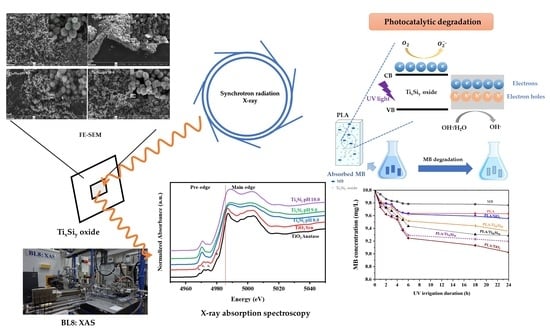
Structural Characterization of Titanium–Silica Oxide Using Synchrotron Radiation X-ray Absorption Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Titania–Silica Binary Oxide (TixSiy Oxide), Silica, and Titanium Dioxide
2.3. Characterization of Titanium–Silicon Oxide
2.3.1. 29Si Solid-State Nuclear Magnetic Resonance Spectroscopy (29Si Solid-State NMR)
2.3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3.3. Field Emission Scanning Electron Microscopy (FE-SEM)
2.3.4. X-ray Diffraction (XRD)
2.3.5. X-ray Absorption Near-Edge Structure Spectroscopy (XANES) and Extended X-ray Absorption Fine Structure Spectroscopy (EXAFS)
2.3.6. X-ray Fluorescence (XRF)
2.3.7. Particle Size Distribution
2.3.8. Specific Surface Area (BET)
2.3.9. Photocatalytic Activity Characterization
3. Results
3.1. Effect of pH
3.2. Effect of Ti/Si Ratio
3.3. Photocatalytic Degradation of Methylene Blue (MB)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasalingam, S.; Peng, R.; Koodali, R.T. Review Article: Removal of Hazardous Pollutants from Wastewaters: Applications of TiO2-SiO2 Mixed Oxide Materials. J. Nanomater. 2014, 2014, 1–42. [Google Scholar] [CrossRef]
- Wang, X.; Xue, J.; Wang, X.; Liu, X. Heterogeneous Ag-TiO2-SiO2 composite materials as novel catalytic systems for selective epoxidation of cyclohexene byH2O2. PLoS ONE 2017, 12, e0176332. [Google Scholar] [CrossRef]
- Sahu, D.R.; Hong, L.Y.; Wang, S.-C.; Huang, J.-L. Synthesis, analysis and characterization of ordered mesoporous TiO2/SBA-15 matrix: Effect of calcination temperature. Microporous Mesoporous Mater. 2009, 117, 640–649. [Google Scholar] [CrossRef]
- Petronella, F.; Pagliarulo, A.; Truppi, A.; Lettieri, M.; Masieri, M.; Calia, A.; Curri, M.L.; Comparelli, R. TiO2 Nanocrystal Based Coatings for the Protection of Architectural Stone: The Effect of Solvents in the Spray-Coating Application for a Self-Cleaning Surfaces. Coatings 2018, 8, 356. [Google Scholar] [CrossRef]
- Zhu, Y.; Buonocore, G.G.; Lavorgna, M. Photocatalytic activity of PLA/TIO2 nanocomposites and TIO2-active multilayered hybrid coatings. Ital. J. Food Sci. 2012, 24, 102–106. [Google Scholar]
- Tiainen, H.D.; Wiedmer, H.; Haugen, J. Processing of highly porous TiO2 bone scaffolds with improved compressive strength. J. Eur. Ceram. Soc. 2013, 33, 15–24. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, K.; Liu, Y. Geopolymer-supported photocatalytic TiO2 film: Preparation and characterization. Constr. Build. Mater. 2017, 151, 63–70. [Google Scholar] [CrossRef]
- Allodi, V.; Brutti, S.; Giarola, M.; Sgambetterra, M.; Navarra, M.A.; Panero, S.; Mariotto, G. Structural and Spectroscopic Characterization of a Nanosized Sulfated TiO2 Filler and of Nanocomposite Nafion Membranes. Polymers 2016, 8, 68. [Google Scholar] [CrossRef]
