Supercritical Impregnation of Mango Leaf Extract into PLA 3D-Printed Devices and Evaluation of Their Biocompatibility with Endothelial Cell Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Enhanced Solvent Extraction (ESE)
2.2.1. Determining the Antioxidant Activity
2.2.2. High Performance Liquid Chromatography (HPLC)
2.3. Supercritical Impregnation (SCI)
2.4. 3D Printing
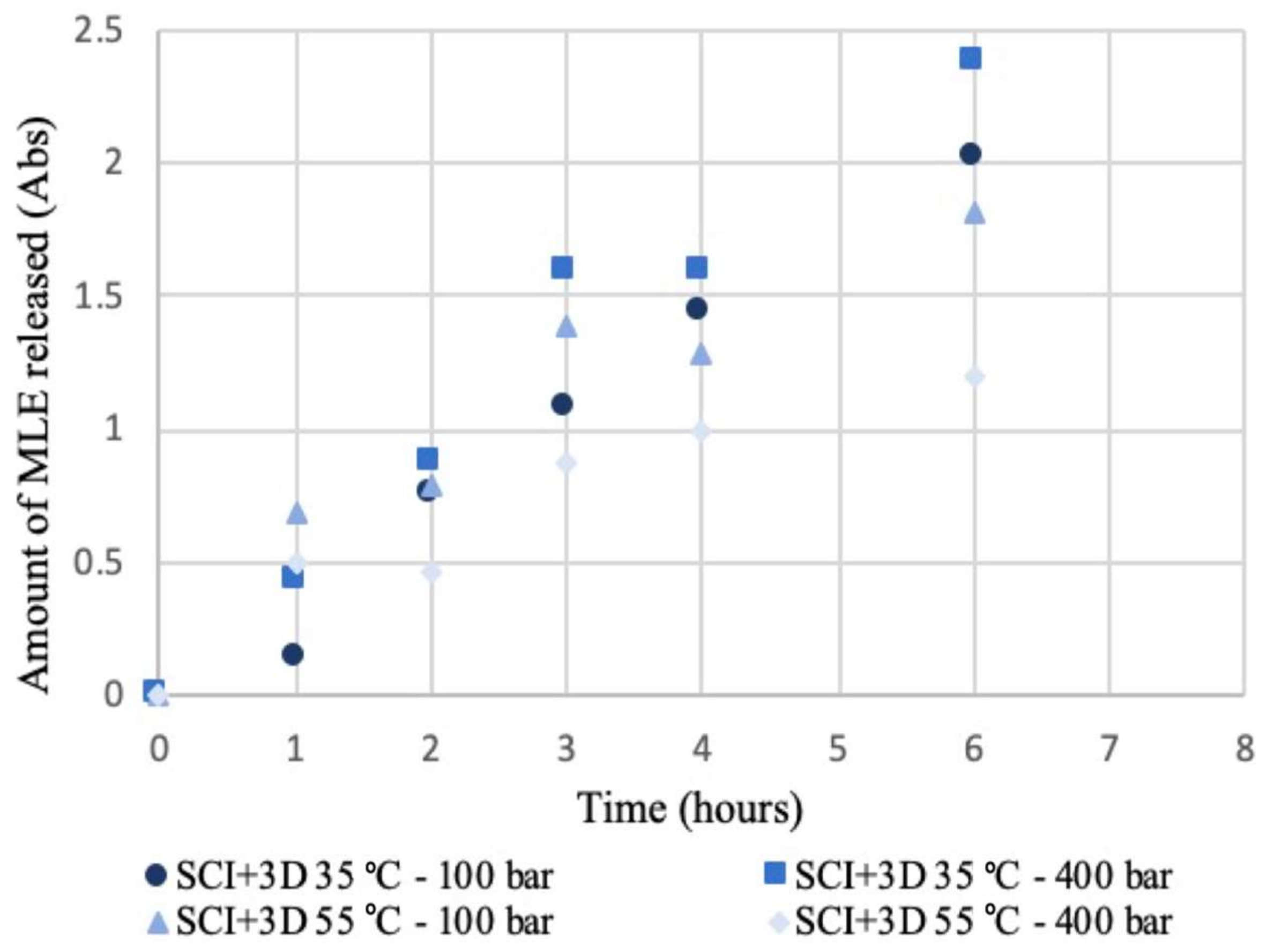
2.5. Release Kinetics Study of Mango Leaf Extract
2.6. Scanning Electron Microscopy (SEM)
2.7. Endothelial Cell Culture on PLA Disks
2.8. Cell Viability and Morphology Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Enhanced Solvent Extraction (ESE)
3.2. Supercritical Impregnation (SCI)
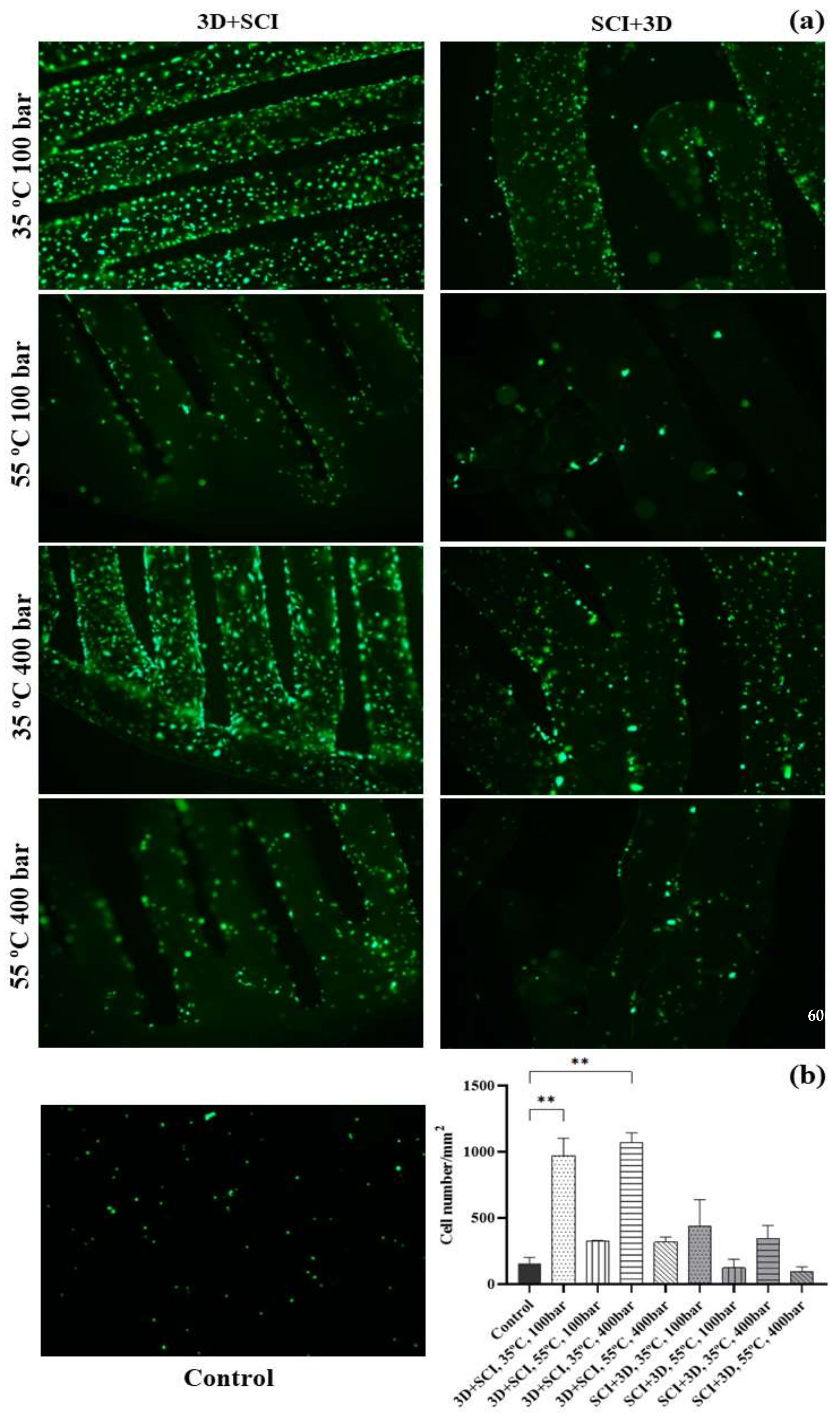
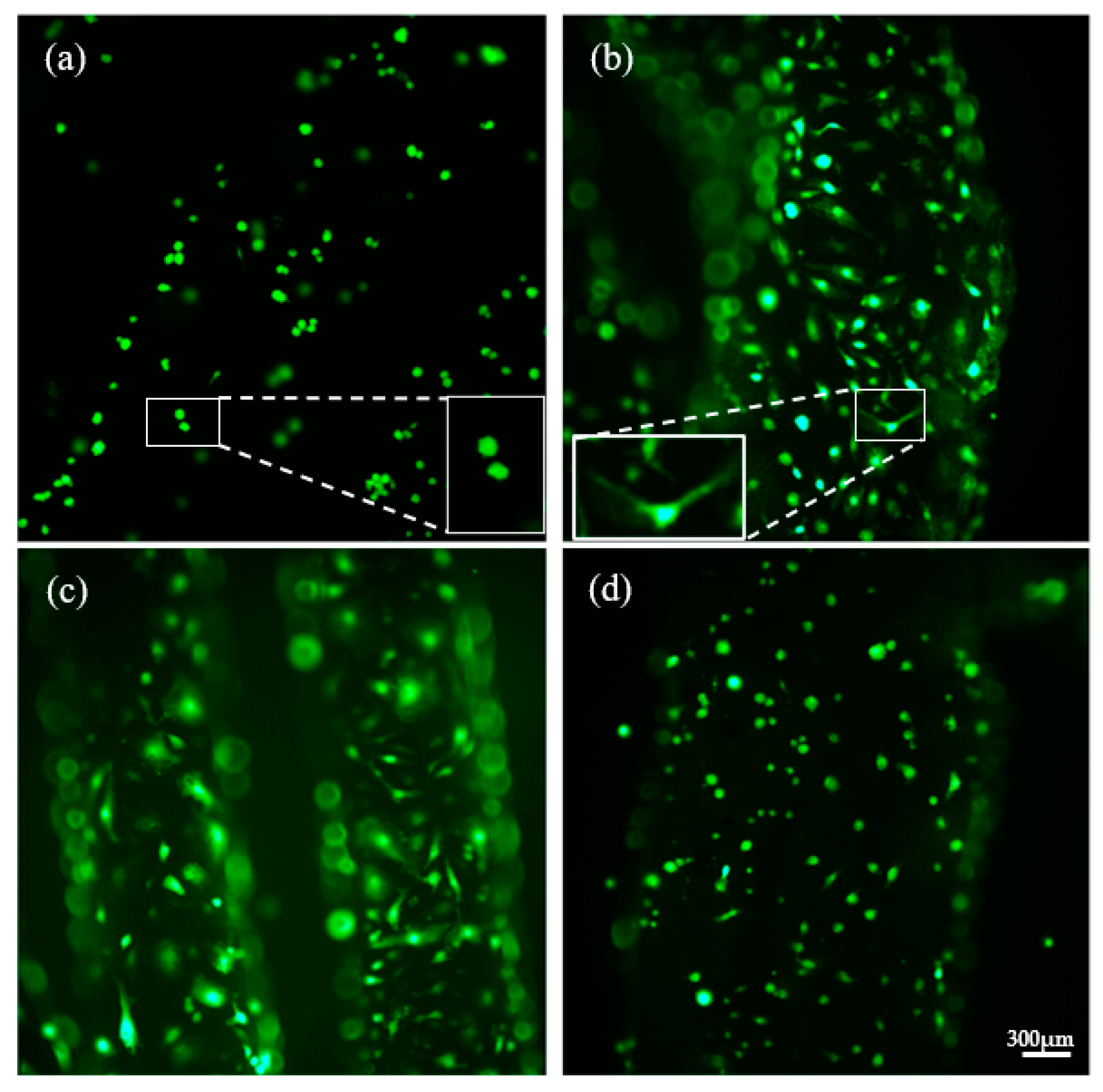
3.3. Cell Viability
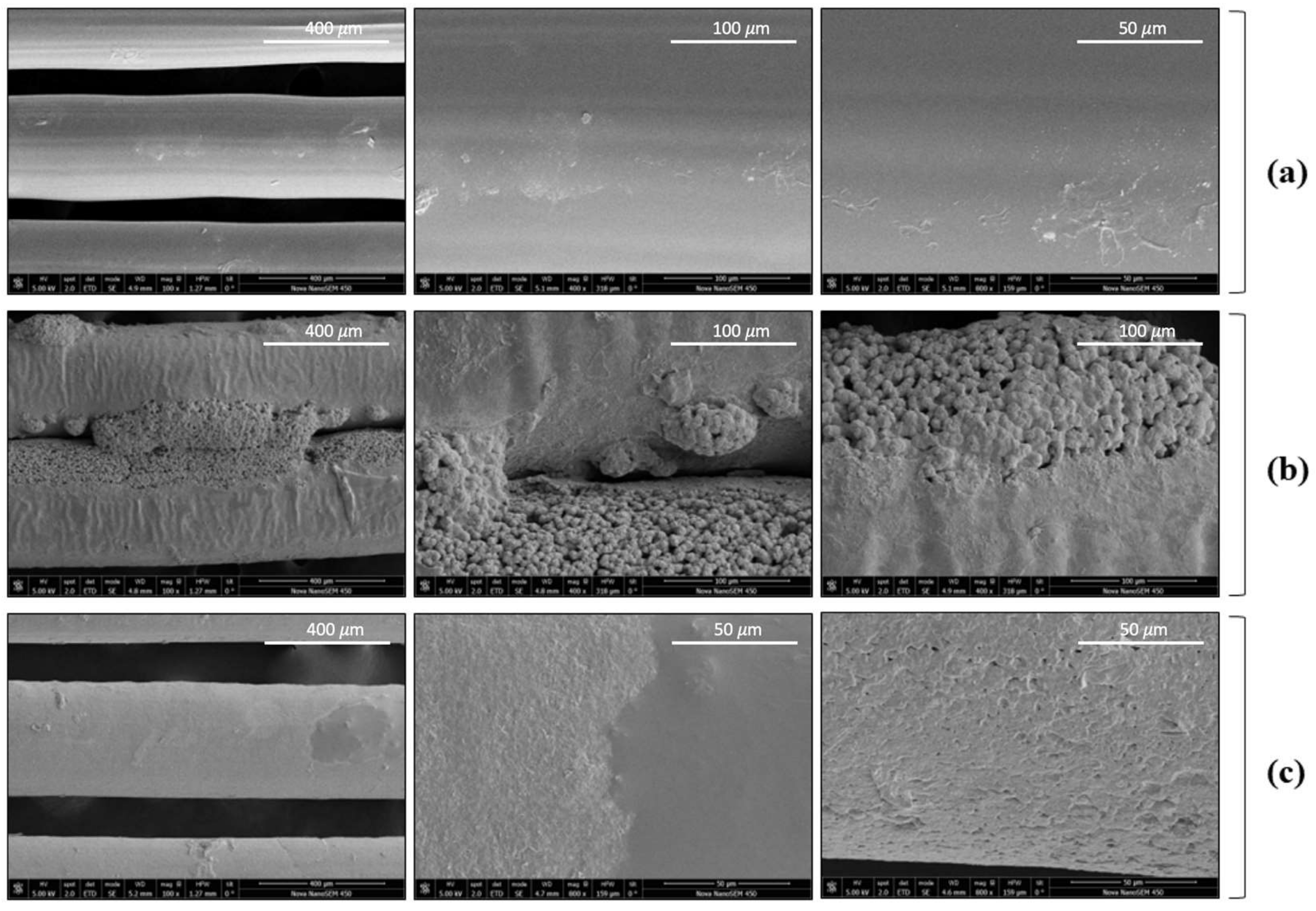
3.4. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birajdar, M.S.; Joo, H.; Koh, W.G.; Park, H. Natural bio-based monomers for biomedical applications: A review. Biomater. Res. 2021, 25, 8. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.K.; Tamer, M.A.; Ghareeb, M.M.; Salem, A.K. Recent Advances in Polymeric Implants. AAPS PharmSciTech 2019, 20, 300. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Medical Applications for 3D Printing: Current and Projected Uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar]
