Synthetic Route to Conjugated Donor–Acceptor Polymer Brushes via Alternating Copolymerization of Bifunctional Monomers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Procedures
2.3.1. Synthesis of Monomers
3-(4-Ethenylphenyl)thiophene (St-D)
N-(benzo[c][1,2,5]thiadiazol-5-yl)pyrrole-2,5-dione (Ma-A)N
2.3.2. Substrate Preparation
2.3.3. APTES Deposition for the Formation of the Initiator Monolayer
2.3.4. Initiator Monolayer for Surface-Initiated RAFT Polymerization
2.3.5. Surface-Initiated RAFT Polymerization
2.3.6. Initiator Monolayer for Surface-Initiated Metal-Free ATRP Polymerization
2.3.7. Surface-Initiated Metal-Free ATRP Polymerization
2.3.8. Oxidative Polymerization
2.3.9. Change of Grafting Density
3. Results and Discussion
3.1. Synthesis of Donor and Acceptor Monomers
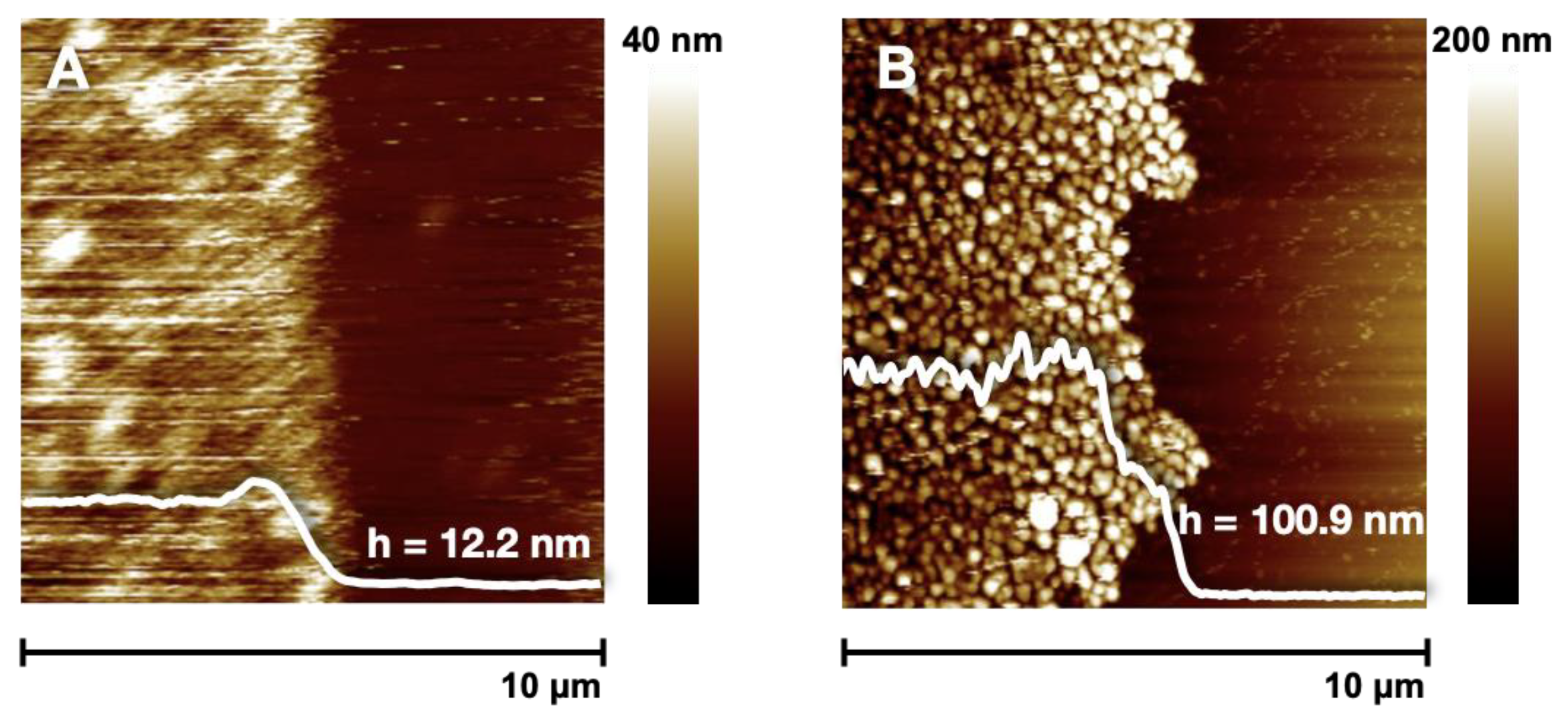
3.2. Synthesis of D–A Polymer Brushes via Surface-Initiated RAFT Polymerization
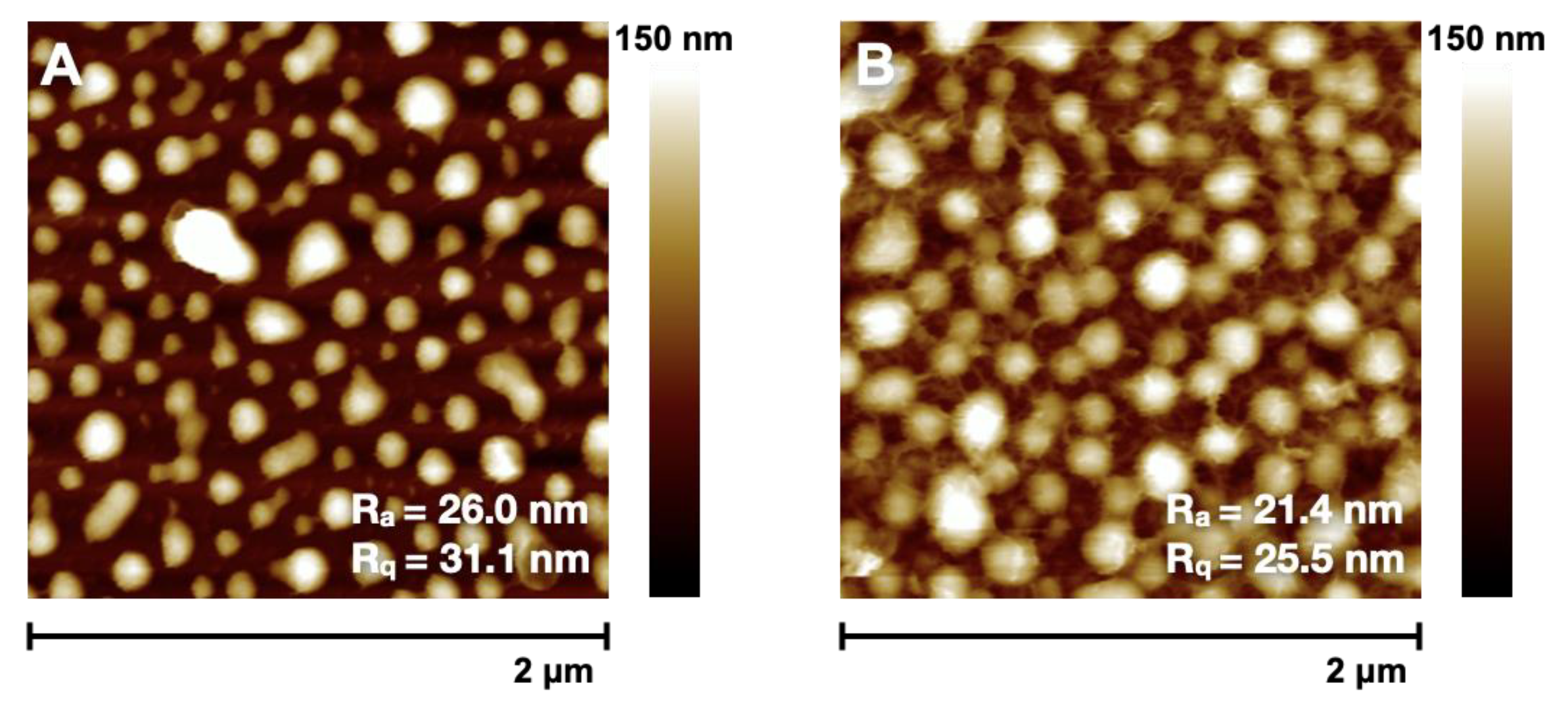
3.3. Changing of Grafting Density
3.4. Synthesis of D–A Polymer Brushes via Metal-Free ATRP
3.5. Oxidative Self-Template Polymerization of Polymer Brushes
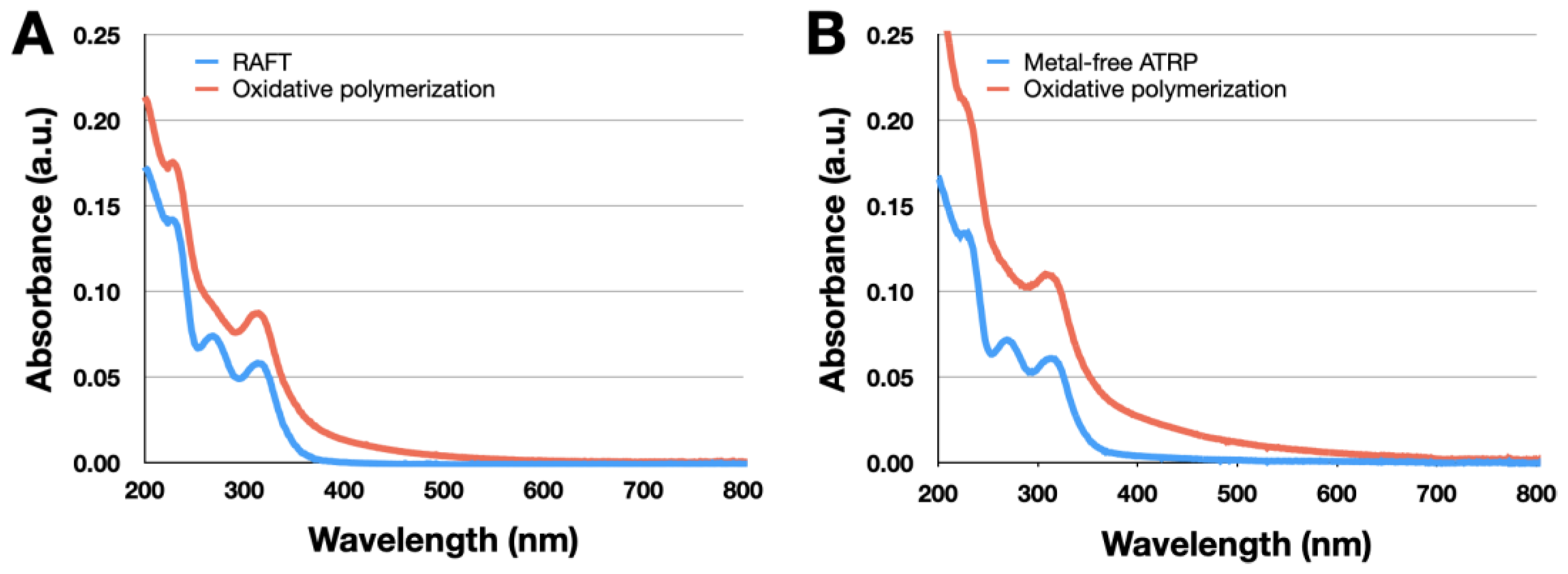
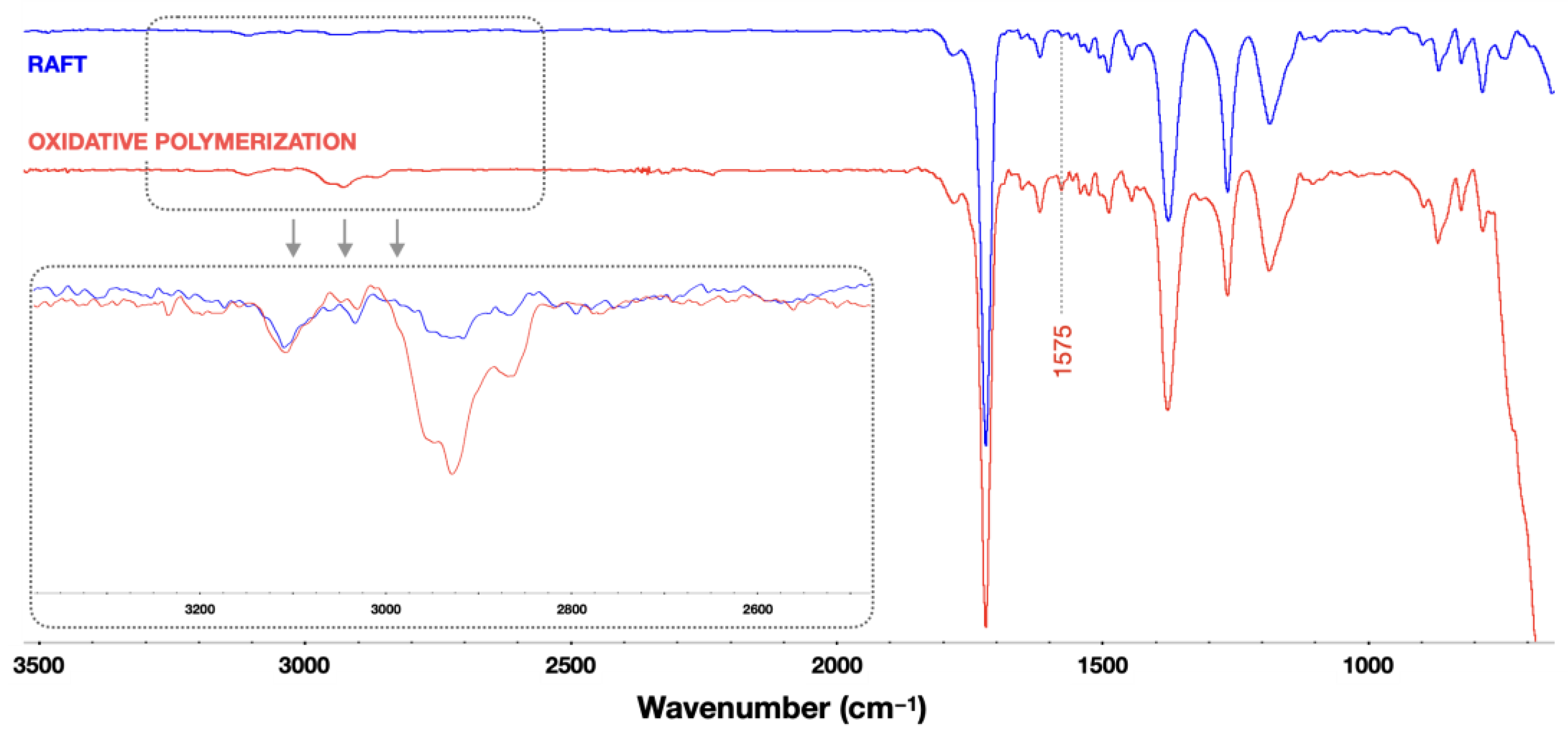
3.6. Spectroscopic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wan, J.; Xia, Y.; Fang, J.; Zhang, Z.; Xu, B.; Wang, J.; Ai, L.; Song, W.; Hui, K.N.; Fan, X.; et al. Solution-Processed Transparent Conducting Electrodes for Flexible Organic Solar Cells with 16.61% Efficiency. Nano-Micro Lett. 2021, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, T.; Zhu, Q.; Zhang, X.; Ethiraj, A.S.; Geng, W.M.; Geng, H.Z. High-Performance Transparent Pedot: Pss/Cnt Films for Oleds. Nanomaterials 2021, 11, 2067. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Li, B.; Cheng, J.; Guan, Q.; Wang, Z.; Ni, W.; Li, C.; Liu, H.; Wang, B. Twisted Yarns for Fiber-Shaped Supercapacitors Based on Wetspun PEDOT:PSS Fibers from Aqueous Coagulation. J. Mater. Chem. A 2016, 4, 11616–11624. [Google Scholar] [CrossRef]
