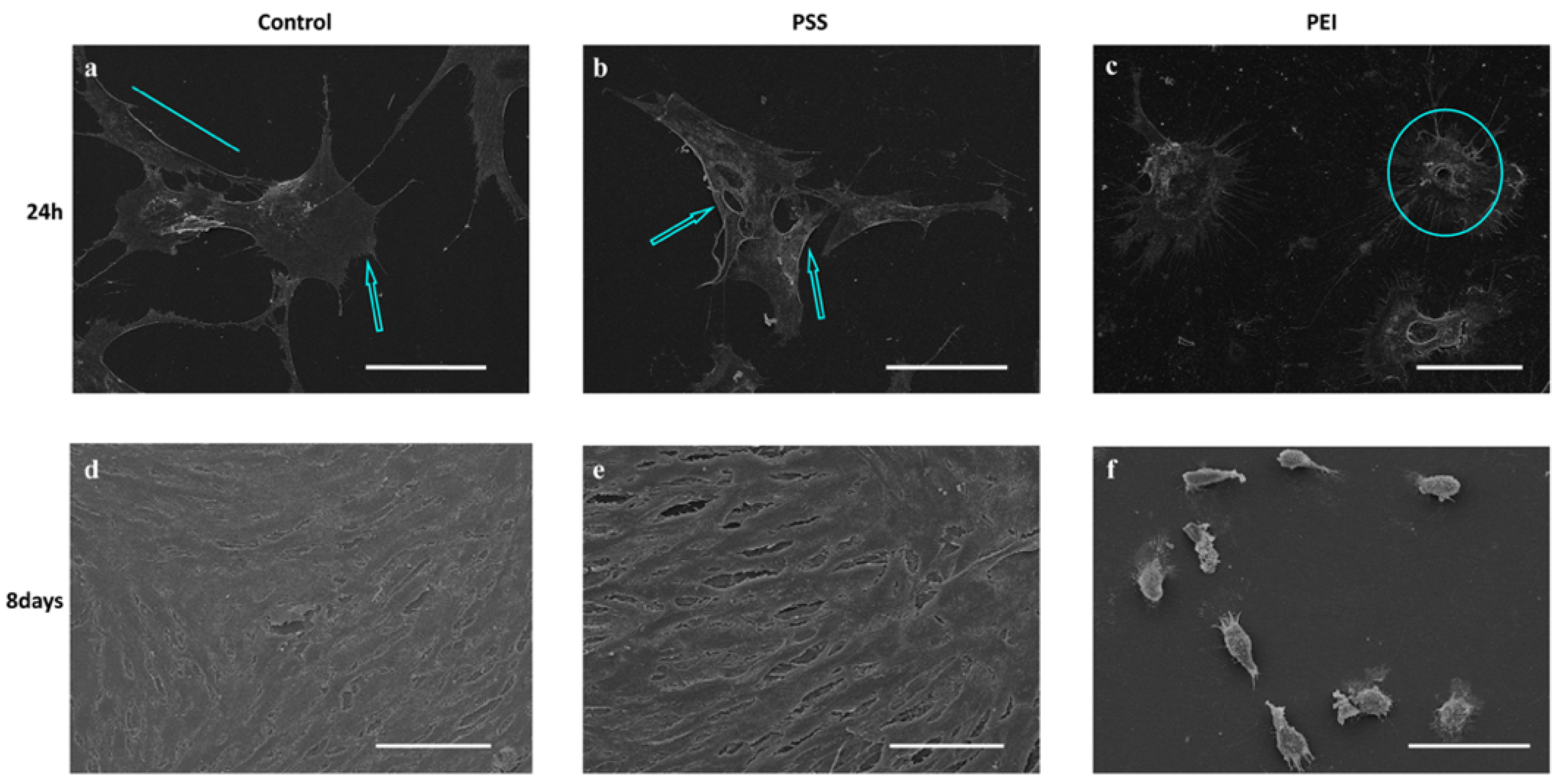
2.3. Scanning Electron Microscopy
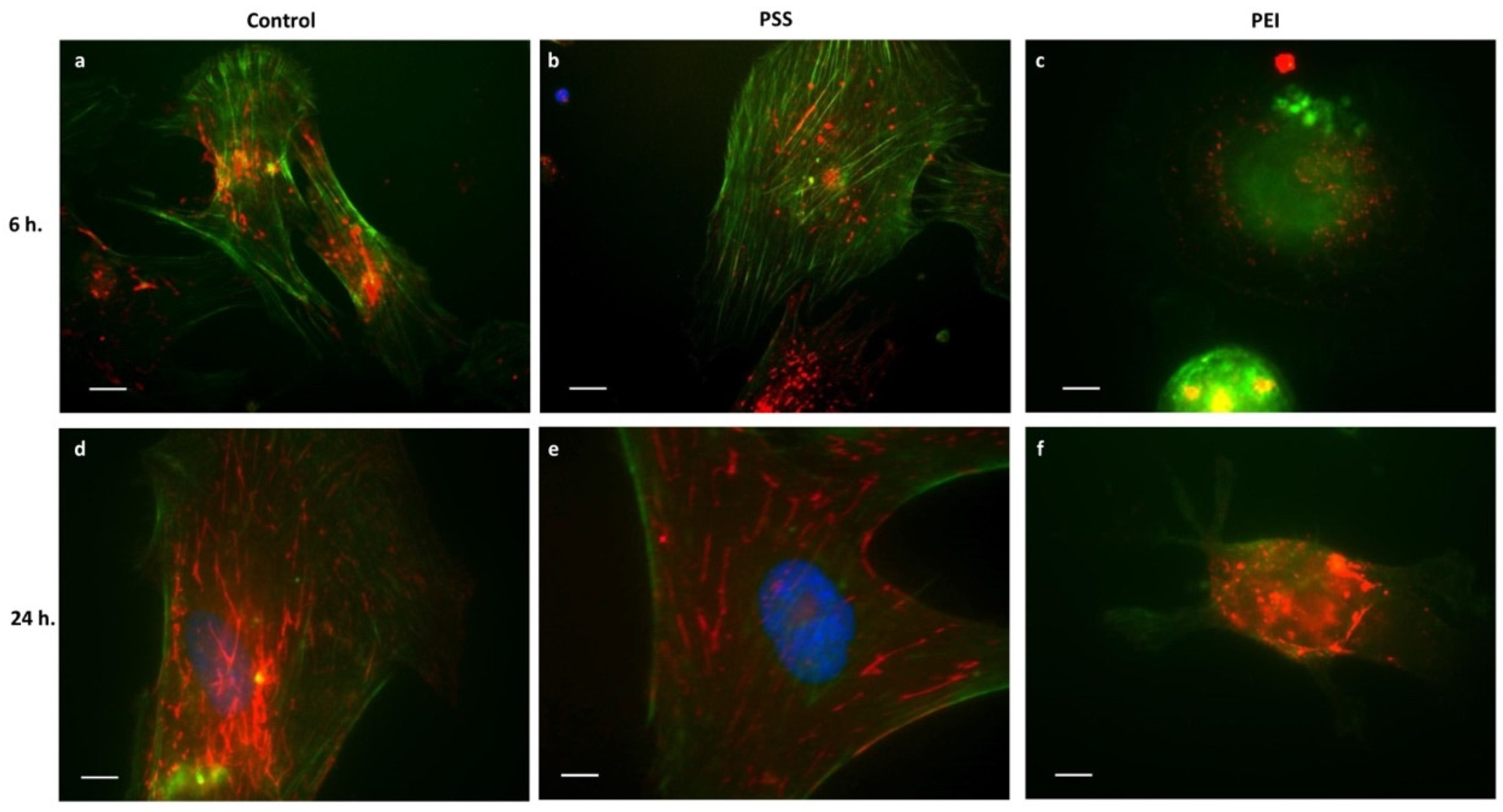
2.4. Cytoskeleton and Adhesion Molecules
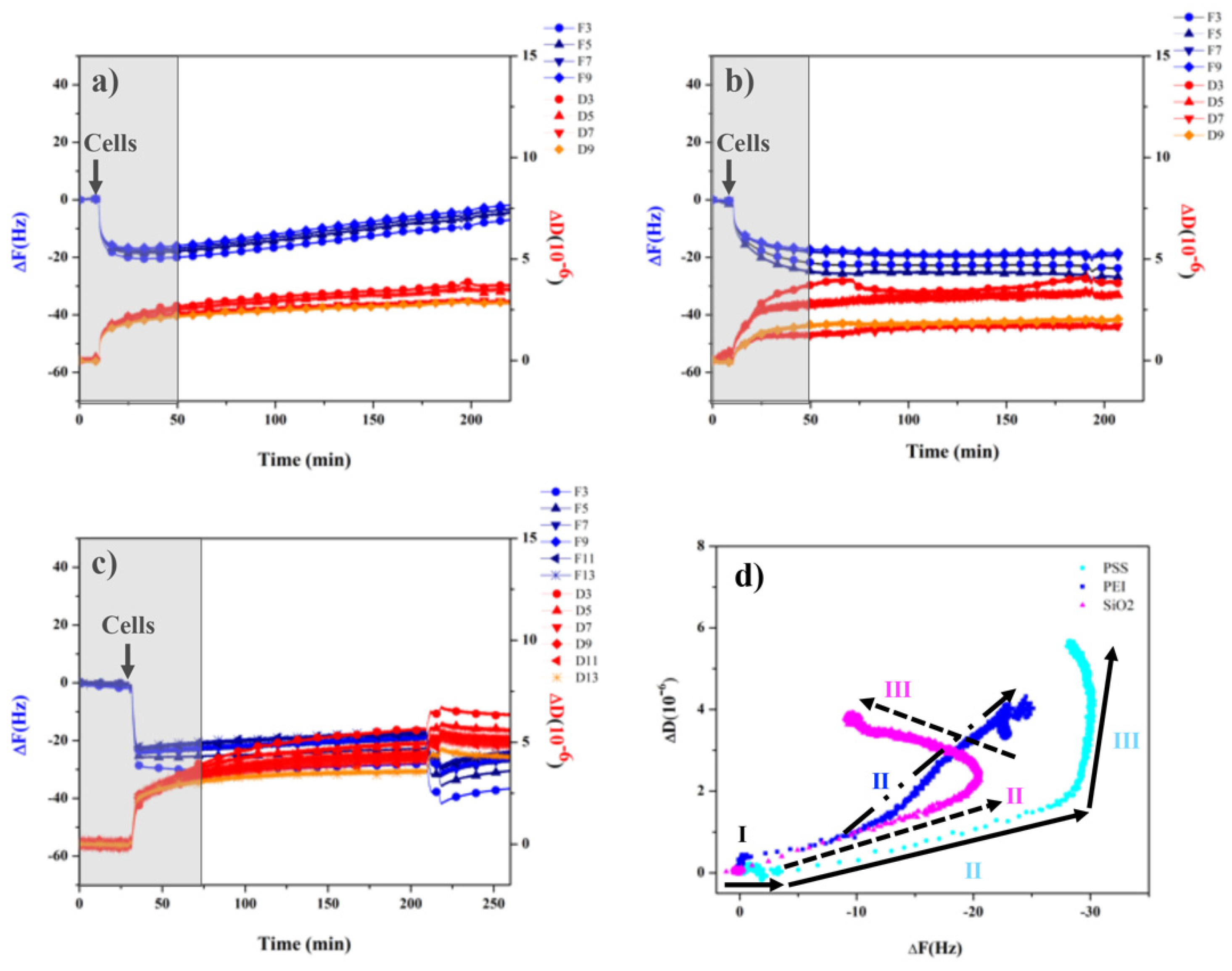
2.5. Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D)
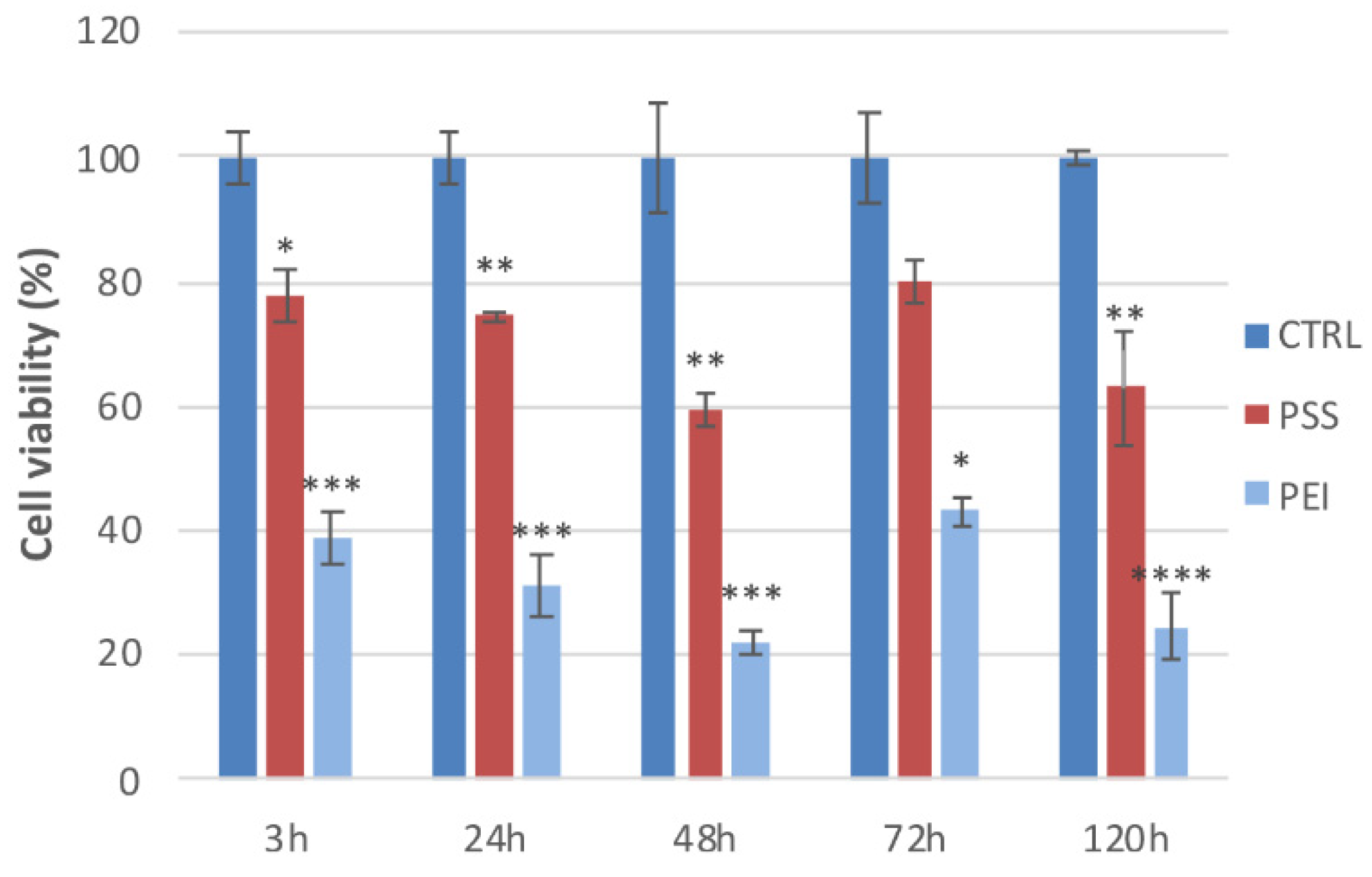
2.6. Cell Viability and Cell Cycle Assessment
2.7. ATP Evaluation and Mitochondrial Transmembrane Potential (ΔΨ)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Morphological Analysis
3.2. Cytoskeleton and Adhesion Molecules
3.2.1. Microfilaments and Microtubules
3.2.2. Integrins
3.2.3. Focal Adhesion Kinase and Paxillin
3.3. Monitoring Cell Behavior with QCD-D Experiments
3.4. Cell Viability and Cell Cycle
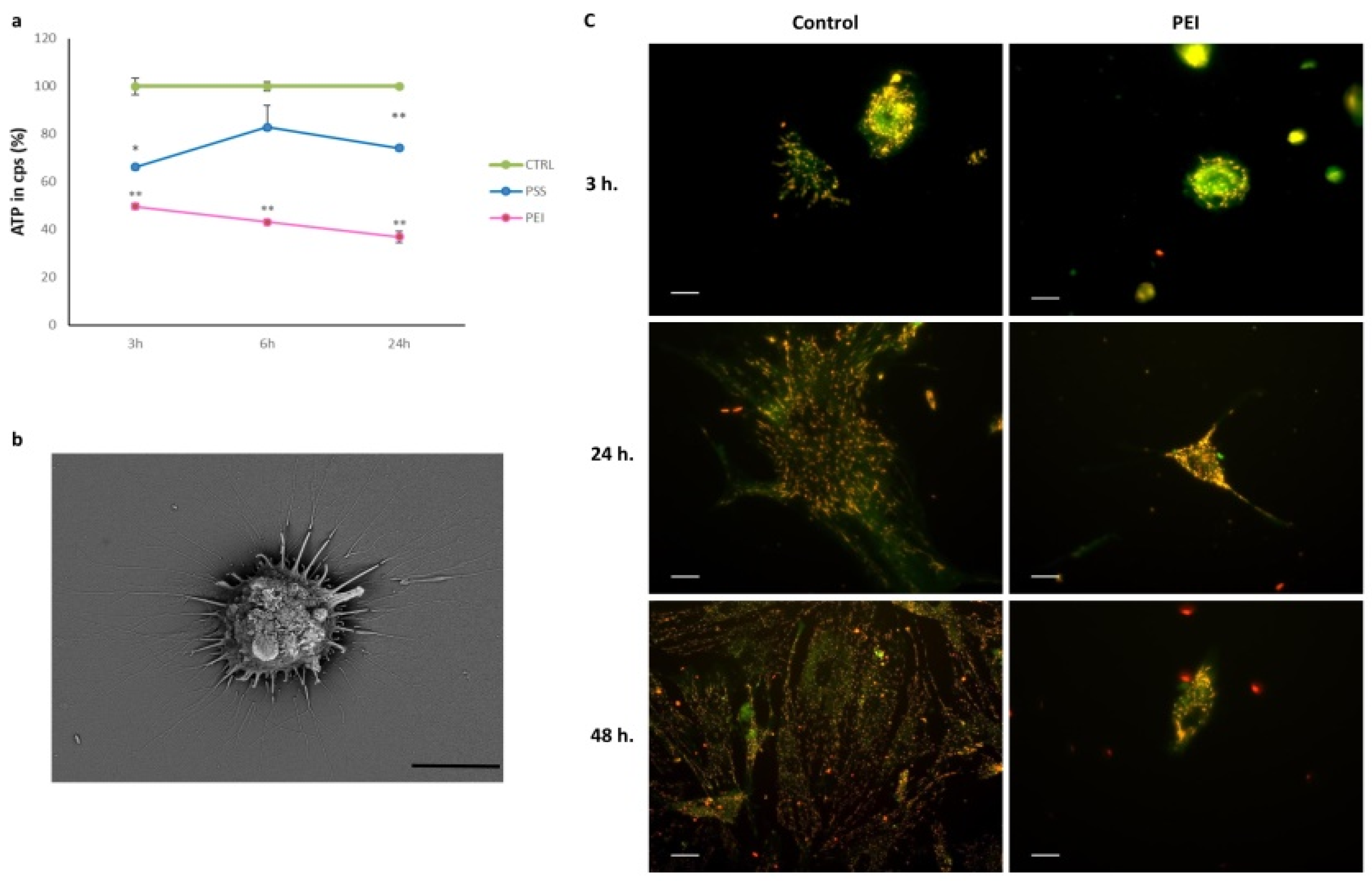
3.5. ATP Evaluation and Mitochondrial Transmembrane Potential (ΔΨ)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, J.M.; Reis, R.L.; Mano, J.F. Biomimetic Extracellular Environment Based on Natural Origin Polyelectrolyte Multilayers. Small 2016, 12, 4308–4342. [Google Scholar] [CrossRef] [PubMed]
- Gribova, V.; Auzely-Velty, R.; Picart, C. Polyelectrolyte Multilayer Assemblies on Materials Surfaces: From Cell Adhesion to Tissue Engineering, Chem. Mater. 