- Bertuna, A.; Comini, E.; Poli, N.; Zappa, D.; Sberveglieri, G. Titanium Dioxide Nanostructures Chemical Sensor. Procedia Eng. 2016, 168, 313–316. [Google Scholar] [CrossRef][Green Version]
- Zapata, P.A.; Palza, H.; Cruz, L.S.; Lieberwirth, I.; Catalina, F.; Corrales, T.; Rabagliati, F.M. Polyethylene and poly(ethylene-co-1-octadecene) composites with TiO2 based nanoparticles by metallocenic “in situ” polymerization. Polymer 2013, 54, 2690–2698. [Google Scholar] [CrossRef]
- Fonseca, C.; Ochoa, A.; Ulloa, M.T.; Alvarez, E.; Canales, D.; Zapata, P.A. Poly(lactic acid)/TiO2 nanocomposites as alternative biocidal and antifungal materials. Mater. Sci. Eng. C 2015, 57, 314–320. [Google Scholar] [CrossRef]
- Wu, F.; Lan, X.; Ji, D.; Liu, Z.; Yang, W.; Yang, M. Grafting polymerization of polylactic acid on the surface of nano-SiO2 and properties of PLA/PLA-grafted-SiO2 nanocomposites. J. Appl. Polym. Sci. 2013, 129, 3019–3027. [Google Scholar] [CrossRef]
- Serenko, O.A.; Muzafarov, A.M. Polymer composites with surface modified SiO2 nanoparticles: Structures, properties, and promising applications. Polym. Sci. Ser. C 2016, 58, 93–101. [Google Scholar] [CrossRef]
- Ha, S.-W.; Weitzmann, M.N.; Beck, G.R., Jr. Chapter 4—Dental and Skeletal Applications of Silica-Based Nanomaterials A2—Subramani, Karthikeyan. In Nanobiomaterials in Clinical Dentistry; Ahmed, W., Hartsfield, J.K., Eds.; William Andrew Publishing: Norwich, NY, USA, 2013; pp. 69–91. [Google Scholar]
- Gao, X.; Wachs, I.E. Titania–silica as catalysts: Molecular structural characteristics and physico-chemical properties. Catal. Today 1999, 51, 233–254. [Google Scholar] [CrossRef]
- Pabón, E.; Retuert, J.; Quijada, R.; Zarate, A. TiO2–SiO2 mixed oxides prepared by a combined sol–gel and polymer inclusion method. Microporous Mesoporous Mater. 2004, 67, 195–203. [Google Scholar] [CrossRef]
- Pabón, E.; Retuert, J.; Quijada, R. Synthesis of mixed silica–titania by the sol–gel method using polyethylenimine: Porosity and catalytic properties. J. Porous Mater. 2007, 14, 151–158. [Google Scholar]
- Milne, C.J.; Penfold, T.J.; Chergui, M. Recent experimental and theoretical developments in time-resolved X-ray spectroscopies. Coord. Chem. Rev. 2014, 277–278, 44–68. [Google Scholar]
- Niltharach, A.; Kityakarn, S.; Worayingyong, A.; Thienprasert, J.T.; Klysubun, W.; Songsiriritthigul, P.; Limpijumnong, S. Structural characterizations of sol–gel synthesized TiO2 and Ce/TiO2 nanostructures. Phys. B Condens. Matter 2012, 407, 2915–2918. [Google Scholar] [CrossRef]
- Kim, W.B.; Choi, S.H.; Lee, J.S. Quantitative Analysis of Ti−O−Si and Ti−O−Ti Bonds in Ti−Si Binary Oxides by the Linear Combination of XANES. J. Phys. Chem. B 2000, 104, 8670–8678. [Google Scholar] [CrossRef]
- Matsuo, S.; Sakaguchi, N.; Wakita, H. Pre-edge features of Ti K-edge X-ray absorption near-edge structure for the local structure of sol-gel titanium oxides. Anal. Sci. 2005, 21, 805–809. [Google Scholar] [CrossRef]