- Bhadra, J.; Al-Thani, N.J.; Madi, N.K.; Al-Maadeed, M.A. High Performance Sulfonic Acid Doped Polyaniline–Polystyrene Blend Ammonia Gas Sensors. J. Mater. Sci. Mater. Electron. 2016, 27, 8206–8216. [Google Scholar] [CrossRef]
- Hofmann, A.I.; Östergren, I.; Kim, Y.; Fauth, S.; Craighero, M.; Yoon, M.H.; Lund, A.; Müller, C. All-Polymer Conducting Fibers and 3D Prints via Melt Processing and Templated Polymerization. ACS Appl. Mater. Interfaces 2020, 12, 8713–8721. [Google Scholar] [CrossRef]
- Wibowo, A.; Vyas, C.; Cooper, G.; Qulub, F.; Suratman, R.; Mahyuddin, A.I.; Dirgantara, T.; Bartolo, P. 3D Printing of Polycaprolactone-Polyaniline Electroactive Scaffolds for Bone Tissue Engineering. Materials 2020, 13, 512. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.; Ganapathy, V.; Kim, Y.H.; Song, K.D.; Park, H.G.; Jun, Y.; Yoo, P.J.; Park, J.H. Nanopatterned Conductive Polymer Films as a Pt, TCO-Free Counter Electrode for Low-Cost Dye-Sensitized Solar Cells. Nanoscale 2013, 5, 7838–7843. [Google Scholar] [CrossRef]
- Anderson, T.E.; Culver, E.W.; Almyahi, F.; Dastoor, P.C.; Rasmussen, S.C. Poly(2,3-Dihexylthieno[3,4-b]Pyrazine-Alt-2,3-Dihexylquinoxaline): Processible, Low-Bandgap, Ambipolar-Acceptor Frameworks via Direct Arylation Polymerization. Synlett 2018, 29, 2542–2546. [Google Scholar] [CrossRef] [Green Version]
- Havinga, E.E.; ten Hoeve, W.; Wynberg, H. Alternate Donor-Acceptor Small-Band-Gap Semiconducting Polymers; Polysquaraines and Polycroconaines. Synth. Met. 1993, 55, 299–306. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Zhang, J.; Li, Y. Low Bandgap Polymers by Copolymerization of Thiophene with Benzothiadiazole. Macromol. Rapid Commun. 2009, 30, 45–51. [Google Scholar] [CrossRef]
- Ajayaghosh, A. Donor-Acceptor Type Low Band Gap Polymers: Polysquaraines and Related Systems. Chem. Soc. Rev. 2003, 32, 181–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.Z.; Cheon, H.J.; Choi, C.; Yoon, S.; Kang, M.; Cho, J.; Jang, Y.H.; Kwon, S.K.; Chung, D.S.; Kim, Y.H. Molecular Engineering of a Donor-Acceptor Polymer to Realize Single Band Absorption toward a Red-Selective Thin-Film Organic Photodiode. ACS Appl. Mater. Interfaces 2019, 11, 28106–28114. [Google Scholar] [CrossRef] [PubMed]
