An Overview of In Vitro Drug Release Methods for Drug-Eluting Stents
Abstract
1. Introduction
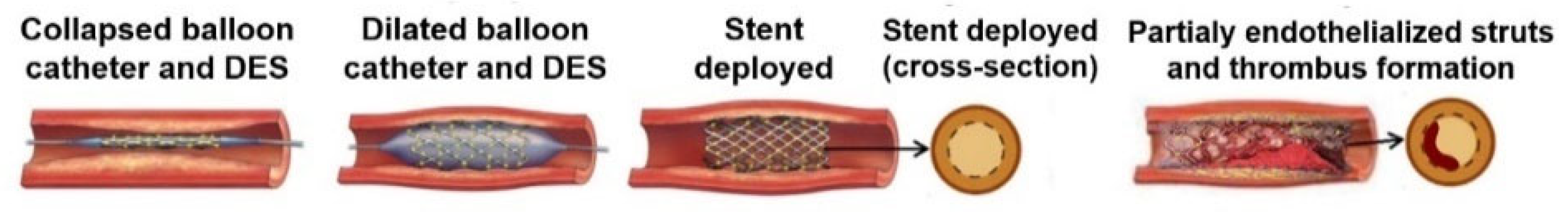
1.1. Evolution of Cardiovascular Stents
1.2. Laboratory Methods for the Development of DES
2. Different Geometrical Models of Drug-Eluting Stents
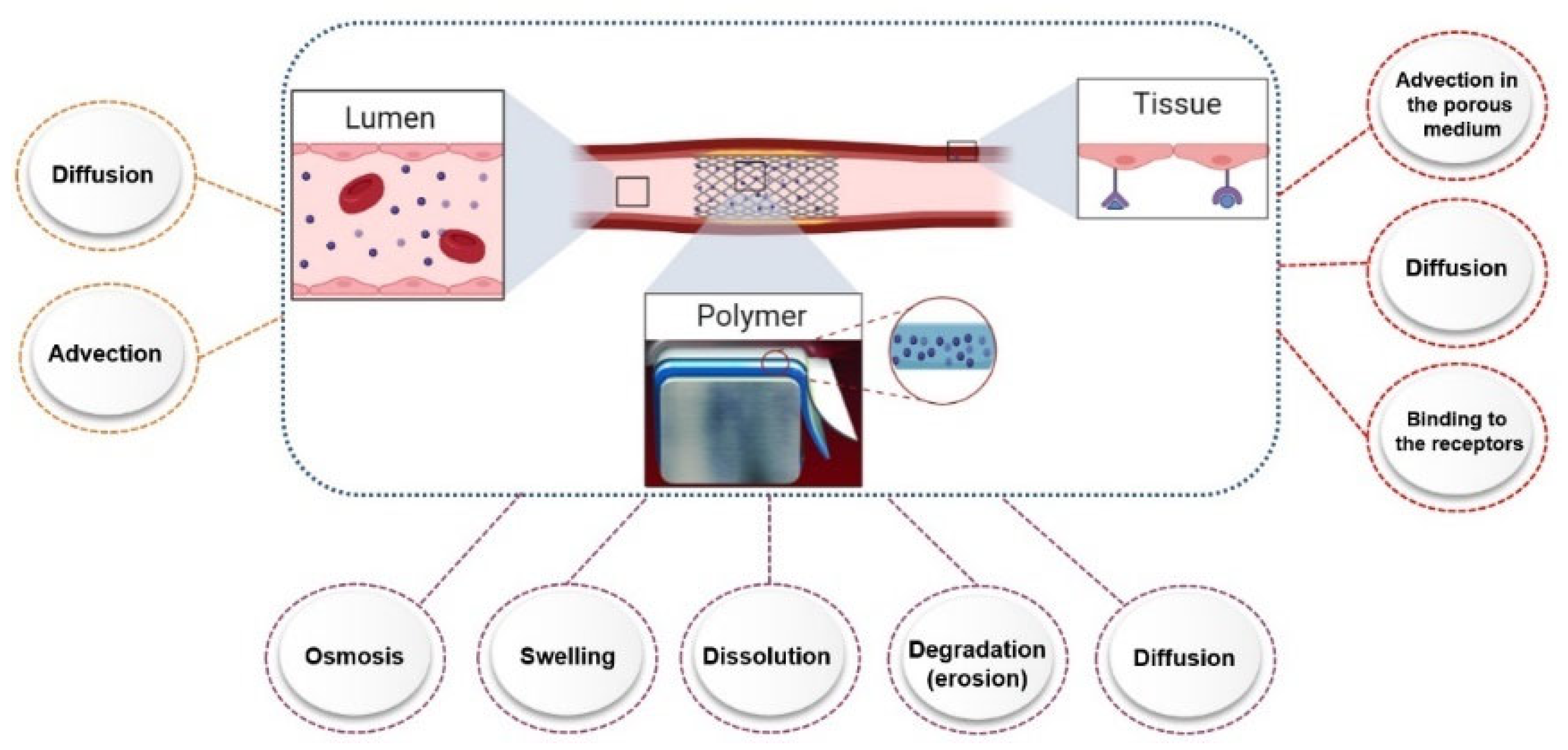
3. Factors Affecting Drug Release from Drug-Eluting Stents (In Vitro)
3.1. Release Compartment
3.1.1. Artificial Blood
3.1.2. Artificial Tissue
3.2. Release Test Methods
3.2.1. Static Condition
3.2.2. Dynamic Condition
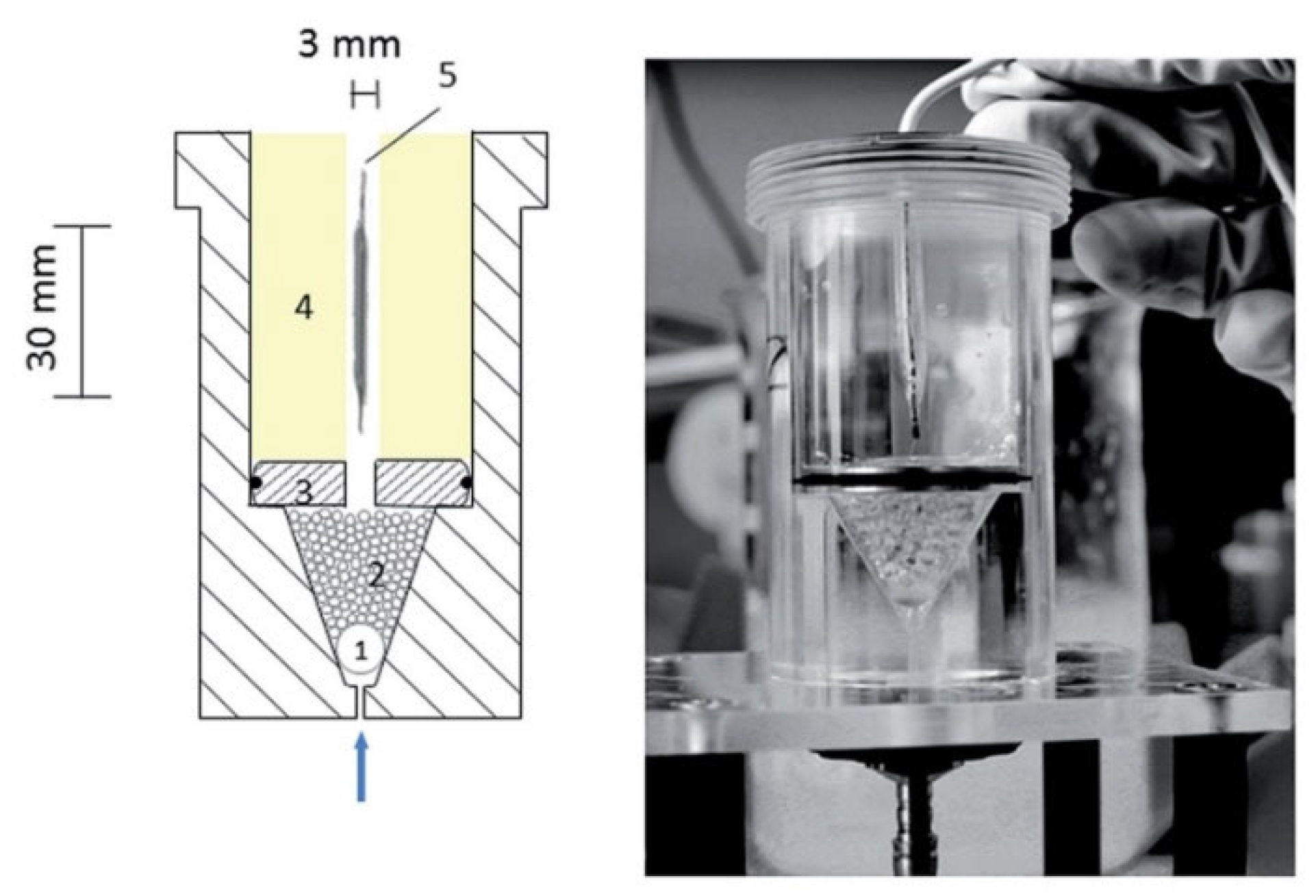
3.3. Apparatus for Release Testing
3.4. Analytical Tools to Determine Drug Release
3.4.1. High-Performance Liquid Chromatography
3.4.2. UV-Vis-Detector
3.4.3. Fluorescence Detector
3.4.4. Raman Spectroscopy
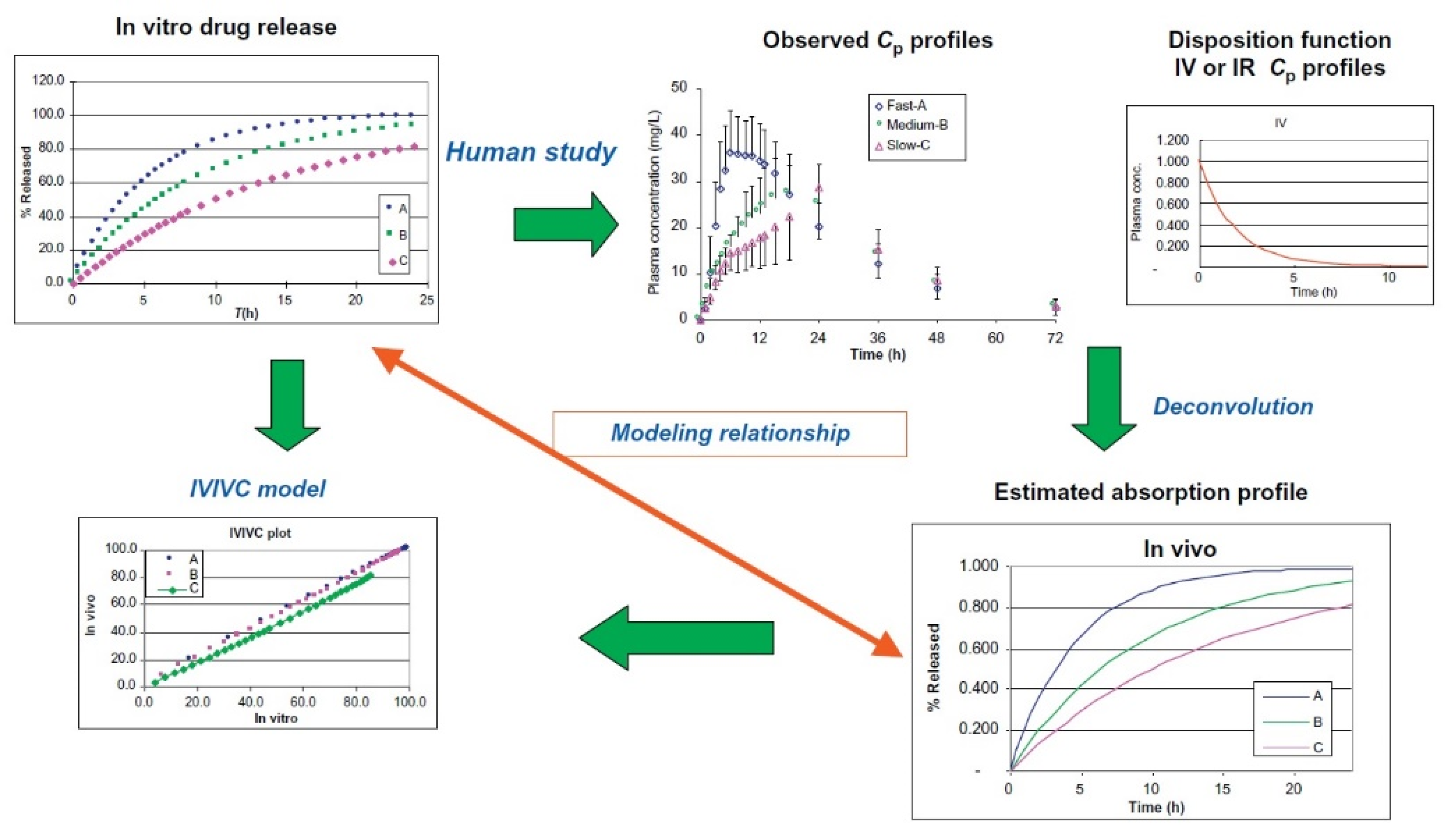
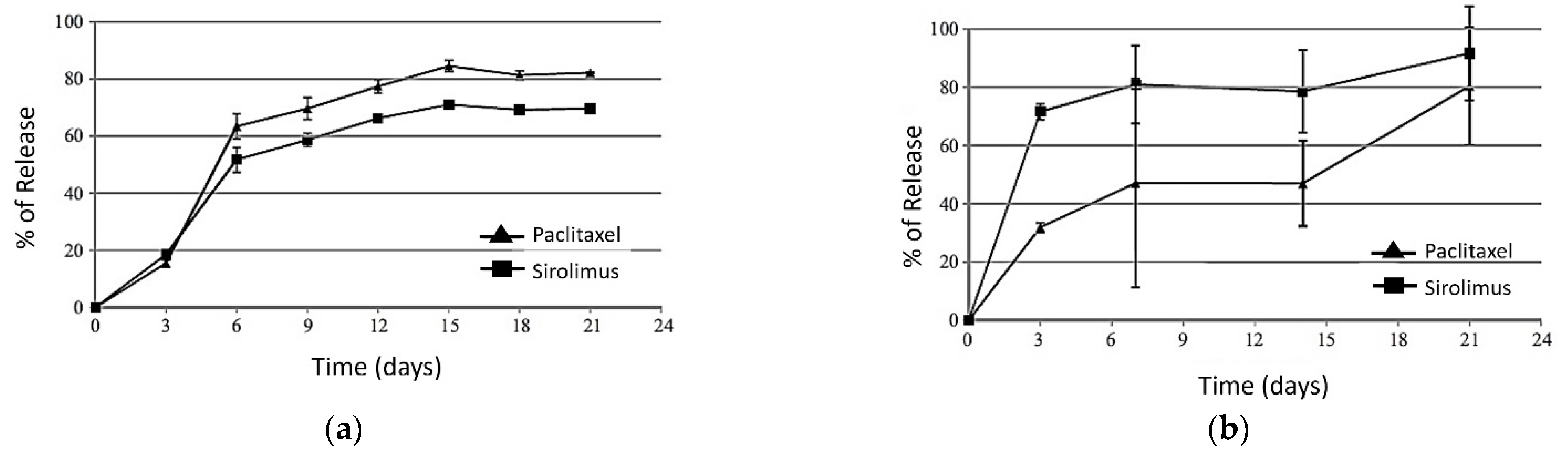
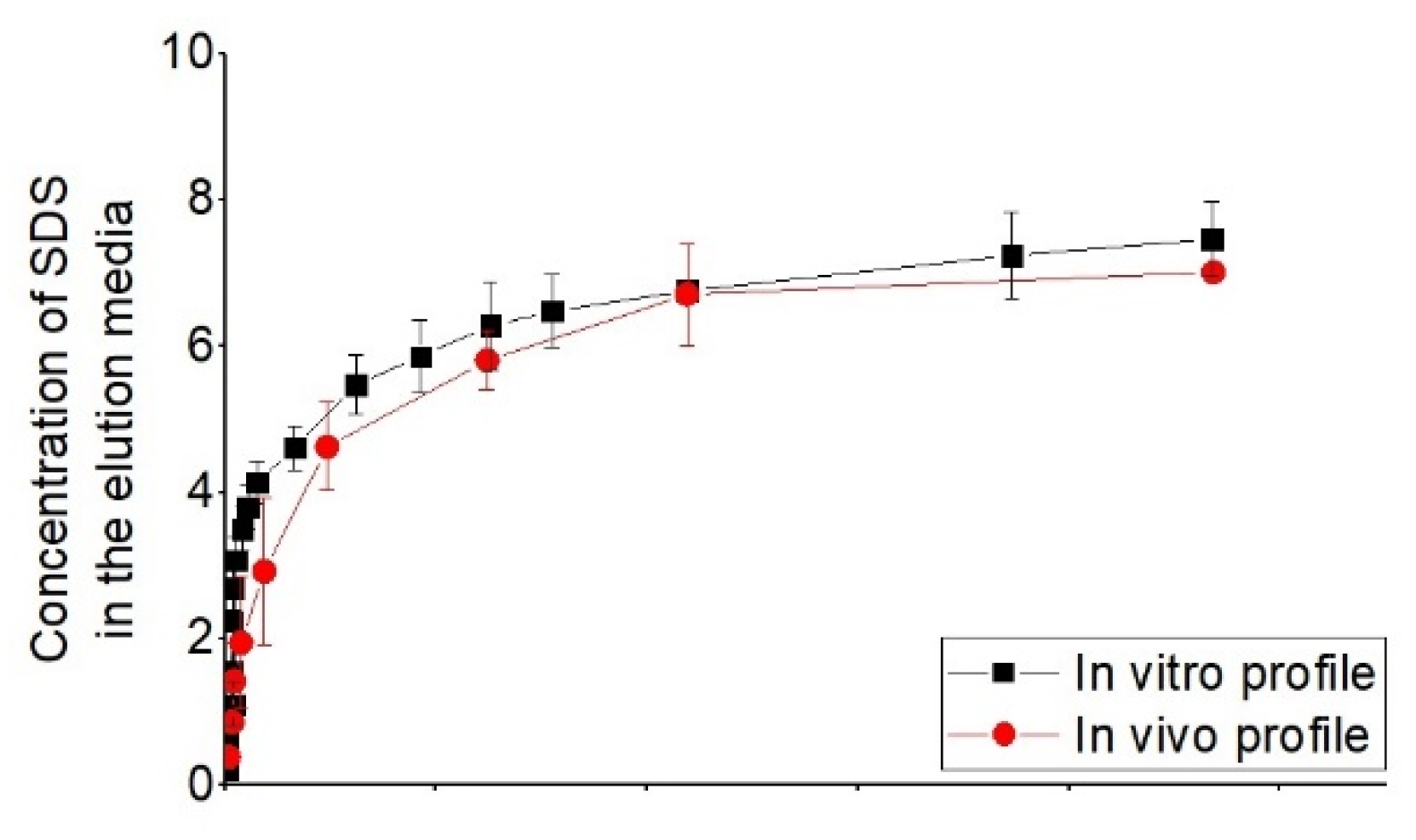
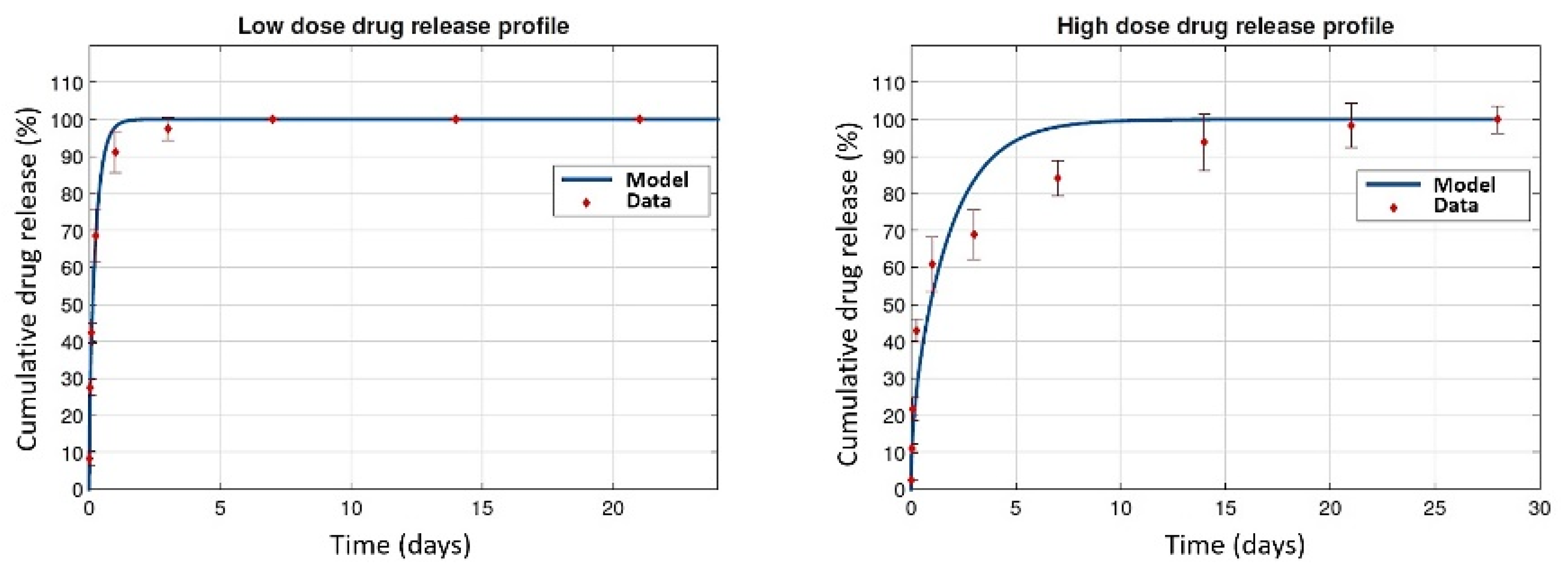
3.5. In Vitro–In Vivo Correlations
4. Study Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, P.D.; Awan, M. Stent selection for percutaneous coronary intervention. J. Contin. Cardiol. Educ. 2017, 3, 64–69. [Google Scholar] [CrossRef]
- Ganly, S. Development of a Gene-Eluting Stent for the Treatment of In-Stent Restenosis. Ph.D. Thesis, NUI, Galway, Ireland, 2016. [Google Scholar]
- Yin, R.X.; Yang, D.Z.; Wu, J.Z. Nanoparticle drug- and gene-eluting stents for the prevention and treatment of coronary restenosis. Theranostics 2014, 4, 175–200. [Google Scholar] [CrossRef]
- Kempin, W.; Kaule, S.; Reske, T.; Grabow, N.; Petersen, S.; Nagel, S.; Schmitz, K.P.; Weitschies, W.; Seidlitz, A. In vitro evaluation of paclitaxel coatings for delivery via drug-coated balloons. Eur. J. Pharm. Biopharm. 2015, 96, 322–328. [Google Scholar] [CrossRef]
- Robertson, K.E. Alternative Approaches to the Prevention of Coronary In-Stent Restenosis. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2014. [Google Scholar]
- Zheng, F.; Xiong, W.; Sun, S.; Zhang, P.; Zhu, J.J. Recent advances in drug release monitoring. Nanophotonics 2019, 8, 391–413. [Google Scholar] [CrossRef]
- Hu, T.; Yang, J.; Cui, K.; Rao, Q.; Yin, T.; Tan, L.; Zhang, Y.; Li, Z.; Wang, G. Controlled Slow-Release Drug-Eluting Stents for the Prevention of Coronary Restenosis: Recent Progress and Future Prospects. ACS Appl. Mater. Interfaces 2015, 7, 11695–11712. [Google Scholar] [CrossRef]
- Torrado, J.; Buckley, L.; Durán, A.; Trujillo, P.; Toldo, S.; Valle Raleigh, J.; Abbate, A.; Biondi-Zoccai, G.; Guzmán, L.A. Restenosis, Stent Thrombosis, and Bleeding Complications: Navigating bof polymeric vascular scaffoldetween Scylla and Charybdis. J. Am. Coll. Cardiol. 2018, 71, 1676–1695. [Google Scholar] [CrossRef]
- Ghousifam, N.; Mortazavian, H.; Bhowmick, R.; Vasquez, Y.; Blum, F.D.; Gappa-Fahlenkamp, H. A three-dimensional in vitro model to demonstrate the haptotactic effect of monocyte chemoattractant protein-1 on atherosclerosis-associated monocyte migration. Int. J. Biol. Macromol. 2017, 97, 141–147. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, J.; Gong, L.; Li, X.; Yang, Y.; Luo, Y. Biomedicine & Pharmacotherapy Voltage-gated sodium channel inhibitor reduces atherosclerosis by modulating monocyte/macrophage subsets and suppressing macrophage proliferation. Biomed. Pharmacother. 2019, 120, 109352. [Google Scholar] [CrossRef]
- Iqbal, J.; Chamberlain, J.; Francis, S.E.; Gunn, J. Role of Animal Models in Coronary Stenting. Ann. Biomed. Eng. 2016, 44, 453–465. [Google Scholar] [CrossRef]