- Zhu, X.; Li, H.; Huang, L.; Zhang, M.; Fan, W.; Cui, L. 3D printing promotes the development of drugs. Biomed. Pharmacother. 2020, 131, 110664. [Google Scholar] [CrossRef]
- Nugroho, W.T.; Dong, Y.; Pramanik, A. Dimensional accuracy and surface finish of 3D printed polyurethane (PU) dog-bone samples optimally manufactured by fused deposition modelling (FDM). Rapid Prototyp. J. 2022, in press. [Google Scholar] [CrossRef]
- Zhu, Y.; Ramadani, E.; Egap, E. Thiol ligand capped quantum dot as an efficient and oxygen tolerance photoinitiator for aqueous phase radical polymerization and 3D printing under visible light. Polym. Chem. 2021, 12, 5106–5116. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, J.; Corrigan, N.; Boyer, C. Controlling mechanical properties of 3D printed polymer composites through photoinduced reversible addition–fragmentation chain transfer (RAFT) polymerization. Polym. Chem. 2022, 13, 44–57. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; López-Biedma, A.; Sánchez-Quesada, C.; Casas, L.; Mantell, C.; Gaforio, J.J.; Martínez De La Ossa, E.J. Selective antitumoural action of pressurized mango leaf extracts against minimally and highly invasive breast cancer. Food Funct. 2017, 8, 3610–3620. [Google Scholar] [CrossRef]