- Alias, S.S.; Mohamad, A.A. ZnO: Effect of pH on the Sol–Gel Process. In Synthesis of Zinc Oxide by Sol–Gel Method for Photoelectrochemical Cells; SpringerBriefs in Materials; Springer: Singapore, 2014; pp. 9–25. [Google Scholar]
- Kim, T.G.; An, G.S.; Han, J.S.; Hur, J.U.; Park, B.G.; Choi, S.-C. Synthesis of Size Controlled Spherical Silica Nanoparticles via Sol-Gel Process within Hydrophilic Solvent. J. Korean Ceram. Soc. 2017, 54, 49–54. [Google Scholar] [CrossRef]
- Karkare, M.M. Choice of precursor not affecting the size of anatase TiO2 nanoparticles but affecting morphology under broader view. Int. Nano Lett. 2014, 4, 111. [Google Scholar] [CrossRef]
- Klysubun, W.; Tarawarakarn, P.; Thamsanong, N.; Amonpattaratkit, P.; Cholsuk, C.; Lapboonrueng, S.; Chaichuay, S.; Wongtepa, W. Upgrade of SLRI BL8 beamline for XAFS spectroscopy in a photon energy range of 1–13 keV. Radiat. Phys. Chem. 2020, 175, 108145. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. THENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Schraml-Marth, M.; Walther, K.L.; Wokaun, A.; Handy, B.E.; Baiker, A. Porous silica gels and TiO2/SiO2 mixed oxides prepared via the sol-gel process: Characterization by spectroscopic techniques. J. Non-Cryst. Solids 1992, 143, 93–111. [Google Scholar] [CrossRef]
- Degirmenci, V.; Erdem, Ö.F.; Ergun, O.; Yilmaz, A.; Michel, D.; Uner, D. Synthesis and NMR Characterization of Titanium and Zirconium Oxides Incorporated in SBA-15. Top. Catal. 2008, 49, 204–208. [Google Scholar] [CrossRef]
- Dirken, P.J.; Smith, M.E.; Whitfield, H.J. 17O and 29Si Solid State NMR Study of Atomic Scale Structure in Sol-Gel-Prepared TiO2-SiO2 Materials. J. Phys. Chem. 1995, 99, 395–401. [Google Scholar] [CrossRef]
- Zeitler, V.A.; Brown, C.A. The Infrared Spectra of Some Ti–O–Si, Ti–O–Ti and Si–O–Si Compounds. J. Phys. Chem. 1957, 61, 1174–1177. [Google Scholar] [CrossRef]
- Praveen, P. Structural, Functional and Optical Characters of TiO2 Nanocrystallites: Anatase and Rutile Phases. St.Joseph’s J. Humanit. Sci. 2019, 6, 43–53. [Google Scholar]
- Chen, Y.-F.; Lee, C.-Y.; Yeng, M.-Y.; Chiu, H.-T. The effect of calcination temperature on the crystallinity of TiO2 nanopowders. J. Cryst. Growth 2003, 247, 363–370. [Google Scholar] [CrossRef]
- Vasquez, J.; Lozano, H.; Lavayen, V.; Lira-Cantú, M.; Gómez-Romero, P.; Santa Ana, M.A.; Benavente, E.; Gonzalez, G. High-yield preparation of titanium dioxide nanostructures by hydrothermal conditions. J. Nanosci. Nanotechnol. 2009, 9, 1103–1107. [Google Scholar] [CrossRef]
- Yamashita, H.; Kawasaki, S.; Ichihashi, Y.; Harada, M.; Takeuchi, M.; Anpo, M.; Stewart, G.; Fox, M.A.; Louis, C.; Che, M. Characterization of Titanium−Silicon Binary Oxide Catalysts Prepared by the Sol−Gel Method and Their Photocatalytic Reactivity for the Liquid-Phase Oxidation of 1-Octanol. J. Phys. Chem. B 1998, 102, 5870–5875. [Google Scholar] [CrossRef]