- Satuluri, P.; Makam, K.; Vijaykumar, S.; Wasty, N.; Kaid, K. Delayed Stent Deformity Secondary To Unrecognized Ballon Angioplasty behind the Stent Struts. J. Am. Coll. Cardiol. 2019, 73, 2848. [Google Scholar] [CrossRef]
- Liou, K.; Jepson, N.; Cao, C.; Luo, R.; Pala, S.; Ooi, S.Y. Drug-eluting Balloon Versus Second Generation Drug Eluting Stents in the Treatment of In-stent Restenosis: A Systematic Review and Meta-analysis. Heart Lung Circ. 2016, 25, 1184–1194. [Google Scholar] [CrossRef]
- Sun, D.; Zheng, Y.; Yin, T.; Tang, C.; Yu, Q.; Wang, G. Coronary drug-eluting stents: From design optimization to newer strategies. J. Biomed. Mater. Res. Part A 2014, 102, 1625–1640. [Google Scholar] [CrossRef]
- Wang, P.J.; Ferralis, N.; Conway, C.; Grossman, J.C.; Edelman, E.R. Strain-induced accelerated asymmetric spatial degradation of polymeric vascular scaffolds. Proc. Natl. Acad. Sci. USA 2018, 115, 2640–2645. [Google Scholar] [CrossRef]
- Bozsak, F.; Chomaz, J.M.; Barakat, A.I. Modeling the transport of drugs eluted from stents: Physical phenomena driving drug distribution in the arterial wall. Biomech. Model. Mechanobiol. 2014, 13, 327–347. [Google Scholar] [CrossRef]
- Kamberi, M.; Rapoza, R. Stability testing of drug eluting stents. J. Drug Deliv. Sci. Technol. 2016, 35, 58–68. [Google Scholar] [CrossRef]
- Garg, S.; Serruys, P.W. Coronary stents: Current status. J. Am. Coll. Cardiol. 2010, 56, S1–S42. [Google Scholar] [CrossRef]
- Brown, J.; O’Brien, C.C.; Lopes, A.C.; Kolandaivelu, K.; Edelman, E.R. Quantification of thrombus formation in malapposed coronary stents deployed in vitro through imaging analysis. J. Biomech. 2018, 71, 296–301. [Google Scholar] [CrossRef]
- Premer, C.; Kanelidis, A.J.; Hare, J.M.; Schulman, I.H. Rethinking Endothelial Dysfunction as a Crucial Target in Fighting Heart Failure. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Kim, M.C.; Kim, I.S.; Jeong, M.H.; Sim, D.S.; Hong, Y.J.; Kim, J.H.; Ahn, Y.; Cho, J.G.; Park, J.C. Incidence of cardiac death and recurrent stent thrombosis after treatment for angiographically confirmed stent thrombosis. J. Cardiol. 2019, 74, 267–272. [Google Scholar] [CrossRef]
- Kim, S.M.; Park, K.S.; Lih, E.; Hong, Y.J.; Kang, J.H.; Kim, I.H.; Jeong, M.H.; Joung, Y.K.; Han, D.K. Fabrication and characteristics of dual functionalized vascular stent by spatio-temporal coating. J. Acta Biomater. 2016, 38, 143–152. [Google Scholar] [CrossRef]
- Singh, G.; Bs, N.A.S.; Schreiter, S.W. Widowmaker Right Coronary Artery Treated With Drug-Eluting Stent Implantation. JACC Case Rep. 2019, 1, 421. [Google Scholar] [CrossRef]
- Jang, W.J.; Yang, J.H.; Song, Y.B.; Hahn, J.Y.; Chun, W.J.; Oh, J.H.; Kim, W.S.; Lee, Y.T.; Yu, C.W.; Lee, H.J.; et al. Second-generation drug-eluting stenting versus coronary artery bypass grafting for treatment of coronary chronic total occlusion. J. Cardiol. 2019, 73, 432–437. [Google Scholar] [CrossRef]
- Farghadan, A.; Arzani, A. The combined effect of wall shear stress topology and magnitude on cardiovascular mass transport. Int. J. Heat Mass Transf. 2019, 131, 252–260. [Google Scholar] [CrossRef]
- Barakat, A.I. Blood flow and arterial endothelial dysfunction: Mechanisms and implications. Comptes Rendus Phys. 2013, 14, 479–496. [Google Scholar] [CrossRef]
- Lafont, A.; Yang, Y. Magnesium stent scaffolds: DREAMS become reality. Lancet 2016, 387, 3–4. [Google Scholar] [CrossRef]
- Baron, S.J.; Lei, Y.; Chinnakondepalli, K.; Vilain, K.; Magnuson, E.A.; Kereiakes, D.J.; Ellis, S.G.; Stone, G.W.; Cohen, D.J. Economic Outcomes of Bioresorbable Vascular Scaffolds Versus Everolimus-Eluting Stents in Patients Undergoing Percutaneous Coronary Intervention: 1-Year Results from the ABSORB III Trial. JACC Cardiovasc. Interv. 2017, 10, 774–782. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Chen, S.; Yu, D.; Sun, W.; Xin, S. Biodegradable magnesium alloy stents as a treatment for vein graft restenosis. Yonsei Med. J. 2019, 60, 429–439. [Google Scholar] [CrossRef]
