Tri-Layered Doxycycline-, Collagen- and Bupivacaine-Loaded Poly(lactic-co-glycolic acid) Nanofibrous Scaffolds for Tendon Rupture Repair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Fabrication of Biomolecule-Loaded PLGA Nanofibers
2.2. Characterization of Integrated PLGA Nanofibers
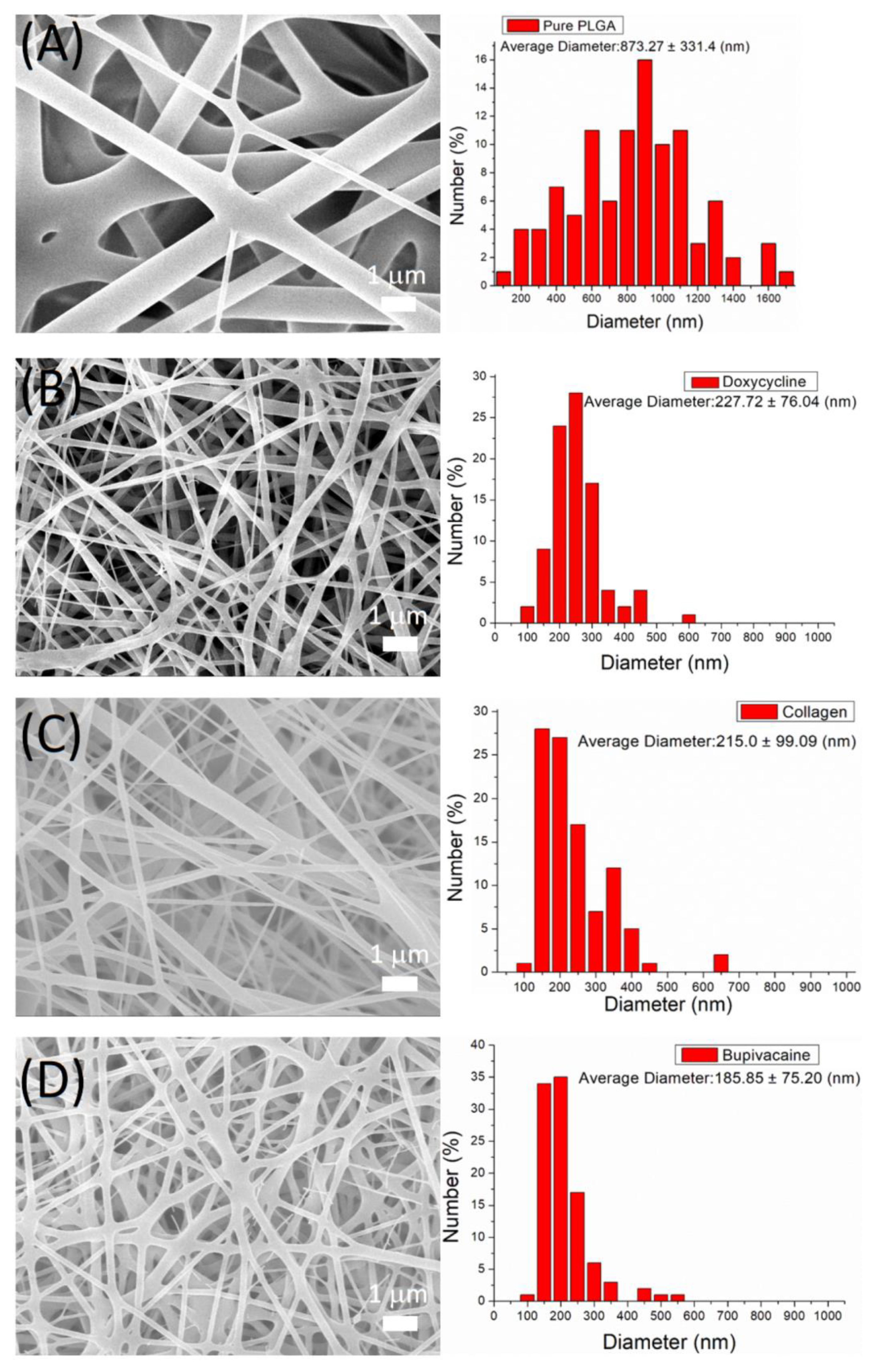
2.2.1. Scanning Electron Microscopy
2.2.2. Wetting Angles
2.2.3. Differential Scanning Calorimetry Assessment
2.2.4. Tensile Strengths of Electrospun Nanofibrous Scaffolds
2.3. In Vitro Drug Elution of PLGA Nanofibers
2.4. Experimental Model of Achilles Tendon Injury and Repair
2.5. Bioactivities Examination
2.6. In Vivo Drug Elution Characterization
2.7. Specimen Assessments
2.7.1. Gross Specimen Assessment
2.7.2. Mechanical Property Assessment
2.7.3. Histology and Immunohistochemistry (IHC) Assay
2.8. Statistical Analyses
3. Results
3.1. Characterization of DCB PLGA Nanofibers
3.2. In Vitro Drug Elution of Pharmaceuticals-Loaded PLGA Nanofibers
3.3. In Vivo Drug Elution Characterization
3.4. Bioactivity and Water/Food Intake
3.5. Mechanical Property Assessment of Repaired Tendons
3.6. Histological and Immunohistochemistry (IHC) Assessments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thorpe, C.T.; Screen, H.R. Tendon Structure and Composition. Adv. Exp. Med. Biol. 2016, 20, 3–10. [Google Scholar] [CrossRef]
- Franchi, M.; Trirè, A.; Quaranta, M.; Orsini, E.; Ottani, V. Collagen structure of tendon relates to function. Sci. World J. 2007, 30, 404–420. [Google Scholar] [CrossRef]
- Buckley, M.R.; Evans, E.B.; Matuszewski, P.E.; Chen, Y.L.; Satchel, L.N.; Elliott, D.M.; Soslowsky, L.J.; Dodge, G.R. Distributions of types I, II and III collagen by region in the human supraspinatus tendon. Connect. Tissue Res. 2013, 54, 374–379. [Google Scholar] [CrossRef]
- Clayton, R.A.E.; Court-Brown, C.M. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury 2008, 39, 1338–1344. [Google Scholar] [CrossRef]
- Leppilahti, J.; Puranen, J.; Orava, S. Incidence of Achilles tendon rupture. Acta Orthop. Scand. 1996, 67, 277–279. [Google Scholar] [CrossRef] [Green Version]
- Voleti, P.B.; Buckley, M.R.; Soslowsky, L.J. Tendon healing: Repair and regeneration. Annu. Rev. Biomed. Eng. 2012, 14, 47–71. [Google Scholar] [CrossRef]
- Smith, R.K.; Birch, H.; Patterson-Kane, J.; Firth, E.C.; Williams, L.; Cherdchutham, W.; van Weeren, W.R.; Goodship, A.E. Should equine athletes commence training during skeletal development? Changes in tendon matrix associated with development, ageing, function and exercise. Equine Vet. J. Suppl. 1999, 30, 201–209. [Google Scholar] [CrossRef]
- Gonçalves-Neto, J.; Witzel, S.S.; Teodoro, W.R.; Carvalho-Júnior, A.E.; Fernandes, T.D.; Yoshinari, H.H. Changes in collagen matrix composition in human posterior tibial tendon dysfunction. Joint Bone Spine 2002, 69, 189–194. [Google Scholar] [CrossRef]