2012, 24, 854−869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, I.; Walz, J.A.; Mace, C.R.; Peterson, A.M. Early hMSC morphology and proliferation on model polyelectrolyte multilayers. Colloids Surf. B Biointerfaces 2019, 178, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Martinez, J.S.; Keller, T.C.S., III; Schlenoff, J.B. Cytotoxicity of Free versus Multilayered Polyelectrolytes. Biomacromolecules 2011, 12, 4063–4070. [Google Scholar] [CrossRef] [Green Version]
- Johannsmann, D. The Quartz Crystal Microbalance in Soft Matter Research; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Reviakine, I.; Johannsmann, D.; Richter, R.P. Hearing What You Cannot See and Visualizing What You Hear: Interpreting Quartz Crystal Microbalance Data from Solvated Interfaces. Anal. Chem. 2011, 83, 8838–8848. [Google Scholar] [CrossRef]
- Dixon, M.C. Quartz Crystal Microbalance with Dissipation Monitoring: Enabling Real-Time Characterization of Biological Materials and Their Interactions. J. Biomol. Tech. 2008, 19, 151–158. [Google Scholar]
- Fredriksson, C.; Kihlman, S.; Rodahl, M.; Kasemo, B. The Piezoelectric Quartz Crystal Mass and Dissipation Sensor: A Means of Studying Cell Adhesion. Langmuir 1998, 14, 248–251. [Google Scholar] [CrossRef]