- McGinty, S.; Pontrelli, G. A general model of coupled drug release and tissue absorption for drug delivery devices. J. Control. Release 2015, 217, 327–336. [Google Scholar] [CrossRef]
- Rikhtegar, F.; Edelman, E.R.; Olgac, U.; Poulikakos, D.; Kurtcuoglu, V. Drug deposition in coronary arteries with overlapping drug-eluting stents. J. Control. Release 2016, 238, 1–9. [Google Scholar] [CrossRef]
- Bozsak, F.; Gonzalez-Rodriguez, D.; Sternberger, Z.; Belitz, P.; Bewley, T.; Chomaz, J.M.; Barakat, A.I. Optimization of drug delivery by drug-eluting stents. PLoS ONE 2015, 10, e0130182. [Google Scholar] [CrossRef]
- Livingston, M.; Tan, A. Coating Techniques and Release Kinetics of Drug-Eluting Stents. J. Med. Devices 2016, 10, MED-15-1164. [Google Scholar] [CrossRef]
- Pruessmann, K.; Wentzlaff, M.; Schilling, R.; Seidlitz, A. Influence of Dissolution Vessel Geometry and Dissolution Medium on In Vitro Dissolution Behaviour of Triamterene-Coated Model Stents in Different Test Setups. AAPS PharmSciTech 2019, 20, 27. [Google Scholar] [CrossRef]
- Basalus, M.W.Z.; Tandjung, K.; van Houwelingen, K.G.; Stoel, M.G.; de Man, F.H.A.F.; Louwerenburg, J.W.; Saïd, S.A.M.; Linssen, G.C.M.; Kleijne, M.A.W.J.; van der Palen, J.; et al. TWENTE Study: The Real-World Endeavor Resolute Versus Xience V Drug-Eluting Stent Study in Twente: Study design, rationale and objectives. Neth. Heart J. Mon. J. Neth. Soc. Cardiol. Neth. Heart Found. 2010, 18, 360–364. [Google Scholar] [CrossRef][Green Version]
- Master, A.M.; Rodriguez, M.E.; Kenney, M.E.; Oleinick, N.L.; Sen Gupta, A. Delivery of the photosensitizer Pc 4 in PEG–PCL micelles for in vitro PDT studies. J. Pharm. Sci. 2010, 99, 2386–2398. [Google Scholar] [CrossRef]
- Pang, J.; Luan, Y.; Li, F.; Cai, X.; Du, J.; Li, Z. Ibuprofen-loaded poly(lactic-co-glycolic acid) films for controlled drug release. Int. J. Nanomed. 2011, 6, 659–665. [Google Scholar] [CrossRef]
- Steele, T.W.J.; Huang, C.L.; Widjaja, E.; Boey, F.Y.C.; Loo, J.S.C.; Venkatraman, S.S. The effect of polyethylene glycol structure on paclitaxel drug release and mechanical properties of PLGA thin films. Acta Biomater. 2011, 7, 1973–1983. [Google Scholar] [CrossRef]
- Zheng, Q.; Chu, Z.; Li, X.; Kang, H.; Yang, X.; Fan, Y. The effect of fluid shear stress on the in vitro release kinetics of sirolimus from PLGA films. Polymers 2017, 9, 618. [Google Scholar] [CrossRef]
- Vey, E.; Rodger, C.; Booth, J.; Claybourn, M.; Miller, A.F.; Saiani, A. Degradation kinetics of poly(lactic-co-glycolic) acid block copolymer cast films in phosphate buffer solution as revealed by infrared and Raman spectroscopies. Polym. Degrad. Stab. 2011, 96, 1882–1889. [Google Scholar] [CrossRef]
- O’Brien, B.T.; Kolachalama, C.V. Impact of flow pulsatility on arterial drug distribution in stent-based therapy. J. Cont. Rel. 2013, 23, 1–7. [Google Scholar] [CrossRef]
- O’Brien, C.C.; Finch, C.H.; Barber, T.J.; Martens, P.; Simmons, A. Analysis of Drug Distribution from a Simulated Drug-Eluting Stent Strut Using an In Vitro Framework. Ann. Biomed. Eng. 2012, 40, 2687–2696. [Google Scholar]
- Vijayaratnam, P.R.S.; Reizes, J.A.; Barber, T.J. Flow-Mediated Drug Transport from Drug-Eluting Stents is Negligible: Numerical and In-vitro Investigations. Ann. Biomed. Eng. 2019, 47, 878–890. [Google Scholar] [CrossRef]