- Riley, G.P.; Harrall, R.L.; Constant, C.R.; Chard, M.D.; Cawston, T.E.; Hazleman, B.L. Tendon degeneration and chronic shoulder pain: Changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann. Rheum. Dis. 1994, 53, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Kauwe, M. Acute Achilles Tendon Rupture: Clinical Evaluation, Conservative Management, and Early Active Rehabilitation. Clin. Podiatr. Med. Surg. 2017, 34, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Dilger, C.P.; Chimenti, R.L. Nonsurgical Treatment Options for Insertional Achilles Tendinopathy. Foot Ankle Clin. 2019, 24, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.; Didembourg, M.; Wood, A.; Devi, A.; Dinsdale, R.; Hazeldine, J.; Alsousou, J.; Keene, D.J.; Hulley, P.; Wagland, S.; et al. Characteristics of L-PRP preparations for treating Achilles tendon rupture within the PATH-2 study. Platelets 2021, 17, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Barboni, B.; Russo, V.; Curini, V.; Mauro, A.; Martelli, A.; Muttini, A.; Bernabò, N.; Valbonetti, L.; Marchisio, M.; Di Giacinto, O.; et al. Achilles tendon regeneration can be improved by amniotic epithelial cell allotransplantation. Cell Transpl. 2012, 21, 2377–2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, C.J.; Lee, D.; Ho, J.; Liu, S.J. Doxycycline-Embedded Nanofibrous Membranes Help Promote Healing of Tendon Rupture. Int. J. Nanomed. 2020, 9, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Tsai, W.C.; Hsu, C.C.; Chou, S.W.; Chung, C.Y.; Chen, J.; Pang, J.H. Effects of celecoxib on migration, proliferation and collagen expression of tendon cells. Connect. Tissue Res. 2007, 48, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Forslund, C.; Bylander, B.; Aspenberg, P. Indomethacin and celecoxib improve tendon healing in rats. Acta Orthop. Scand. 2003, 74, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Zhang, S.; Li, Q.; Yang, J.; Dong, W.; Wang, S.; Cheng, Y.; Al-Qwbani, M.; Wang, Q.; Yu, B. Effects of celecoxib on proliferation and tenocytic differentiation of tendon-derived stem cells. Biochem. Biophys. Res. Commun. 2014, 450, 762–766. [Google Scholar] [CrossRef]
- Abgollahiyan, P.; Oroojalian, F.; Mokhtarzadeh, A. The triad of nanotechnology, cell signalling, and scaffold implantation for the successful repair of damaged organs: An overview on soft-tissue engineering. J. Contr. Release 2021, 332, 460–492. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine. J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Contr. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Yu, Y.H.; Liu, S.J. Polyetheretherketone for orthopedic applications: A review. Curr. Opin. Chem. Eng. 2021, 1, 100687. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Norelli, J.B.; Graver, A.; Ekstein, C.; Schwartz, J.; Chowdhury, F.; Drakos, M.C.; Grande, D.A.; Chahine, N.O. Therapeutic effects of doxycycline on the quality of repaired and unrepaired Achilles tendons. Am. J. Sports Med. 2017, 45, 2872–2881. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.W.; Barr, J.; Greenwald, R.; Lane, L.B.; Dines, J.S.; Dines, D.M.; Drakos, M.C.; Grande, D.A.; Chahine, N.O. Enhancement of Achilles tendon repair mediated by matrix metalloproteinase inhibition via systemic administration of doxycycline. J. Orthop. Res. 2014, 32, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef]
- Shafiei, F.T.; McAllister, R.K.; Lopez, J. Bupivacaine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022; Available online: https://www.ncbi.nlm.nih.gov/books/NBK532883/ (accessed on 5 May 2022).