- Galli Marxer, C.; Collaud Coen, M.; Greber, T.; Greber, U.F.; Schlapbach, L. Cell spreading on quartz crystal microbalance elicits positive frequency shifts indicative of viscosity changes. Anal. Bioanal. Chem. 2003, 377, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Fatisson, J.; Azari, F.; Tufenkji, N. Real-time QCM-D monitoring of cellular responses to different cytomorphic agents. Biosens. Bioelectron. 2011, 26, 3207–3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunot, C.; Ponsonnet, L.; Lagneau, C.; Farge, P.; Picart, C.; Grosgogeat, B. Cytotoxicity of polyethyleneimine (PEI), precursor base layer of polyelectrolyte multilayer films. Biomaterials 2007, 28, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.L.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Bershadsky, A.; Chausovsky, A.; Becker, E.; Lyubimova, A.; Geiger, B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr. Biol. 1996, 6, 1279–1289. [Google Scholar] [CrossRef] [Green Version]
- Binarova, P.; Tuszynski, J. Tubulin: Structure, functions and roles in disease. Cells 2019, 8, 1294–1301. [Google Scholar] [CrossRef] [Green Version]
- Etienne-Manneville, S. Microtubules in cell migration. Annu. Rev. Cell Dev. Biol. 2013, 29, 471–499. [Google Scholar] [CrossRef]
- Bodakuntla, S.; Jijumon, A.S.; Villablanca, C.; Gonzalez-Billault, C.; Janke, C. Microtubule-associated proteins: Structuring the cytoskeleton. Trends Cell Biol. 2019, 29, 804–819. [Google Scholar] [CrossRef]