- Steckler, T.T.; Henriksson, P.; Mollinger, S.; Lundin, A.; Salleo, A.; Andersson, M.R. Very Low Band Gap Thiadiazoloquinoxaline Donor-Acceptor Polymers as Multi-Tool Conjugated Polymers. J. Am. Chem Soc. 2014, 136, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Cendra, C.; Balhorn, L.; Zhang, W.; O’Hara, K.; Bruening, K.; Tassone, C.J.; Steinrück, H.G.; Liang, M.; Toney, M.F.; McCulloch, I.; et al. Unraveling the Unconventional Order of a High-Mobility Indacenodithiophene-Benzothiadiazole Copolymer. ACS Macro Lett. 2021, 10, 1306–1314. [Google Scholar] [CrossRef]
- Li, G.; Xie, Z.; Wang, Q.; Chen, X.; Zhang, Y.; Wang, X. Asymmetric Acceptor–Donor–Acceptor Polymers with Fast Charge Carrier Transfer for Solar Hydrogen Production. Chem.-A Eur. J. 2021, 27, 939–943. [Google Scholar] [CrossRef]
- Hedström, S.; Henriksson, P.; Wang, E.; Andersson, M.R.; Persson, P. Light-Harvesting Capabilities of Low Band Gap Donor-Acceptor Polymers. Phys. Chem. Chem. Phys. 2014, 16, 24853–24865. [Google Scholar] [CrossRef]
- Holliday, S.; Li, Y.; Luscombe, C.K. Recent Advances in High Performance Donor-Acceptor Polymers for Organic Photovoltaics. Prog. Polym. Sci. 2017, 70, 34–51. [Google Scholar] [CrossRef]
- Kim, M.; Ryu, S.U.; Park, S.A.; Choi, K.; Kim, T.; Chung, D.; Park, T. Donor–Acceptor-Conjugated Polymer for High-Performance Organic Field-Effect Transistors: A Progress Report. Adv. Funct. Mater. 2020, 30, 1904545. [Google Scholar] [CrossRef]
- Sonar, P.; Williams, E.L.; Singh, S.P.; Dodabalapur, A. Thiophene-Benzothiadiazole-Thiophene (D-A-D) Based Polymers: Effect of Donor/Acceptor Moieties Adjacent to D-A-D Segment on Photophysical and Photovoltaic Properties. J. Mater. Chem. 2011, 21, 10532–10541. [Google Scholar] [CrossRef]