- Sánchez-Gomar, I.; Benítez-Camacho, J.; Cejudo-Bastante, C.; Casas, L.; Moreno-Luna, R.; Mantell, C.; Durán-Ruiz, M.C. Pro-angiogenic effects of natural antioxidants extracted from mango leaf, olive leaf and red grape pomace over endothelial colony-forming cells. Antioxidants 2022, 11, 851. [Google Scholar] [CrossRef]
- Tamai, H.; Igaki, K.; Kyo, E.; Kosuga, K.; Kawashima, A.; Matsui, S.; Komori, H.; Tsuji, T.; Motohara, S.; Uehata, H. Initial and 6-Month Results of Biodegradable Poly- l -Lactic Acid Coronary Stents in Humans. Circulation 2000, 102, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Rosales, J.M.; Cejudo, C.; Verano, L.; Casas, L.; Mantell, C.; de la Ossa, E.J.M. Supercritical Impregnation of PLA Filaments with Mango Leaf Extract to Manufacture Functionalized Biomedical Devices by 3D Printing. Polymers 2021, 13, 2125. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.Z.; Chen, Y.C.; Moreno-Luna, R.; Khademhosseini, A.; Melero-Martin, J.M. Transdermal regulation of vascular network bioengineering using a photopolymerizable methacrylated gelatin hydrogel. Biomaterials 2013, 34, 6785–6796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, R.Z.; Moreno-Luna, R.; Muñoz-Hernandez, R.; Li, D.; Jaminet, S.C.S.; Greene, A.K.; Melero-Martin, J.M. Human white adipose tissue vasculature contains endothelial colonyforming cells with robust in vivo vasculogenic potential. Angiogenesis 2013, 1, 735–744. [Google Scholar] [CrossRef] [Green Version]