- Vives, S.; Meunier, C. Influence of the synthesis route on sol–gel SiO2–TiO2 (1:1) xerogels and powders. Ceram. Int. 2008, 34, 37–44. [Google Scholar] [CrossRef]
- Yu, H.-F.; Wang, S.-M. Effects of water content and pH on gel-derived TiO2–SiO2. J. Non-Cryst. Solids 2000, 261, 260–267. [Google Scholar] [CrossRef]
- Yoldas, B.E. Formation of titania-silica glasses by low temperature chemical polymerization. J. Non-Cryst. Solids 1980, 38, 81–86. [Google Scholar] [CrossRef]
- Alias, S.S.; Ismail, A.B.; Mohamad, A.A. Effect of pH on ZnO nanoparticle properties synthesized by sol–gel centrifugation. J. Alloys Compd. 2010, 499, 231–237. [Google Scholar] [CrossRef]
- Wu, L.; Wu, Y.; Lü, Y. Self-assembly of small ZnO nanoparticles toward flake-like single crystals. Mater. Res. Bull. 2006, 41, 128–133. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Li Hsiung, T.; Paul Wang, H.; Wang, H.C. XANES studies of photocatalytic active species in nano TiO2–SiO2. Radiat. Phys. Chem. 2006, 75, 2042–2045. [Google Scholar] [CrossRef]
- Rossi, T.C.; Grolimund, D.; Nachtegaal, M.; Cannelli, O.; Mancini, G.F.; Bacellar, C.; Kinschel, D.; Rouxel, J.R.; Ohannessian, N.; Pergolesi, D.; et al. X-ray absorption linear dichroism at the Ti K-edge of anatase TiO2 single crystals. Phys. Rev. B 2019, 100, 245207. [Google Scholar] [CrossRef]
- Farges, F.; Brown, G.E.; Rehr, J.J. Ti K-edge XANES studies of Ti coordination and disorder in oxide compounds: Comparison between theory and experiment. Phys. Rev. B 1997, 56, 1809–1819. [Google Scholar] [CrossRef]
- Davis, R.J.; Liu, Z. Titania−Silica: A Model Binary Oxide Catalyst System. Chem. Mater. 1987, 9, 2311–2324. [Google Scholar] [CrossRef]
- Sharma, A.; Varshney, M.; Park, J.; Ha, T.-K.; Chae, K.-H.; Shin, H.-J. XANES, EXAFS and photocatalytic investigations on copper oxide nanoparticles and nanocomposites. RSC Adv. 2015, 5, 21762–21771. [Google Scholar] [CrossRef]
- Khemthong, P.; Photai, P.; Grisdanurak, N. Structural properties of CuO/TiO2 nanorod in relation to their catalytic activity for simultaneous hydrogen production under solar light. Int. J. Hydrogen Energy 2013, 38, 15992–16001. [Google Scholar] [CrossRef]
- Loshmanov, A.A.; Sigaev, V.N.; Khodakovskaya, R.Y.; Pavlushkin, N.M.; Yamzin, I.I. Small-angle neutron scattering on silica glasses containing titania. J. Appl. Crystallogr. 1974, 7, 207–210. [Google Scholar] [CrossRef]
- Sandstrom, D.R.; Lytle, F.W.; Wei, P.S.P.; Greegor, R.B.; Wong, J.; Schultz, P. Coordination of Ti in TiO2–SiO2 glass by X-ray absorption spectroscopy. J. Non-Cryst. Solids 1980, 41, 201–207. [Google Scholar] [CrossRef]
- Neurock, M.; Manzer, L.E. Theoretical insights on the mechanism of alkene epoxidation by H2O2 with titanium silicalite. Chem. Commun. 1996, 10, 1133–1134. [Google Scholar] [CrossRef]