- Du, Z.; Bao, X.; Andersen, C.P.; Didriksen, C.B.; Wang, J.; Lin, M.C.; Cao, Z.; Yu, D. Fluorination on Electron-Deficient Units of Benzothiadiazole-Based Donor-Acceptor Conjugated Polymers for Novel Fullerene-Based Organic Solar Cells. Sol. Energy 2021, 220, 864–872. [Google Scholar] [CrossRef]
- Yuen, J.D.; Wudl, F. Strong Acceptors in Donor-Acceptor Polymers for High Performance Thin Film Transistors. Energy Environ. Sci. 2013, 6, 392–406. [Google Scholar] [CrossRef]
- Bhat, G.; Liu, Q.; Kielar, M.; Hamada, Y.; Michinobu, T.; Sah, P.; Ko Kyaw, A.K.; Pandey, A.K.; Sonar, P. Energy-Level Manipulation in Novel Indacenodithiophene-Based Donor-Acceptor Polymers for Near-Infrared Organic Photodetectors. ACS Appl. Mater. Interfaces 2021, 13, 29866–29875. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.; Park, H.; Kim, T.; Hwang, S.H.; Seo, D.; Chung, T.D.; Choi, T.L. Universal Suzuki-Miyaura Catalyst-Transfer Polymerization for Precision Synthesis of Strong Donor/Acceptor-Based Conjugated Polymers and Their Sequence Engineering. J. Am. Chem Soc. 2021, 143, 11180–11190. [Google Scholar] [CrossRef] [PubMed]
- Jellison, J.L.; Lee, C.-H.; Zhu, X.; Wood, J.D.; Plunkett, K.N. Electron Acceptors Based on an All-Carbon Donor-Acceptor Copolymer. Angew. Chem. 2012, 124, 12487–12490. [Google Scholar] [CrossRef]
- Wakioka, M.; Torii, N.; Saito, M.; Osaka, I.; Ozawa, F. Donor-Acceptor Polymers Containing 4,8-Dithienylbenzo[1,2-b:4,5-b′]Dithiophene via Highly Selective Direct Arylation Polymerization. ACS Appl. Polym. Mater. 2021, 3, 830–836. [Google Scholar] [CrossRef]
- Krebs, F.C.; Nyberg, R.B.; Jørgensen, M. Influence of Residual Catalyst on the Properties of Conjugated Polyphenylenevinylene Materials: Palladium Nanoparticles and Poor Electrical Performance. Chem. Mater. 2004, 16, 1313–1318. [Google Scholar] [CrossRef]
- Garrett, C.E.; Prasad, K. The Art of Meeting Palladium Specifications in Active Pharmaceutical Ingredients Produced by Pd-Catalyzed Reactions. Adv. Synth. Catal. 2004, 346, 889–900. [Google Scholar] [CrossRef]