- Song, J.; Kouidri, S.; Bakir, F. Numerical study on flow topology and hemodynamics in tortuous coronary artery with symmetrical and asymmetrical stenosis. Biocybern. Biomed. Eng. 2021, 41, 142–155. [Google Scholar] [CrossRef]
- Chabi, F. Etude Numérique et Expérimentale du Transfert de Masse, par Advection et Diffusion en Écoulement Pulsé, sur des Stents Actifs; Ecole Nationale Supérieure d’Arts et Métiers—ENSAM: Paris, France, 2016. [Google Scholar]
- Seidlitz, A.; Nagel, S.; Semmling, B.; Grabow, N.; Martin, H.; Senz, V.; Harder, C.; Sternberg, K.; Schmitz, K.-P.; Kroemer, H.K.; et al. Examination of drug release and distribution from drug-eluting stents with a vessel-simulating flow-through cell. Eur. J. Pharm. Biopharm. 2011, 78, 36–48. [Google Scholar] [CrossRef]
- Vijayaratnam, P.R.S.; O’Brien, C.C.; Reizes, J.A.; Barber, T.J.; Edelman, E.R. The impact of blood rheology on drug transport in stented arteries: Steady simulations. PLoS ONE 2015, 10, e0128178. [Google Scholar] [CrossRef]
- Abbasnezhad, N.; Zirak, N.; Shirinbayan, M.; Tcharkhtchi, A.; Bakir, F. On the importance of physical and mechanical properties of PLGA films during drug release. J. Drug Deliv. Sci. Technol. 2021, 63, 102446. [Google Scholar] [CrossRef]
- Wang, X.; Venkatraman, S.S.; Boey, F.Y.C.; Loo, J.S.C.; Tan, L.P. Controlled release of sirolimus from a multilayered PLGA stent matrix. Biomaterials 2006, 27, 5588–5595. [Google Scholar] [CrossRef]
- Zirak, N.; Maadani, A.; Salahinejad, E.; Abbasnezhad, N.; Shirinbayan, M. Fabrication, drug delivery kinetics and cell viability assay of PLGA-coated vancomycin-loaded silicate porous microspheres. Ceram. Int. 2022, 48, 48–54. [Google Scholar] [CrossRef]
- Abbasnezhad, N.; Shirinbayan, M.; Chabi, F.; Champmartin, S.; Tcharkhtchi, A.; Bakir, F. Viscoelastic Behavior of Drug-Loaded Polyurethane. Polym 2021, 13, 2608. [Google Scholar] [CrossRef]
- Nelson, F.C.; Stachel, S.J.; Eng, C.P.; Sehgal, S.N. Manipulation of the C (22)-C (27) region of rapamycin: Stability issues and biological implications. Bioorg. Med. Chem. Lett. 1999, 9, 295–300. [Google Scholar] [CrossRef]
- Naseerali, C.P.; Hari, P.R.; Sreenivasan, K. The release kinetics of drug eluting stents containing sirolimus as coated drug: Role of release media. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 709–712. [Google Scholar] [CrossRef]
- Raval, A.; Parikh, J.; Engineer, C. Mechanism of controlled release kinetics from medical devices. Braz. J. Chem. Eng. 2010, 27, 211–225. [Google Scholar] [CrossRef]
- Jelonek, K.; Karpeta, P.; Jaworska, J.; Pastusiak, M.; Włodarczyk, J.; Kasperczyk, J.; Dobrzyński, P. Comparison of extraction methods of sirolimus from polymeric coatings of bioresorbable vascular scaffolds. Mater. Lett. 2018, 214, 220–223. [Google Scholar] [CrossRef]
- Merciadez, M.; Alquier, L.; Mehta, R.; Patel, A.; Wang, A. A novel method for the elution of sirolimus (Rapamycin) in drug-eluting stents. Dissolut. Technol. 2011, 18, 37–42. [Google Scholar] [CrossRef]
- McGinty, S. A decade of modelling drug release from arterial stents. Math. Biosci. 2014, 257, 80–90. [Google Scholar] [CrossRef]
- Neubert, A.; Sternberg, K.; Nagel, S.; Harder, C.; Schmitz, K.; Kroemer, H.K.; Weitschies, W. Development of a vessel-simulating fl ow-through cell method for the in vitro evaluation of release and distribution from drug-eluting stents. J. Control. Release 2008, 130, 2–8. [Google Scholar] [CrossRef]
- Semmling, B.; Nagel, S.; Sternberg, K.; Weitschies, W.; Seidlitz, A. Long-term stable hydrogels for biorelevant dissolution testing of drug-eluting stents. J. Pharm Technol Drug Res. 2013, 2, 19. [Google Scholar] [CrossRef]