- Scherk, C.G.; Ostermann, A.; Achterhold, K.; Iakovleva, O.; Nazikkol, C.; Krebs, B.; Knapp, E.W.; Meyer-Klaucke, W.; Parak, F.G. The X-ray absorption spectroscopy Debye-Waller factors of an iron compound and of met-myoglobin as a function of temperature. Eur. Biophys. J. 2001, 30, 393–403. [Google Scholar]
- Wellenreuther, G.; Parthasarathy, V.; Meyer-Klaucke, W. Towards a black-box for biological EXAFS data analysis. II. Automatic BioXAS Refinement and Analysis (ABRA). J. Synchrotron Radiat. 2010, 17, 25–35. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Yuan, L.; Zhang, M.; Zhou, W. Viscoelastic interfacial properties of compatibilized poly(ε-caprolactone)/polylactide blend. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 756–765. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity Auflage, 2nd ed.; John Wiley & Sons, Ltd: Hoboken, NJ, USA; Academic Press: London, UK, 1982. [Google Scholar]
- Zhang, X.; Zhang, F.; Chan, K.-Y. Synthesis of titania–silica mixed oxide mesoporous materials, characterization and photocatalytic properties. Appl. Catal. A Gen. 2005, 284, 193–198. [Google Scholar] [CrossRef]
- Van Grieken, R.; Aguado, J.; López-Muñoz, M.J.; Marugán, J. Sol-Gel Titania and Titania-Silica Mixed Oxides Photocatalysts. Solid State Phenom. 2010, 162, 221–238. [Google Scholar] [CrossRef]
- Kosuge, K. Titanium-Containing Porous Silica Prepared by a Modified Sol−Gel Method. J. Phys. Chem. B 1999, 103, 3563–3569. [Google Scholar] [CrossRef]






















| Samples | TTIP (mol) | TEOS (mol) | C2H5OH (mol) | HCl/NH4OH (mol) | H2O (mol) |
|---|---|---|---|---|---|
| SiO2 | - | 0.120 | 1.889 | 0.060 b | 0.440 |
| Ti50Si50 pH 8.0 | 0.011 | 0.109 | 1.889 | 0.025 b | 0.440 |
| Ti50Si50pH 9.0 | 0.011 | 0.109 | 1.889 | 0.060 b | 0.440 |
| Ti50Si50 pH 10.0 | 0.011 | 0.109 | 1.889 | 0.075 b | 0.440 |
| TiO2 | 0.120 | - | 4.293 | 0.081 a | 0.702 |
| Samples | TTIP (mol) | TEOS (mol) | C2H5OH (mol) | NH4OH/HCl (mol) | H2O (mol) |
|---|---|---|---|---|---|
| SiO2 | - | 0.120 | 1.889 | 0.060 b | 0.440 |
| Ti70Si30 | 0.020 | 0.100 | 1.889 | 0.060 b | 0.440 |
| Ti50Si50 | 0.011 | 0.109 | 1.889 | 0.060 b | 0.440 |
| Ti40Si60 | 0.008 | 0.112 | 1.889 | 0.060 b | 0.440 |
| TiO2 | 0.120 | - | 4.293 | 0.081 a | 0.702 |
| Sample | Shell | Bond | ΔE (eV) | CN | R(Å) | σ2 | R-Factor |
|---|---|---|---|---|---|---|---|
| Ti50Si50 pH 8.0 | 1 | Ti-O | 4.11 ± 3.34 | 4.14 ± 0.18 | 1.99 ± 0.02 | 0.0053 ± 0.0013 | 0.0176 |
| 2 | Ti-Si | 1.27 ± 0.44 | 2.79 ± 0.03 | 0.0018 ± 0.0019 | |||
| Ti50Si50 pH 9.0 | 1 | Ti-O | 2.72 ± 2.03 | 4.07 ± 0.12 | 1.83 ± 0.01 | 0.0042 ± 0.0008 | 0.0082 |
| 2 | Ti-Si | 1.00 ± 0.13 | 2.77 ± 0.02 | 0.0020 ± 0.0020 | |||
| Ti50Si50 pH 10.0 | 1 | Ti-O | 6.70 ± 3.29 | 4.08 ± 0.19 | 1.94 ± 0.02 | 0.0049 ± 0.0024 | 0.0158 |
| 2 | Ti-Si | 1.52 ± 0.22 | 2.81 ± 0.03 | 0.0013 ± 0.0031 | |||