- Patel, D.G.; Feng, F.; Ohnishi, Y.Y.; Abboud, K.A.; Hirata, S.; Schanze, K.S.; Reynolds, J.R. It Takes More than an Imine: The Role of the Central Atom on the Electron-Accepting Ability of Benzotriazole and Benzothiadiazole Oligomers. J. Am. Chem Soc. 2012, 134, 2599–2612. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xia, K.; Hou, Y.; Zhang, Q.; Ye, Z.; Lu, J. Designing Flexible, Smart and Self-Sustainable Supercapacitors for Portable/Wearable Electronics: From Conductive Polymers. Chem. Soc. Rev. 2021, 50, 12702–12743. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Han, Y. Inhibition of Dewetting of Thin Polymer Films. Prog. Mater. Sci. 2012, 57, 947–979. [Google Scholar] [CrossRef]
- Whiting, G.L.; Snaith, H.J.; Khodabakhsh, S.; Andreasen, J.W.; Breiby, D.W.; Nielsen, M.M.; Greenham, N.C.; Friend, R.H.; Huck, W.T.S. Enhancement of Charge-Transport Characteristics in Polymeric Films Using Polymer Brushes. Nano Lett. 2006, 6, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Szuwarzyński, M.; Wolski, K.; Kruk, T.; Zapotoczny, S. Macromolecular Strategies for Transporting Electrons and Excitation Energy in Ordered Polymer Layers. Prog. Polym. Sci. 2021, 121, 101433. [Google Scholar] [CrossRef]
- Ponder, J.F.; Gregory, S.A.; Atassi, A.; Menon, A.K.; Lang, A.W.; Savagian, L.R.; Reynolds, J.R.; Yee, S.K. Significant Enhancement of the Electrical Conductivity of Conjugated Polymers by Post-Processing Side Chain Removal. J. Am. Chem Soc. 2022, 144, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Szuwarzyński, M.; Wolski, K.; Zapotoczny, S. Enhanced Stability of Conductive Polyacetylene in Ladder-like Surface-Grafted Brushes. Polym. Chem. 2016, 7, 5664–5670. [Google Scholar] [CrossRef]
- Wolski, K.; Gruszkiewicz, A.; Zapotoczny, S. Conductive Polythiophene-Based Brushes Grafted from an ITO Surface via a Self-Templating Approach. Polym. Chem. 2015, 6, 7505–7513. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.A. Surface-Initiated Controlled Radical Polymerization: State-of-the-Art, Opportunities, and Challenges in Surface and Interface Engineering with Polymer Brushes. Chem. Rev. 2017, 117, 1105–1318. [Google Scholar] [CrossRef] [Green Version]