- Guija-Poma, E.; Inocente-Camones, M.N.; Ponce-Pardo, J.; Zarzosa-Norabuena, E. Evaluation of the 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) technique to determine antioxidant capacity. Med. Horiz. 2015, 15, 57–60. [Google Scholar] [CrossRef] [Green Version]
- Lerma-Torres, J.M.; Navarro-Ocaña, A.; Calderón-Santoyo, M.; Hernández-Vázquez, L.; Ruiz-Montañez, G.; Ragazzo-Sánchez, J.A. Preparative scale extraction of mangiferin and lupeol from mango (Mangifera indica L.) leaves and bark by different extraction methods. J. Food Sci. Technol. 2019, 56, 4625–4631. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.M.; Rathod, V.K. Utilization of waste dried Mangifera indica leaves for extraction of mangiferin by conventional batch extraction and advance three-phase partitioning. Green Processing Synth. 2016, 5, 79–85. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; De La Ossa, E.M. Use of high-pressure techniques to produce Mangifera indica L. leaf extracts enriched in potent antioxidant phenolic compounds. Innov. Food Sci. Emerg. Technol. 2015, 29, 94–106. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Rodríguez, M.; de la Ossa, E.M. Extraction of antioxidant compounds from different varieties of Mangifera indica leaves using green technologies. J. Supercrit. Fluids 2012, 72, 168–175. [Google Scholar] [CrossRef]
- Milovanovic, S.; Markovic, D.; Mrakovic, A.; Kuska, R.; Zizovic, I.; Frerich, S.; Ivanovic, J. Supercritical CO2—Assisted production of PLA and PLGA foams for controlled thymol release. Mater. Sci. Eng. C 2019, 99, 394–404. [Google Scholar] [CrossRef]
- Sugiura, K.; Ogawa, S.; Tabata, I.; Hori, T. Impregnation of tranilast to the poly(lactic acid) fiber with supercritical carbon dioxide and the release behavior of tranilast. J. Fiber Sci. Technol. 2005, 61, 159–165. [Google Scholar] [CrossRef] [Green Version]
- García-Casas, I.; Montes, A.; Valor, D.; Pereyra, C.; de la Ossa, E.J.M. Foaming of polycaprolactone and its impregnation with quercetin using supercritical CO2. Polymers 2019, 11, 1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda-Villa, P.P.; Gañán, N.A.; Martini, R.E.; Goñi, M.L. Supercritical CO2-assisted impregnation of polylactic acid films with R-carvone: Effect of processing on loading, mass transfer kinetics, and final properties. J. CO2 Util. 2022, 61, 102029. [Google Scholar] [CrossRef]