- Tzafriri, A.R.; Garcia-Polite, F.; Li, X.; Keating, J.; Balaguer, J.M.; Zani, B.; Bailey, L.; Markham, P.; Kiorpes, T.C.; Carlyle, W.; et al. Defining drug and target protein distributions after stent-based drug release: Durable versus deployable coatings. J. Control. Release 2018, 274, 102–108. [Google Scholar] [CrossRef]
- Bandomir, J.; Kaule, S.; Schmitz, K.P.; Sternberg, K.; Petersen, S.; Kragl, U. Usage of different vessel models in a flow-through cell: In vitro study of a novel coated balloon catheter. RSC Adv. 2015, 5, 11604–11610. [Google Scholar] [CrossRef]
- Martin, D.M.; Boyle, F.J. Drug-eluting stents for coronary artery disease: A review. Med. Eng. Phys. 2011, 33, 148–163. [Google Scholar] [CrossRef]
- Lovich, M.A.; Creel, C.; Hong, K.; Hwang, C.; Edelman, E.R. Carrier proteins determine local pharmacokinetics and arterial distribution of paclitaxel. J. Pharm. Sci. 2001, 90, 1324–1335. [Google Scholar] [CrossRef]
- Semmling, B.; Nagel, S.; Sternberg, K.; Weitschies, W.; Seidlitz, A. Development of Hydrophobized Alginate Hydrogels for the Vessel-Simulating Flow-Through Cell and Their Usage for Biorelevant Drug-Eluting Stent Testing. AAPS PharmSciTech 2013, 14, 1209–1218. [Google Scholar] [CrossRef][Green Version]
- Beier, S.; Ormiston, J.; Webster, M.; Cater, J.; Norris, S.; Medrano-Gracia, P.; Young, A.; Cowan, B. Hemodynamics in Idealized Stented Coronary Arteries: Important Stent Design Considerations. Ann. Biomed. Eng. 2016, 44, 315–329. [Google Scholar] [CrossRef]
- Hwang, C.-W.; Wu, D.; Edelman, E.R. Physiological transport forces govern drug distribution for stent-based delivery. Circulation 2001, 104, 600–605. [Google Scholar] [CrossRef]
- Huang, Y.; Venkatraman, S.S.; Boey, F.Y.C.; Lahti, E.M.; Umashankar, P.R.; Mohanty, M.; Arumugam, S.; Khanolkar, L.; Vaishnav, S. In vitro and in vivo performance of a dual drug-eluting stent (DDES). Biomaterials 2010, 31, 4382–4391. [Google Scholar] [CrossRef]
- Khan, W.; Farah, S.; Nyska, A.; Domb, A.J. Carrier free rapamycin loaded drug eluting stent: In vitro and in vivo evaluation. J. Control. Release 2013, 168, 70–76. [Google Scholar] [CrossRef]
- Abbasnezhad, N.; Shirinbayan, M.; Tcharkhtchi, A.; Bakir, F. In vitro study of drug release from various loaded polyurethane samples and subjected to different non-pulsed flow rates. J. Drug Deliv. Sci. Technol. 2020, 55, 101500. [Google Scholar] [CrossRef]
- Abbasnezhad, N.; Zirak, N.; Shirinbayan, M.; Kouidri, S.; Salahinejad, E.; Tcharkhtchi, A.; Bakir, F. Controlled release from polyurethane films: Drug release mechanisms. J. Appl. Polym. Sci. 2020, 138, 50083. [Google Scholar] [CrossRef]
- Acharya, G.; Park, K. Mechanisms of controlled drug release from drug-eluting stents. Adv. Drug Deliv. Rev. 2006, 58, 387–401. [Google Scholar] [CrossRef]
- Abbasnezhad, N.; Kebdani, M.; Shirinbayan, M.; Champmartin, S.; Tcharkhtchi, A.; Kouidri, S.; Bakir, F. Development of a model based on physical mechanisms for the explanation of drug release: Application to diclofenac release from polyurethane films. Polymers 2021, 13, 1230. [Google Scholar] [CrossRef]
- Minghetti, P.; Cilurzo, F.; Selmin, F.; Casiraghi, A.; Grignani, A.; Montanari, L. Sculptured drug-eluting stent for the on-site delivery of tacrolimus. Eur. J. Pharm. Biopharm. 2009, 73, 331–336. [Google Scholar] [CrossRef]
- Arafat, M.; Fouladian, P.; Wignall, A.; Song, Y.; Parikh, A.; Albrecht, H.; Prestidge, C.A.; Garg, S.; Blencowe, A. Development and In Vitro Evaluation of 5-Fluorouracil-Eluting Stents for the Treatment of Colorectal Cancer and Cancer-Related Obstruction. Pharmaceutics 2020, 13, 17. [Google Scholar] [CrossRef]