- Ritsema van Eck, G.C.; Chiappisi, L.; de Beer, S. Fundamentals and Applications of Polymer Brushes in Air. ACS Appl. Polym. Mater. 2022, 4, 3062–3087. [Google Scholar] [CrossRef]
- Wolski, K.; Gruszkiewicz, A.; Wytrwal-Sarna, M.; Bernasik, A.; Zapotoczny, S. The Grafting Density and Thickness of Polythiophene-Based Brushes Determine the Orientation, Conjugation Length and Stability of the Grafted Chains. Polym. Chem. 2017, 8, 6250–6262. [Google Scholar] [CrossRef]
- Koler, A.; Krajnc, P. Surface Modification of Hypercrosslinked Vinylbenzyl Chloride PolyHIPEs by Grafting via RAFT. Macromol. Chem. Phys. 2021, 222, 2000381. [Google Scholar] [CrossRef]
- Wei, W.; Balamurugan, A.; Dwyer, J.H.; Gopalan, P. Substrate-Independent Approach to Dense Cleavable Polymer Brushes by Nitroxide-Mediated Polymerization. ACS Macro Lett. 2018, 7, 100–104. [Google Scholar] [CrossRef]
- Wolski, K.; Szuwarzyński, M.; Kopeć, M.; Zapotoczny, S. Ordered Photo- and Electroactive Thin Polymer Layers. Eur. Polym. J. 2015, 65, 155–170. [Google Scholar] [CrossRef]
- Alonzi, M.; Lanari, D.; Marrocchi, A. Synthesis of Polymeric Semiconductors by a Surface-Initiated Approach. RSC Adv. 2013, 3, 23909. [Google Scholar] [CrossRef]
- Saito, R. Combination of Template Polymerization and Atom Transfer Radical Polymerization: Strategy for Synthesis of Specifically Structural Polymers. Polymer 2008, 49, 2625–2631. [Google Scholar] [CrossRef] [Green Version]
- Grobelny, A.; Grobelny, A.; Zapotoczny, S. Precise Stepwise Synthesis of Donor-Acceptor Conjugated Polymer Brushes Grafted from Surfaces. Int. J. Mol. Sci. 2022, 23, 6162. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Ghosh, S.; Paul, S.; Khan, M.E.H.; De, P. Styrene-Maleimide/Maleic Anhydride Alternating Copolymers: Recent Advances and Future Perspectives. Macromol. Rapid Commun. 2021, 42, 2100501. [Google Scholar] [CrossRef]