- Verano Naranjo, L.; Cejudo Bastante, C.; Casas Cardoso, L.; Mantell Serrano, C.; Martínez de la Ossa Fernández, E.J. Supercritical impregnation of ketoprofen into polylactic acid for biomedical application: Analysis and modeling of the release kinetic. Polymers 2021, 13, 1982. [Google Scholar] [CrossRef] [PubMed]
- Nofar, M.; Park, C.B. Poly (lactic acid). Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Tu, J.B.; Ma, R.Z.; Dong, Q.; Jiang, F.; Hu, X.Y.; Li, Q.Y.; Pattar, P.; Zhang, H. Induction of apoptosis in infantile hemangioma endothelial cells by propranolol. Exp. Ther. Med. 2013, 6, 574–578. [Google Scholar] [CrossRef] [Green Version]
- Khaidakov, M.; Wang, X.; Mehta, J.L. Potential Involvement of LOX-1 in Functional Consequences of Endothelial Senescence. PLoS ONE 2011, 6, e20964. [Google Scholar] [CrossRef] [Green Version]
- Boer, S.; Bowman, M.; Notley, C.; Mo, A.; Lima, P.; Jong, A.; Dirven, R.; Weijers, E.; Lillicrap, D.; James, P.; et al. Endothelial characteristics in healthy endothelial colony forming cells; generating a robust and valid ex vivo model for vascular disease. J. Thromb. Haemost. 2020, 18, 2721–2731. [Google Scholar] [CrossRef]
- Ismail, M.B.; Rajendran, P.; AbuZahra, H.M.; Veeraraghavan, V.P. Mangiferin Inhibits Apoptosis in Doxorubicin-Induced Vascular Endothelial Cells via the Nrf2 Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 4259. [Google Scholar] [CrossRef]