- Jones, M.C.; Zha, J.; Humphries, M.J. Connections between the cell cycle, cell adhesion and the cytoskeleton. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180227–201802237. [Google Scholar] [CrossRef]
- Kadry, Y.A.; Calderwood, D.A. Chapter 22: Structural and signaling functions of integrins. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183206–183221. [Google Scholar] [CrossRef]
- Peterson, R.J.; Koval, M. Above the matrix: Functional roles for apically localized integrins. Front. Cell Dev. Biol. 2021, 9, 699407–699421. [Google Scholar] [CrossRef]
- Thamma, U.; Kowal, T.J.; Falk, M.M.; Jain, H. Nanostructure of bioactive glass affects bone cell attachment via protein restructuring upon adsorption. Sci. Rep. 2021, 11, 5763–5777. [Google Scholar] [CrossRef] [PubMed]
- Zwolanek, D.; Flicker, M.; Kirstätter, E.; Zaucke, F.; van Osch, G.J.V.M.; Erben, R.G. β1 Integrins Mediate Attachment of Mesenchymal Stem Cells to Cartilage Lesions. BioRes. Open Access 2015, 4, 39–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huttenlocher, A.; Horwitz, A.R. Integrins in cell migration. Cold Spring Harb. Perspect. Biol. 2011, 3, a005074–a005090. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S. Mechanotransduction—A field pulling together? J. Cell Sci. 2008, 121, 3285–3292. [Google Scholar] [CrossRef] [Green Version]