- Hai, T.A.P.; Matsukuma, H.; Sugimoto, R. Grafting Poly(3-Hexylthiophene) to the Surface of Polypropylene Using Oxidative Polymerization. Polymer 2017, 121, 247–255. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization-A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Choi, J.; Schattling, P.; Jochum, F.D.; Pyun, J.; Char, K.; Theato, P. Functionalization and Patterning of Reactive Polymer Brushes Based on Surface Reversible Addition and Fragmentation Chain Transfer Polymerization. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4010–4018. [Google Scholar] [CrossRef]
- Benetti, E.M.; Reimhult, E.; de Bruin, J.; Zapotoczny, S.; Textor, M.; Vancso, G.J. Poly(methacrylic acid) Grafts Grown from Designer Surfaces: The Effect of Initiator Coverage on Polymerization Kinetics, Morphology, and Properties. Macromolecules 2009, 42, 1640–1647. [Google Scholar] [CrossRef]
- Narupai, B.; Page, Z.A.; Treat, N.J.; McGrath, A.J.; Pester, C.W.; Discekici, E.H.; Dolinski, N.D.; Meyers, G.F.; Read de Alaniz, J.; Hawker, C.J. Simultaneous Preparation of Multiple Polymer Brushes under Ambient Conditions Using Microliter Volumes. Angew. Chem. 2018, 130, 13621–13626. [Google Scholar] [CrossRef]
- Andersson, M.R.; Selse, D.; Berggren, M.; Jaervinen, H.; Hjertberg, T.; Inganaes, O.; Wennerstroem, O.; Oesterholm, J.-E. Regioselective polymerization of 3-(4-octylphenyl)thiophene with FeCl3. Macromolecules 1994, 27, 6503–6506. [Google Scholar] [CrossRef]
- Grześ, G.; Wolski, K.; Uchacz, T.; Bała, J.; Louis, B.; Scheblykin, I.G.; Zapotoczny, S. Ladder-like Polymer Brushes Containing Conjugated Poly(Propylenedioxythiophene) Chains. Int. J. Mol. Sci. 2022, 23, 5886. [Google Scholar] [CrossRef] [PubMed]




| Step | Thickness (nm) | Contact Angle (°) |
|---|---|---|
| APTES | 1.9 ± 0.1 | 44 ± 1 |
| CTA agent | 3.2 ± 0.1 | 64 ± 1 |
| RAFT polymerization | 34.4 ± 0.6 | 69 ± 1 |
| Oxidative polymerization | 41.2 ± 2.0 | 113 ± 1 |