- Soong, Y.Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Cabañas, J. Study of Cell Proliferation on Polymers Impregnated by Supercritical Technology with Natural Extracts. Bachelor’s Thesis, Science Faculty, University of Cádiz, Cádiz, Spain, 2020. [Google Scholar]
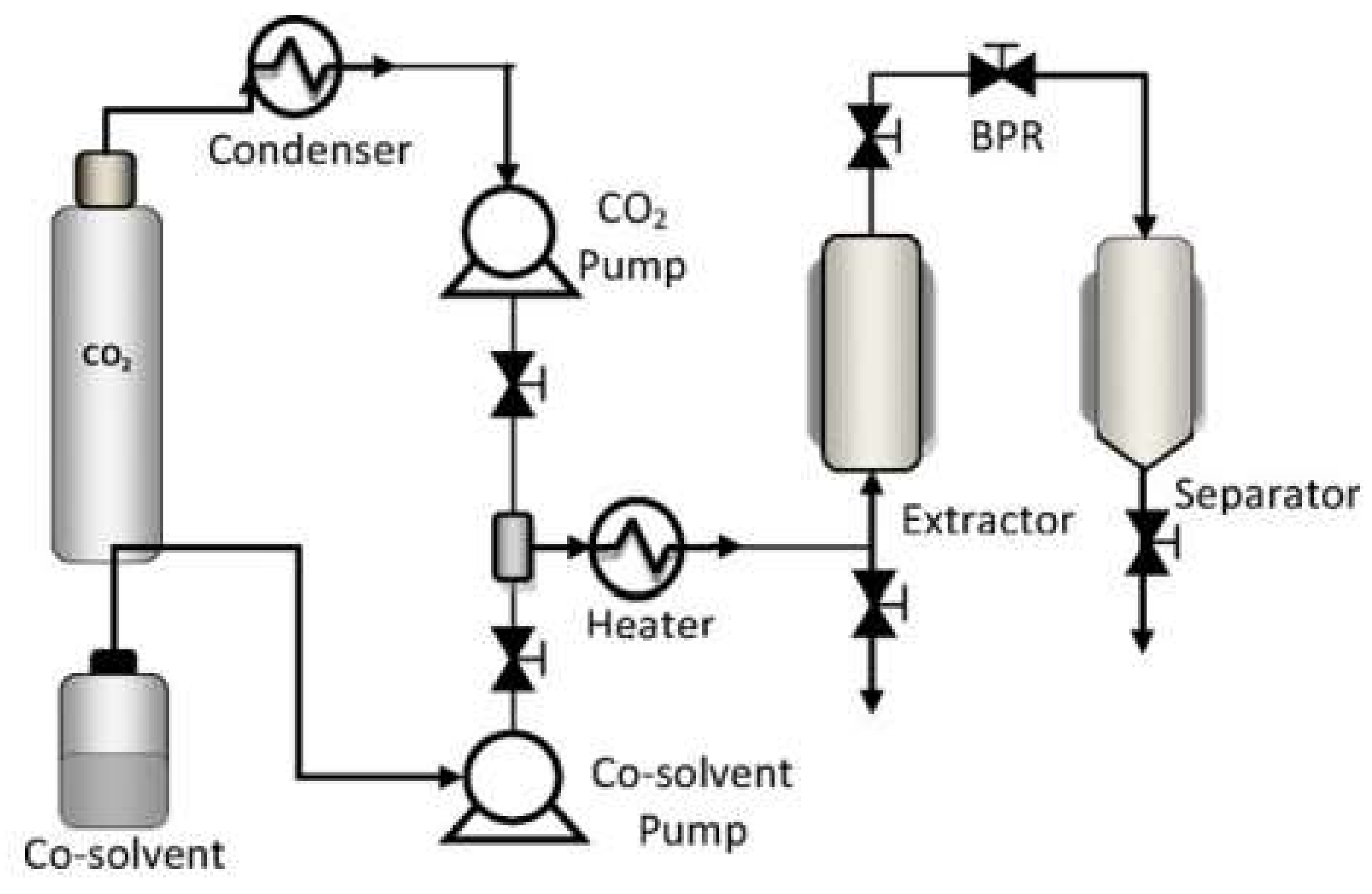




| Setup 1 | Setup 2 | Setup 3 | Setup 4 | |
|---|---|---|---|---|
| Temperature | 35 °C | 35 °C | 55 °C | 55 °C |
| Pressure | 100 bar | 400 bar | 100 bar | 400 bar |
| Layer Height | Thread Thickness | Diameter | Outer Turns | |
|---|---|---|---|---|
| 0.2 mm | 0.4 mm | 1 cm | 1 | |
| Filament temperature | Bed temperature | Printing rate | ||
| 200 °C | 60 °C | 25 mm/s | ||
| ELE Performance (%) | 10.7 ± 1.2 |
|---|---|
| Extract concentration (ppm) | 78200 ± 231 |
| EC50 (ppm) | 29,945 ± 0.14 |
| AAI | 0.7703 ± 0.003 |
| Majority compounds identified (µg/mL) | |
| Gallic acid | 312.6 ± 13.4 |
| Mangiferin | 601.8 ± 18.5 |
| Iriflophenone 3-C-β-D-glucoside | 1403.6 ± 16.4 |
| 35 °C, 100 bar | 35 °C, 400 bar | 55 °C, 100 bar | 55 °C, 400 bar | |
|---|---|---|---|---|
| Diameter (mm) | 1.753 | 1.753 | 1.778 | 1.854 |