| Ti70Si30 | 1 | Ti-O | 6.68 ± 3.23 | 4.15 ± 0.19 | 1.90 ± 0.02 | 0.0051 ± 0.0014 | 0.0198 |
| 2 | Ti-Si | 0.97 ± 0.21 | 2.800 ± 0.04 | 0.0006 ± 0.0050 | |||
| Ti50Si50 | 1 | Ti-O | 2.72 ± 2.03 | 4.07 ± 0.12 | 1.83 ± 0.01 | 0.0042 ± 0.0008 | 0.0082 |
| 2 | Ti-Si | 1.00 ± 0.13 | 2.77 ± 0.02 | 0.0020 ± 0.0020 | |||
| Ti40Si60 | 1 | Ti-O | 6.54 ± 2.27 | 4.09 ± 0.13 | 1.82 ± 0.01 | 0.0048 ± 0.0010 | 0.0103 |
| 2 | Ti-Si | 1.19 ± 0.03 | 2.79 ± 0.03 | 0.0023 ± 0.0017 |
| Sample | Atomic (%) | Atomic Ratio of Ti/Si | |
|---|---|---|---|
| Si | Ti | ||
| SiO2 | 100 | 0 | - |
| Ti50Si50 pH 8.0 | 54.39 | 45.61 | 0.84 |
| Ti50Si50 pH 9.0 | 47.87 | 52.13 | 1.09 |
| Ti50Si50 pH 10.0 | 9.82 | 93.18 | 9.49 |
| Ti70Si30 | 29.79 | 70.21 | 2.36 |
| Ti50Si50 | 47.87 | 52.13 | 1.09 |
| Ti40Si60 | 60.19 | 39.81 | 0.66 |
| TiO2 | 0 | 100 | - |
| Sample | Zetasizer Nano ZS | SEM | |
|---|---|---|---|
| Particle Size, d (nm) | Polydispesity Index, PDI | Value of Average Diameter, dn (nm) | |
| SiO2 | 147.8 | 0.248 | 144.2 ± 11.3 |
| Ti50Si50 pH 8.0 | 639.3 | 0.627 | 41.5 ± 16.7 |
| Ti50Si50 pH 9.0 | 136.2 | 0.239 | 135.4 ± 12.3 |
| Ti50Si50 pH 10.0 | 397.0 | 0.322 | 114.2 ± 24.2 |
| Ti70Si30 | 573.1 | 0.630 | 149.3 ± 15.5 |
| Ti50Si50 | 136.2 | 0.239 | 135.4 ± 12.3 |
| Ti40Si60 | 825.5 | 0.907 | 131.8 ± 13.3 |
| TiO2 | 40.3 | 0.490 | 27.8 ± 6.3 |
| Sample | Specific Surface Area, SBET (m2g−1) | Pore Volume, Vp (cm3g−1) | Mean Pore Diameter, dp (nm) |
|---|---|---|---|
| SiO2 | 116.90 | 1.06 | 51.08 |
| TixSiy pH 8.0 | 177.02 | 0.11 | 2.56 |
| TixSiy pH 9.0 | 225.68 | 0.33 | 5.85 |
| TixSiy pH 10.0 | 62.75 | 0.42 | 27.31 |
| Ti70Si30 | 569.07 | 1.42 | 10.96 |
| Ti50Si50 | 225.68 | 0.33 | 5.85 |
| Ti40Si60 | 68.34 | 0.36 | 21.97 |
| TiO2 | 74.07 | 0.23 | 12.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teamsinsungvon, A.; Ruksakulpiwat, C.; Amonpattaratkit, P.; Ruksakulpiwat, Y. Structural Characterization of Titanium–Silica Oxide Using Synchrotron Radiation X-ray Absorption Spectroscopy. Polymers 2022, 14, 2729. https://doi.org/10.3390/polym14132729
Teamsinsungvon A, Ruksakulpiwat C, Amonpattaratkit P, Ruksakulpiwat Y. Structural Characterization of Titanium–Silica Oxide Using Synchrotron Radiation X-ray Absorption Spectroscopy. Polymers. 2022; 14(13):2729. https://doi.org/10.3390/polym14132729
Chicago/Turabian StyleTeamsinsungvon, Arpaporn, Chaiwat Ruksakulpiwat, Penphitcha Amonpattaratkit, and Yupaporn Ruksakulpiwat. 2022. "Structural Characterization of Titanium–Silica Oxide Using Synchrotron Radiation X-ray Absorption Spectroscopy" Polymers 14, no. 13: 2729. https://doi.org/10.3390/polym14132729
APA StyleTeamsinsungvon, A., Ruksakulpiwat, C., Amonpattaratkit, P., & Ruksakulpiwat, Y. (2022). Structural Characterization of Titanium–Silica Oxide Using Synchrotron Radiation X-ray Absorption Spectroscopy. Polymers, 14(13), 2729. https://doi.org/10.3390/polym14132729