- Revach, O.Y.; Grosheva, I.; Geiger, B. Biomechanical regulation of focal adhesion and invadopodia formation. J. Cell Sci. 2020, 133, 244848–244858. [Google Scholar] [CrossRef]
- Hu, Y.L.; Lu, S.; Szeto, K.W.; Sun, J.; Wang, Y.; Lasheras, J.C.; Chien, S. FAK and paxillin dynamics at focal adhesions in the protrusions of migrating cells. Sci. Rep. 2014, 4, 6024–6031. [Google Scholar] [CrossRef] [Green Version]
- Castillo, A.B.; Blundo, J.T.; Chen, J.C.; Lee, K.L.; Yereddi, N.R.; Jang, E.; Kumar, S.; Tang, W.J.; Zarrin, S.; Kim, J.B.; et al. Focal Adhesion Kinase Plays a Role in Osteoblast Mechanotransduction In Vitro but Does Not Affect Load-Induced Bone Formation In Vivo. PLoS ONE 2012, 7, e43291–e43302. [Google Scholar] [CrossRef]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef]
- Schaller, M.D. Paxillin: A focal adhesion-associated adaptor protein. Oncogene 2001, 20, 6459–6472. [Google Scholar] [CrossRef] [Green Version]
- Deakin, N.O.; Turner, C.E. Paxillin comes of age. J. Cell Sci. 2008, 121, 2435–2444. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Yano, H.; Uchida, H.; Hashimoto, S.; Schaefer, E.; Sabe, H. Tyrosine Phosphorylation of Paxillin α Is Involved in Temporospatial Regulation of Paxillin-containing Focal Adhesion Formation and F-actin Organization in Motile Cells. J. Biol. Chem. 2000, 275, 27155–27164. [Google Scholar] [CrossRef]