| Increment (%) | 0.17 | 0.17 | 1.6 | 5.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosso, P.; Cejudo, C.; Sánchez-Gomar, I.; Durán-Ruiz, M.C.; Moreno-Luna, R.; Casas, L.; Pereyra, C.; Mantell, C. Supercritical Impregnation of Mango Leaf Extract into PLA 3D-Printed Devices and Evaluation of Their Biocompatibility with Endothelial Cell Cultures. Polymers 2022, 14, 2706. https://doi.org/10.3390/polym14132706
Grosso P, Cejudo C, Sánchez-Gomar I, Durán-Ruiz MC, Moreno-Luna R, Casas L, Pereyra C, Mantell C. Supercritical Impregnation of Mango Leaf Extract into PLA 3D-Printed Devices and Evaluation of Their Biocompatibility with Endothelial Cell Cultures. Polymers. 2022; 14(13):2706. https://doi.org/10.3390/polym14132706
Chicago/Turabian StyleGrosso, Pilar, Cristina Cejudo, Ismael Sánchez-Gomar, Mª Carmen Durán-Ruiz, Rafael Moreno-Luna, Lourdes Casas, Clara Pereyra, and Casimiro Mantell. 2022. "Supercritical Impregnation of Mango Leaf Extract into PLA 3D-Printed Devices and Evaluation of Their Biocompatibility with Endothelial Cell Cultures" Polymers 14, no. 13: 2706. https://doi.org/10.3390/polym14132706
APA StyleGrosso, P., Cejudo, C., Sánchez-Gomar, I., Durán-Ruiz, M. C., Moreno-Luna, R., Casas, L., Pereyra, C., & Mantell, C. (2022). Supercritical Impregnation of Mango Leaf Extract into PLA 3D-Printed Devices and Evaluation of Their Biocompatibility with Endothelial Cell Cultures. Polymers, 14(13), 2706. https://doi.org/10.3390/polym14132706