- Garcia-Fernandez, M.J.; Maton, M.; Benzine, Y.; Tabary, N.; Baptiste, E.J.; Gargouri, M.; Bria, M.; Blanchemain, N.; Karrout, Y. Ciprofloxacin loaded vascular prostheses functionalized with poly-methylbeta-cyclodextrin: The importance of in vitro release conditions. J. Drug Deliv. Sci. Technol. 2019, 53, 101166. [Google Scholar] [CrossRef]
- Ma, X.; Xiao, Y.; Xu, H.; Lei, K.; Lang, M. Preparation, degradation and in vitro release of ciprofloxacin-eluting ureteral stents for potential antibacterial application. Mater. Sci. Eng. C 2016, 66, 92–99. [Google Scholar] [CrossRef]
- Chen, M.-C.; Liang, H.-F.; Chiu, Y.-L.; Chang, Y.; Wei, H.-J.; Sung, H.-W. A novel drug-eluting stent spray-coated with multi-layers of collagen and sirolimus. J. Control. Release 2005, 108, 178–189. [Google Scholar] [CrossRef]
- Prostyakova, A.I.; Zybin, D.I.; Kapustin, D.V. In Vitro Evaluation of Drug Content in and Drug Release Kinetics from Stents with Different Types of Polymer Coating. Pharm. Chem. J. 2019, 52, 1011–1015. [Google Scholar] [CrossRef]
- Sarısözen, C.; Arıca, B.; Hıncal, A.A.; Çalış, S. Development of biodegradable drug releasing polymeric cardiovascular stents and in vitro evaluation. J. Microencapsul. 2009, 26, 501–512. [Google Scholar] [CrossRef]
- Guo, Q.; Knight, P.T.; Mather, P.T. Tailored drug release from biodegradable stent coatings based on hybrid polyurethanes. J. Control. Release 2009, 137, 224–233. [Google Scholar] [CrossRef]
- Ma, X.; Oyamada, S.; Gao, F.; Wu, T.; Robich, M.P.; Wu, H.; Wang, X.; Buchholz, B.; McCarthy, S.; Gu, Z.; et al. Paclitaxel/sirolimus combination coated drug-eluting stent: In vitro and in vivo drug release studies. J. Pharm. Biomed. Anal. 2011, 54, 807–811. [Google Scholar] [CrossRef]
- Kraitzer, A.; Ofek, L.; Schreiber, R.; Zilberman, M. Long-term in vitro study of paclitaxel-eluting bioresorbable core/shell fiber structures. J. Control. Release 2008, 126, 139–148. [Google Scholar] [CrossRef]
- Seidlitz, A.; Schick, W.; Reske, T.; Senz, V.; Grabow, N.; Petersen, S.; Nagel, S.; Weitschies, W. In vitro study of sirolimus release from a drug-eluting stent: Comparison of the release profiles obtained using different test setups. Eur. J. Pharm. Biopharm. 2015, 93, 328–338. [Google Scholar] [CrossRef]
- Benard, N.; Coisne, D.; Donal, E.; Perrault, R. Experimental study of laminar blood flow through an artery treated by a stent implantation: Characterisation of intra-stent wall shear stress. J. Biomech. 2003, 36, 991–998. [Google Scholar] [CrossRef]
- Seidlitz, A.; Nagel, S.; Semmling, B.; Grabow, N.; Sternberg, K.; Weitschies, W. Biorelevant dissolution testing of drug-eluting stents: Experiences with a modified flow-through cell setup. Dissolut. Technol. 2011, 18, 26–35. [Google Scholar] [CrossRef]
- Benard, N.; Perrault, R.; Coisne, D. Computational approach to estimating the effects of blood properties on changes in intra-stent flow. Ann. Biomed. Eng. 2006, 34, 1259–1271. [Google Scholar] [CrossRef]
- Ku, D.N.; Giddens, D.P. Pulsatile flow in a model carotid bifurcation. Arteriosclerosis 1983, 3, 31–39. [Google Scholar] [CrossRef]
- Zhu, X.; Braatz, R.D. A mechanistic model for drug release in PLGA biodegradable stent coatings coupled with polymer degradation and erosion. J. Biomed. Mater. Res. 2014, 7, 2269–2279. [Google Scholar] [CrossRef]
- Fotaki, N.; Klein, S. In Vitro Drug Release Testing of Special Dosage Forms; John Wiley & Sons: Hoboken, NJ, USA, 2019; ISBN 1118341473. [Google Scholar]
- Hermans, A.; Dorozynski, P.; Muzzio, F.J.; Li, H.; Nielsen, S.; Chen, S.; Reppas, C.; Klein, S.; Patel, S.k.; Wacker, M.; et al. Workshop report: USP workshop on advancements in in vitro performance testing of drug products. Dissolut. Technol. 2020, 27, 52–70. [Google Scholar] [CrossRef]