- Goicoechea, S.M.; Zinn, A.; Awadia, S.S.; Snyder, K.; Garcia-Mata, R. A RhoG-mediated signaling pathway that modulates invadopodia dynamics in breast cancer cells. J. Cell Sci. 2017, 130, 1064–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.T. Nuclear FAK: A new mode of gene regulation from cellular adhesions. Mol. Cells 2013, 36, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Yi, Q.; Tang, L. The roles of nuclear focal adhesion kinase (FAK) on cancer: A focused review. J. Exp. Clin. Cancer Res. 2019, 38, 250–261. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Bai, H.; Yang, P. Real-time monitoring of mechanical changes during dynamic adhesion of erythrocytes to endothelial cells by QCM-D. Chem. Commun. 2015, 51, 11449–11451. [Google Scholar] [CrossRef]
- Bohmer, R.M.; Scharf, E.; Assoian, R.K. Cytoskeletal integrity is required throughout the mitogen stimulation phase of the cell cycle and mediates the anchorage-dependent expression of cyclin D1. Mol. Biol. Cell 1996, 7, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Zinn, A.; Goicoechea, S.M.; Kreider-Letterman, G.; Maity, D.; Awadia, S.; Cedeno-Rosario, L.; Chen, Y.; Garcia-Mata, R. The small Gtpase RhoG regulates microtubule-mediated focal adhesion disassembly. Sci. Rep. 2019, 9, 5163–5178. [Google Scholar] [CrossRef] [Green Version]