| %C | %N | %O | %S | S-C: S-N | N:S | ||
|---|---|---|---|---|---|---|---|
| S-C | S-N | ||||||
| Experimental | 77.1 ± 0.4 | 8.7 ± 0.4 | 8.8 ± 0.1 | 2.6 ± 0.1 | 2.7 ± 0.1 | 0.96 | 1.61 |
| Theoretical | 75.9 | 10.3 | 6.9 | 3.4 | 3.4 | 1.00 | 1.50 |
| Polymerization Time (h) | Brush Thickness Based on Spectroscopic Ellipsometry (nm) | Brush Thickness Based on AFM (nm) |
|---|---|---|
| 2 | 9.6 ± 0.5 | 12.2 ± 0.8 |
| 4 | 34.3 ± 0.7 | 40.8 ± 0.5 |
| 6 | 41.6 ± 2.1 | 49.8 ± 0.6 |
| 10 | 52.4 ± 2.0 | 64.9 ± 0.7 |
| 12 | 76.8 ± 2.2 | 72.6 ± 0.6 |
| 24 | 96.2 ± 1.4 | 100.9 ± 2.4 |
| 96 | - | 200.1 ± 2.4 |
| Grafting Density | Brush Thickness Based on Spectroscopic Ellipsometry (nm) | Brush Thickness Based on AFM (nm) | Roughness | |
|---|---|---|---|---|
| Rq | Ra | |||
| 100% | 34.3 ± 0.7 | 40.8 ± 0.5 | 19.5 | 15.9 |
| 80% | 41.9 ± 0.6 | 48.3 ± 0.7 | 20.1 | 16.6 |
| 70% | 14.5 ± 1.1 | 18.6 ± 1.4 | 31.9 | 26.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grobelny, A.; Lorenc, K.; Skowron, Ł.; Zapotoczny, S. Synthetic Route to Conjugated Donor–Acceptor Polymer Brushes via Alternating Copolymerization of Bifunctional Monomers. Polymers 2022, 14, 2735. https://doi.org/10.3390/polym14132735
Grobelny A, Lorenc K, Skowron Ł, Zapotoczny S. Synthetic Route to Conjugated Donor–Acceptor Polymer Brushes via Alternating Copolymerization of Bifunctional Monomers. Polymers. 2022; 14(13):2735. https://doi.org/10.3390/polym14132735
Chicago/Turabian StyleGrobelny, Anna, Karolina Lorenc, Łucja Skowron, and Szczepan Zapotoczny. 2022. "Synthetic Route to Conjugated Donor–Acceptor Polymer Brushes via Alternating Copolymerization of Bifunctional Monomers" Polymers 14, no. 13: 2735. https://doi.org/10.3390/polym14132735
APA StyleGrobelny, A., Lorenc, K., Skowron, Ł., & Zapotoczny, S. (2022). Synthetic Route to Conjugated Donor–Acceptor Polymer Brushes via Alternating Copolymerization of Bifunctional Monomers. Polymers, 14(13), 2735. https://doi.org/10.3390/polym14132735