- Kumbar, S.G.; Nukavarapu, S.P.; James, R.; Nair, L.S.; Laurencin, C.T. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 2008, 29, 4100–4107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.H.; Hsu, Y.H.; Chou, Y.C.; Fan, C.L.; Ueng, S.W.; Kau, Y.C.; Liu, S.J. Sustained relief of pain from osteosynthesis surgery of rib fracture by using biodegradable lidocaine-eluting nanofibrous membranes. Nanomedicine 2016, 12, 1785–1793. [Google Scholar] [CrossRef]
- Tai, I.C.; Fu, Y.C.; Wang, C.K.; Chang, J.K.; Ho, M.L. Local delivery ofcontrolled-release simvastatin/PLGA/HAp microspheres enhances bone repair. Int. J. Nanomed. 2013, 8, 3895–3905. [Google Scholar] [CrossRef] [Green Version]
- Kogawa, A.C.; Zoppi, A.; Quevedo, M.A.; Salgado, H.R.N.; Longhi, M.R. Increasing doxycycline hyclate photostability by complexation with β-cyclodextrin. AAPS Pharm. Sci. Tech. 2014, 15, 1209–1217. [Google Scholar] [CrossRef] [Green Version]
- Jug, M.; Maestrelli, F.; Bragagni, M.; Mura, P. Preparation and solid-state characterization of bupivacaine hydrochloride cyclodextrin complexes aimed for buccal delivery. J. Pharm. Biomed. Anal. 2010, 52, 9–18. [Google Scholar] [CrossRef]
- Hope, M.; Saxby, T.S. Tendon healing. Foot Ankle Clin. 2007, 12, 553–567. [Google Scholar] [CrossRef]
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodeling. J. Musculoskelet Neuronal Interact. 2006, 6, 181–190. [Google Scholar] [PubMed]
- Güngörmüş, C.; Kolankaya, D.; Aydin, E. Histopathological and biomechanical evaluation of tenocyte seeded allografts on rat Achilles tendon regeneration. Biomaterials 2015, 51, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Pajala, A.; Melkko, J.; Leppilahti, J.; Ohtonen, P.; Soini, Y.; Risteli, J. Tenascin-C and type I and III collagen expression in total Achilles tendon rupture. An immunohistochemical study. Histol. Histopathol. 2009, 24, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Abdul Alim, M.; Domeij-Arverud, E.; Nilsson, G.; Edman, G.; Ackermann, P.W. Achilles tendon rupture healing is enhanced by intermittent pneumatic compression upregulating collagen type I synthesis. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2021–2029. [Google Scholar] [CrossRef]
- Veronesi, F.; Borsari, V.; Contartese, D.; Xian, J.; Baldini, N.; Fini, M. The clinical strategies for tendon repair with biomaterials: A review on rotator cuff and Achilles tendons. J. Biomed. Mater. Res. Appl. Biomater. 2020, 108, 1826–1843. [Google Scholar] [CrossRef]
- Sensini, A.; Gualandi, C.; Zucchelli, A.; Boyle, L.A.; Kao, A.P.; Reilly, G.C.; Tozzi, G.; Cristofolini, L.; Focarete, M.L. Tendon fascicle-inspired nanofibrous scaffold of polylactic acid/collagen with enhanced 3D-structure and biomechanical properties. Sci. Rep. 2018, 21, 17167. [Google Scholar] [CrossRef]
- Mao, Z.; Fan, B.; Wang, X.; Huang, X.; Guan, J.; Sun, Z.; Xu, B.; Yang, M.; Chen, Z.; Jiang, D.; et al. A systematic review of tissue engineering scaffold in tendon bone healing in vivo. Front. Bioeng. Biotechnol. 2021, 9, 621483. [Google Scholar] [CrossRef]