- Abbasnezhad, N.; Bakir, F.; Shirinbayan, M.; Maurel, B. New mathematical model based on the kinetic profile for the prediction of multistage drug release from delievery systems. Int. J. Pharm. 2020, 10, 1–8. [Google Scholar]
- Paarakh, M.P.; Jose, P.A.N.I.; Setty, C.; Mand Peter, G.V. Release Kinetics—Concepts and Applications. Int. J. Pharm. Res. Technol. 2019, 8, 12–20. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef]
- Medina, J.R.; Cortes, M.; Romo, E. Comparison of the USP Apparatus 2 and 4 for testing the in vitro release performance of ibuprofen generic suspensions. Int. J. Appl. Pharm. 2017, 9, 90–95. [Google Scholar] [CrossRef][Green Version]
- Dhole, S.M.; Khedekar, P.B.; Amnerkar, N.D. Comparison of UV spectrophotometry and high performance liquid chromatography methods for the determination of repaglinide in tablets. Pharm. Methods 2012, 3, 68–72. [Google Scholar] [CrossRef]
- Bourget, P.; Amin, A.; Vidal, F.; Merlette, C.; Troude, P.; Corriol, O. Raman spectroscopy applied to analytical quality control of injectable drugs: Analytical evaluation and comparative economic versus HPLC and UV/visible-FTIR. J. Pharm. Belg. 2013, 3, 32–45. [Google Scholar]
- Bourget, P.; Amin, A.; Vidal, F.; Merlette, C.; Lagarce, F. Comparison of Raman spectroscopy vs. high performance liquid chromatography for quality control of complex therapeutic objects: Model of elastomeric portable pumps filled with a fluorouracil solution. J. Pharm. Biomed. Anal. 2014, 91, 176–184. [Google Scholar] [CrossRef]
- Malinowski, H.; Marroum, P.; Uppoor, V.R.; Gillespie, W.; Ahn, H.Y.; Lockwood, P.; Henderson, J.; Baweja, R.; Hossain, M.; Fleischer, N.; et al. FDA guidance for industry extended release solid oral dosage forms: Development, evaluation, and application of in vitro/in vivo correlations. Dissolution Technol. 1997, 4, 23–32. [Google Scholar] [CrossRef]
- D’Souza, S. A Review of In Vitro Drug Release Test Methods for Nano-Sized Dosage Forms. Adv. Pharm. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Qiu, Y.; Duan, J.Z. In Vitro/In Vivo Correlations: Fundamentals, Development Considerations, and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128024478. [Google Scholar]
- Jacobs, T.; Rossenu, S.; Dunne, A.; Molenberghs, G.; Straetemans, R.; Bijnens, L. Combined models for data from in vitro-in vivo correlation experiments. J. Biopharm. Stat. 2008, 18, 1197–1211. [Google Scholar] [CrossRef]
- Sako, K.; Sawada, T.; Nakashima, H.; Yokohama, S.; Sonobe, T. Influence of water soluble fillers in hydroxypropylmethylcellulose matrices on in vitro and in vivo drug release. J. Control. Release 2002, 81, 165–172. [Google Scholar] [CrossRef]
- Tannergren, C.; Bergendal, A.; Lennernäs, H.; Abrahamsson, B. Toward an increased understanding of the barriers to colonic drug absorption in humans: Implications for early controlled release candidate assessment. Mol. Pharm. 2009, 6, 60–73. [Google Scholar] [CrossRef]
- Lennernäs, H. Regional intestinal drug permeation: Biopharmaceutics and drug development. Eur. J. Pharm. Sci. 2014, 57, 333–341. [Google Scholar] [CrossRef]
- Rohrs, B.R.; Skoug, J.W.; Halstead, G.W. Theory and Case Studies. Vitr.-Vivo Correl. 2013, 423, 17. [Google Scholar]
- Kostewicz, E.S.; Abrahamsson, B.; Brewster, M.; Brouwers, J.; Butler, J.; Carlert, S.; Dickinson, P.A.; Dressman, J.; Holm, R.; Klein, S.; et al. In vitro models for the prediction of in vivo performance of oral dosage forms. Eur. J. Pharm. Sci. 2014, 57, 342–366. [Google Scholar] [CrossRef]