- Gumbiner, B.M. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 1996, 84, 345–357. [Google Scholar] [CrossRef] [Green Version]
- Hall, A.; Parhamifar, L.; Lange, M.K.; Meyle, K.D.; Sanderhoff, M.; Andersen, H.; Roursgaard, M.; Larsen, A.K.; Jensen, P.B.; Christensen, C.; et al. Polyethylenimine architecture-dependent metabolic imprints and perturbation of cellular redox homeostasis. Biochim. Biophys. Acta 2015, 1847, 328–342. [Google Scholar] [CrossRef] [Green Version]
- Hinshaw, D.B.; Armstrong, B.C.; Burger, J.M.; Beals, T.F.; Hyslop, P.A. ATP and microfilaments in cellular oxidant injury. Am. J. Pathol. 1988, 132, 479–488. [Google Scholar]
- Dos Remedios, C.G.; Chhabra, D.; Kekic, M.; Dedova, I.V.; Tsubakihara, M.; Berry, D.A.; Nosworthy, N.J. Actin binding proteins: Regulation of cytoskeletal microfilaments. Physiol. Rev. 2003, 83, 433–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ingber, D.E. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys. J. 1994, 66, 2181–2189. [Google Scholar] [CrossRef] [Green Version]





| G0/G1 (%) | S (%) | G2/M (%) | ||||
|---|---|---|---|---|---|---|
| 48 h | 6 Days | 48 h | 6 Days | 48 h | 6 Days | |