- Pillai, D.S.; Dhinsa, B.S.; Khan, W.S. Tissue engineering in Achilles tendon reconstruction; the role of stem cells, growth factors and scaffolds. Curr. Stem Cell Res. Ther. 2017, 12, 506–512. [Google Scholar] [CrossRef] [Green Version]
- Blomgran, P.; Hammerman, M.; Aspenberg, P. Systemic corticosteroids improve tendon healing when given after the early inflammatory phase. Sci. Rep. 2017, 7, 12468. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Tang, C.; Chen, Y.; Ruan, D.; Zhang, E.; Yin, Z.; Chen, X.; Jiang, Y.; Cai, Y.; Fei, Y.; et al. Pharmacological inhibition of Rac1 activity prevents pathological calcification and enhances tendon regeneration. ACS Biomater. Sci. Eng. 2019, 5, 3511–3522. [Google Scholar] [CrossRef]
- Liu, S.J.; Kau, Y.C.; Chou, C.Y.; Chen, J.K.; Wu, R.C.; Yeh, W.L. Electrospun PLGA/collagen nanofibrous membrane as early-stage wound dressing. J. Membr. Sci. 2010, 355, 53–59. [Google Scholar] [CrossRef]
- Krakauer, T.; Buckley, M. Doxycycline Is anti-inflammatory and inhibits staphylococcal exotoxin-induced cytokines and chemokines. Antimicrob. Agents Chemother. 2003, 47, 3630–3633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, A.L.; des Jardins-Park, H.E.; Duoto, B.A.; Mascharak, S.; Murphy, M.P.; Irizarry, D.M.; Foster, D.S.; Jones, R.E.; Barnes, L.A.; Marshall, C.D.; et al. Doxycycline reduces scar thickness and improves collagen architecture. Ann. Surg. 2020, 272, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Ballieul, R.J.; Jacobs, T.F.; Herregods, S.; Van Sint Jan, P.; Wyler, B.; Vereecke, H.; Almqvist, F.; Herregods, L. The peri-operative use of intra-articular local anesthetics: A review. Acta Anaesthesiol. Belg. 2009, 60, 101–108. [Google Scholar]
- Sekimoto, K.; Tobe, M.; Saito, S. Local anesthetic toxicity: Acute and chronic management. Acute Med. Surg. 2017, 4, 152–160. [Google Scholar] [CrossRef]
- Smoot, J.D.; Bergese, S.D.; Onel, E.; Williams, H.T.; Hedden, W. The efficacy and safety of DepoFoam bupivacaine in patients undergoing bilateral, cosmetic, submuscular augmentation mammaplasty: A randomized, double-blind, active-control study. Aesthet Surg. J. 2012, 32, 69–76. [Google Scholar] [CrossRef]
- Ateş, Y.; Unal, N.; Cuhruk, H.; Erkan, N. Postoperative analgesia in children using preemptive retrobulbar block and local anesthetic infiltration in strabismus surgery. Reg. Anesth. Pain. Med. 1998, 23, 569–574. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.-H.; Shen, S.-J.; Hsu, Y.-H.; Chou, Y.-C.; Yu, P.-C.; Liu, S.-J. Tri-Layered Doxycycline-, Collagen- and Bupivacaine-Loaded Poly(lactic-co-glycolic acid) Nanofibrous Scaffolds for Tendon Rupture Repair. Polymers 2022, 14, 2659. https://doi.org/10.3390/polym14132659
Yu Y-H, Shen S-J, Hsu Y-H, Chou Y-C, Yu P-C, Liu S-J. Tri-Layered Doxycycline-, Collagen- and Bupivacaine-Loaded Poly(lactic-co-glycolic acid) Nanofibrous Scaffolds for Tendon Rupture Repair. Polymers. 2022; 14(13):2659. https://doi.org/10.3390/polym14132659
Chicago/Turabian StyleYu, Yi-Hsun, Shih-Jyun Shen, Yung-Heng Hsu, Ying-Chao Chou, Ping-Chun Yu, and Shih-Jung Liu. 2022. "Tri-Layered Doxycycline-, Collagen- and Bupivacaine-Loaded Poly(lactic-co-glycolic acid) Nanofibrous Scaffolds for Tendon Rupture Repair" Polymers 14, no. 13: 2659. https://doi.org/10.3390/polym14132659