- Kim, D.H.; Kang, S.G.; Choi, J.R.; Byun, J.N.; Kim, Y.C.; Ahn, Y.M. Evaluation of the Biodurability of Polyurethane-Covered Stent Using a Flow Phantom. Korean J. Radiol. 2001, 2, 75–79. [Google Scholar] [CrossRef][Green Version]
- Mcginty, S.; Mccormick, C.; Mckittrick, C.; Kennedy, S.; Wheel, M.; Scullion, B.; Pontrelli, G.; Mckee, S. Combining mathematical modelling with in-vitro experiments to predict in-vivo drug-eluting stent kinetics. In Proceedings of the 5th International Conference on Computational and Mathematical Biomedical Engineering (CMBE2017), Pittsburgh, PA, USA, 10–12 April 2017; pp. 1011–1014. [Google Scholar]



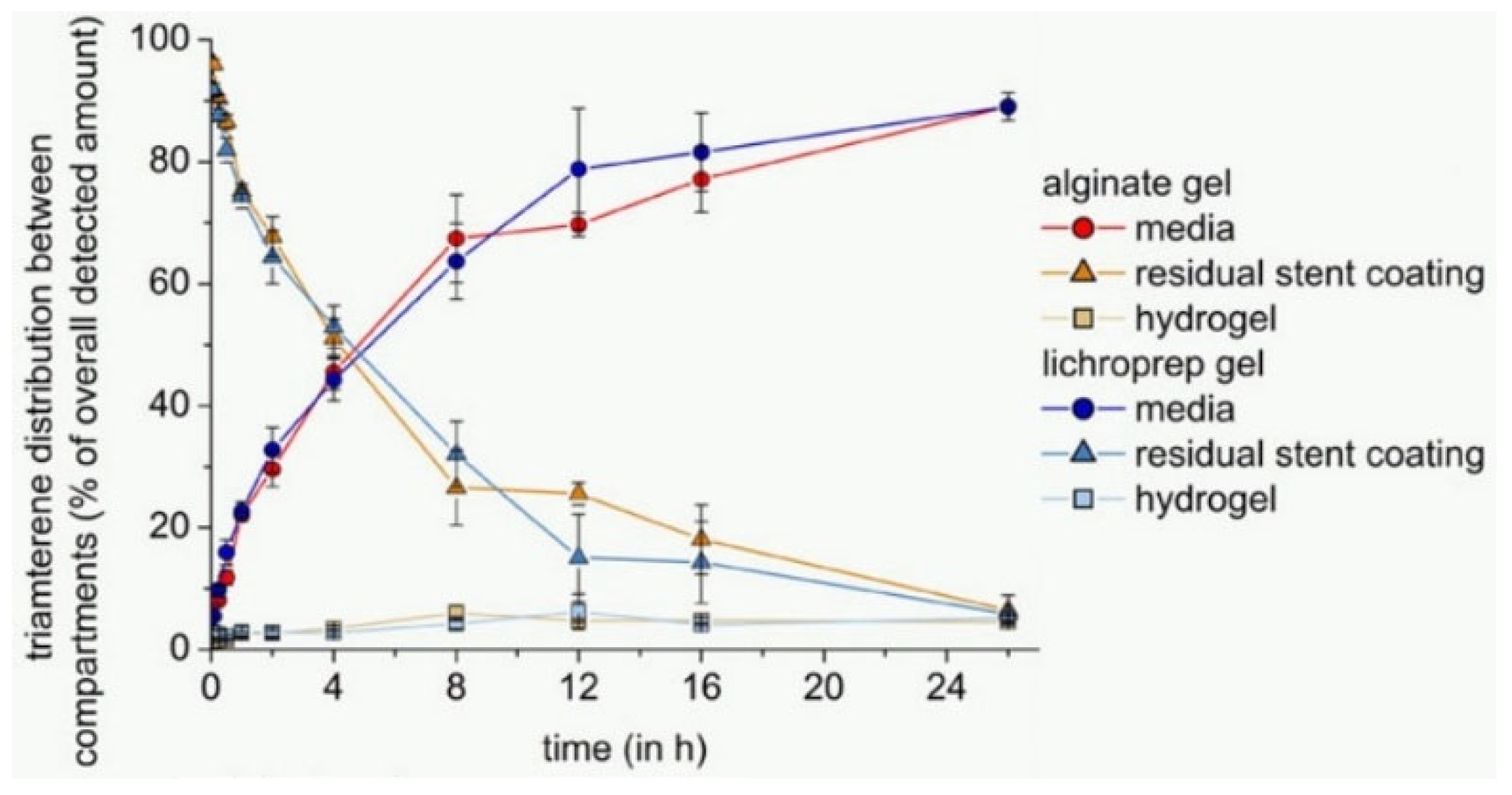
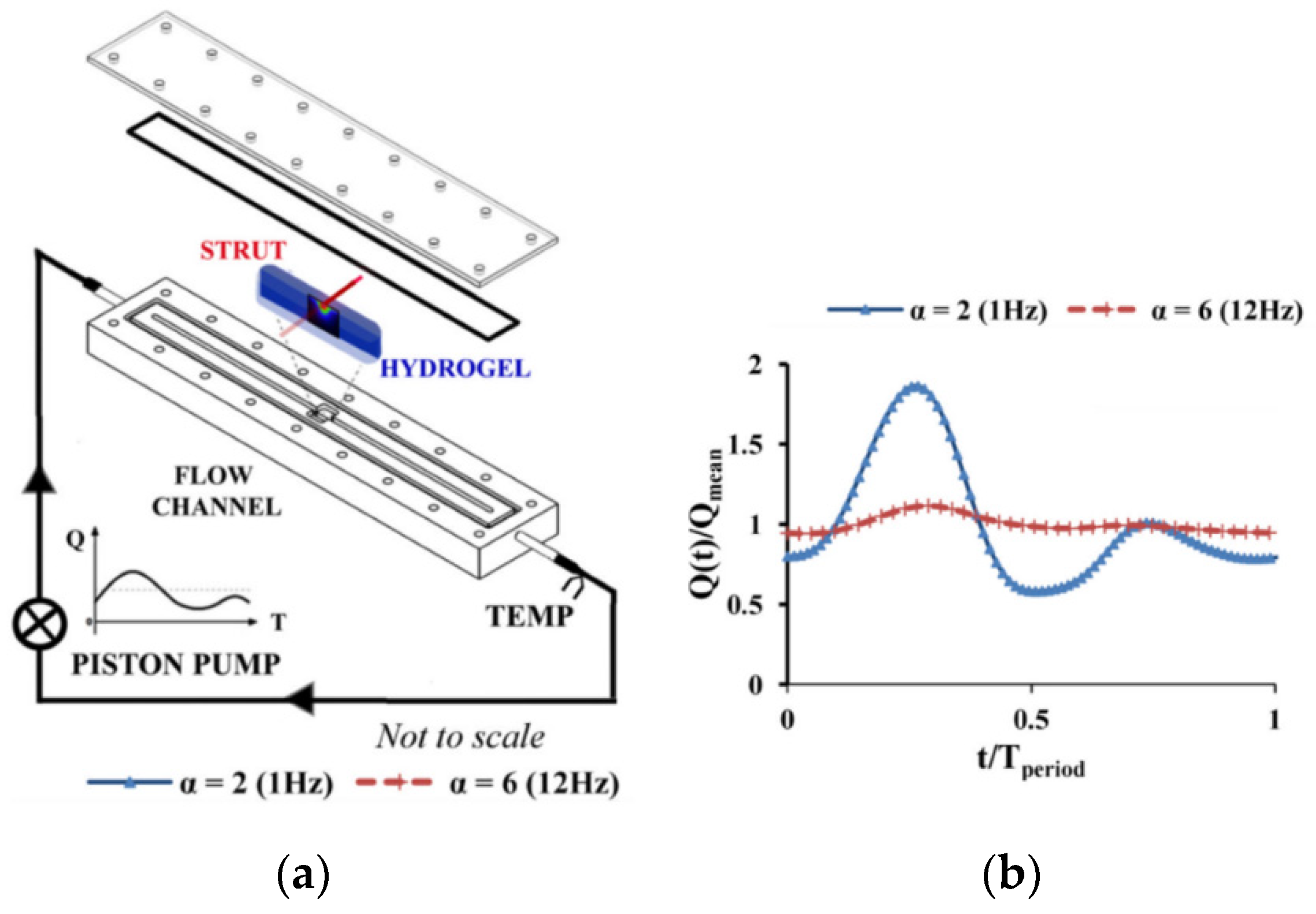
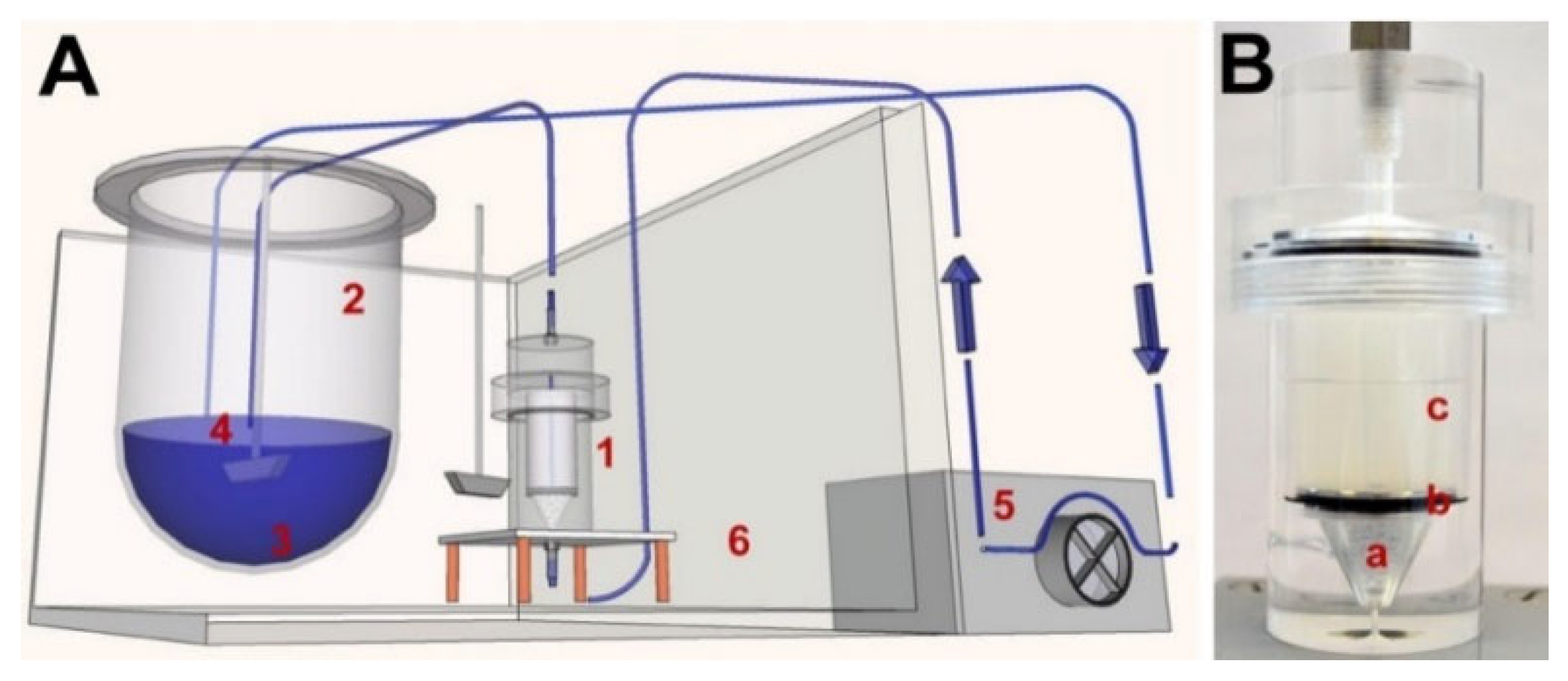
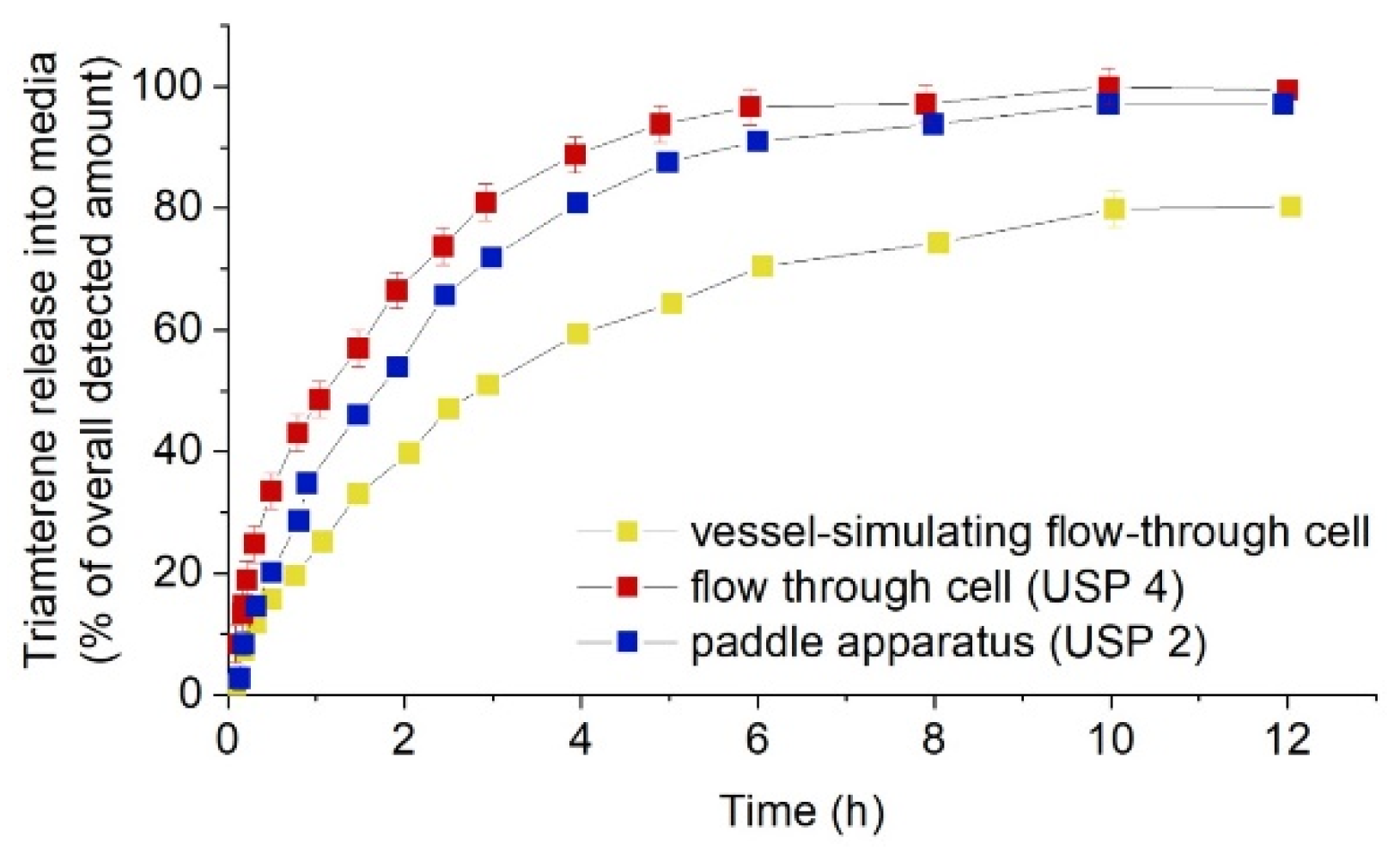
| Artificial Blood | Advantages/Dis-Advantages | Advantages/Dis-Advantages | Advantages/Dis-Advantages |
|---|---|---|---|
| phosphate buffered saline (PBS) [46,47] | pH constant 7.4, the proximity of its ions to the ions of the body (%) | calcium alginate hydrogel [58] | stability at 37 °C, the feasibility to adapt gel strength and elasticity, mild gelling conditions, feasibility of incorporation the diverse substances such as proteins or living cells |
| 9:1 (v/v) of normal saline and isopropanol [53] | suitable medium for in vitro release of sirolimus | 3 wt.% alginate; 2 wt.% agar; 2 wt.% agarose; 10 wt.% PAA; 15 wt.% PVA [59] | agarose: long-term dissolution |
| deionized water, PBS, phosphate-buffer (PB) [34] | deionized water increased the release compared to PBS and PB | calcium alginate; polyacrylamide (PAAm); poly(vinylethy limidazolium bromide [61] | disadvantages of calcium alginate: dissolution of the network by monovalent cations (like Na+) and its susceptibility to microbial contamination |
| surfactant 0.1% P123 (kind of PEO–PPO–PEO block copolymers) in phosphate buffer pH 4.0 [54] | suitable for in vitro release of sirolimus | alginate-based gel containing microparticles LiChroprep® RP-18 or mediumchain triglycerides [64] | additives improved the transfer of hydrophobic drugs into the hydrogel but had no significant effect on the hydrophilic drugs |
| glycerol-water (40/60 vol%, 0.01% surfactant) [41,43] | deionized water, PBS, and PB as the base for preparing hydrogel [34] | more drug transfer to deionized waterbased hydrogels than PBS and PB-based hydrogels | |
| 2% ultra-pure sodium dodecyl sulfate (SDS), in high purity water with 10% gradient-grade acetonitrile (ACN), and buffered to pH 4.5 with phosphate+ 55:45:0.02 water/tetrahydrofuran (THF)/formic acid (v/v) [56] | good correlate between in vitro release profile with in vivo from porcine | ||
| 87% of glycerol and 13% of water [45] | approaching to the viscosity of blood |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasnezhad, N.; Zirak, N.; Champmartin, S.; Shirinbayan, M.; Bakir, F. An Overview of In Vitro Drug Release Methods for Drug-Eluting Stents. Polymers 2022, 14, 2751. https://doi.org/10.3390/polym14132751
Abbasnezhad N, Zirak N, Champmartin S, Shirinbayan M, Bakir F. An Overview of In Vitro Drug Release Methods for Drug-Eluting Stents. Polymers. 2022; 14(13):2751. https://doi.org/10.3390/polym14132751
Chicago/Turabian StyleAbbasnezhad, Navideh, Nader Zirak, Stéphane Champmartin, Mohammadali Shirinbayan, and Farid Bakir. 2022. "An Overview of In Vitro Drug Release Methods for Drug-Eluting Stents" Polymers 14, no. 13: 2751. https://doi.org/10.3390/polym14132751
APA StyleAbbasnezhad, N., Zirak, N., Champmartin, S., Shirinbayan, M., & Bakir, F. (2022). An Overview of In Vitro Drug Release Methods for Drug-Eluting Stents. Polymers, 14(13), 2751. https://doi.org/10.3390/polym14132751