| CTRL | 68.4 | 83.6 | 10.7 | 6.6 | 13.6 | 8.1 |
| PSS | 66.0 | 68.1 | 12.7 | 11.1 | 16.9 | 11.6 |
| PEI | 58.7 | 20.0 | 8.0 | 10.0 | 8.7 | 40.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alba, A.; Villaggio, G.; Messina, G.M.L.; Caruso, M.; Federico, C.; Cambria, M.T.; Marletta, G.; Sinatra, F. Cytostatic Effects of Polyethyleneimine Surfaces on the Mesenchymal Stromal Cell Cycle. Polymers 2022, 14, 2643. https://doi.org/10.3390/polym14132643
Alba A, Villaggio G, Messina GML, Caruso M, Federico C, Cambria MT, Marletta G, Sinatra F. Cytostatic Effects of Polyethyleneimine Surfaces on the Mesenchymal Stromal Cell Cycle. Polymers. 2022; 14(13):2643. https://doi.org/10.3390/polym14132643
Chicago/Turabian StyleAlba, Anna, Giusy Villaggio, Grazia Maria Lucia Messina, Massimo Caruso, Concetta Federico, Maria Teresa Cambria, Giovanni Marletta, and Fulvia Sinatra. 2022. "Cytostatic Effects of Polyethyleneimine Surfaces on the Mesenchymal Stromal Cell Cycle" Polymers 14, no. 13: 2643. https://doi.org/10.3390/polym14132643
APA StyleAlba, A., Villaggio, G., Messina, G. M. L., Caruso, M., Federico, C., Cambria, M. T., Marletta, G., & Sinatra, F. (2022). Cytostatic Effects of Polyethyleneimine Surfaces on the Mesenchymal Stromal Cell Cycle. Polymers, 14(13), 2643. https://doi.org/10.3390/polym14132643







