Chemical and Structural Elucidation of Lignin and Cellulose Isolated Using DES from Bagasse Based on Alkaline and Hydrothermal Pretreatment
Abstract
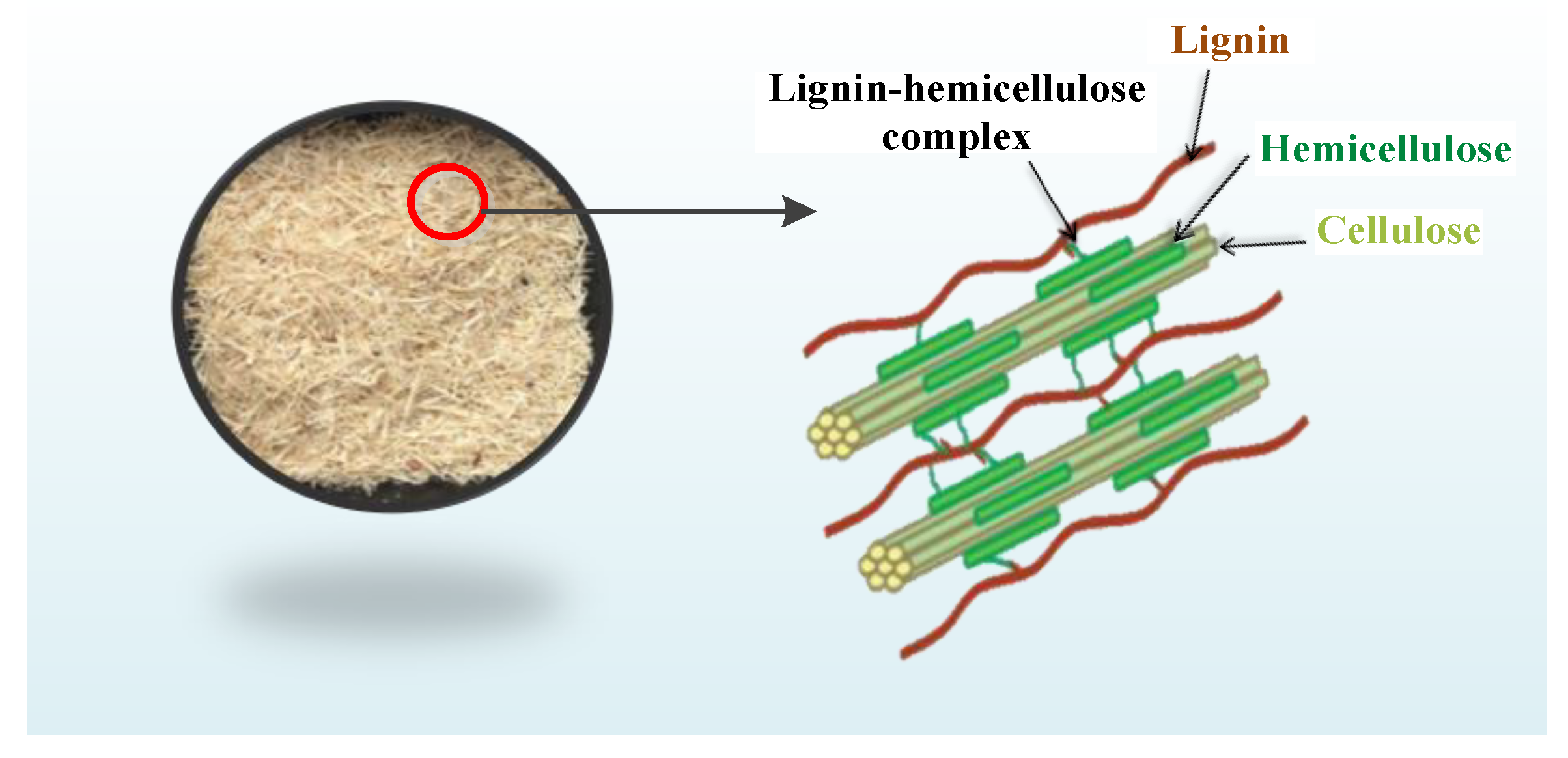
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Experimental Methods
- (1)
- Synthesis of L-DES
- (2)
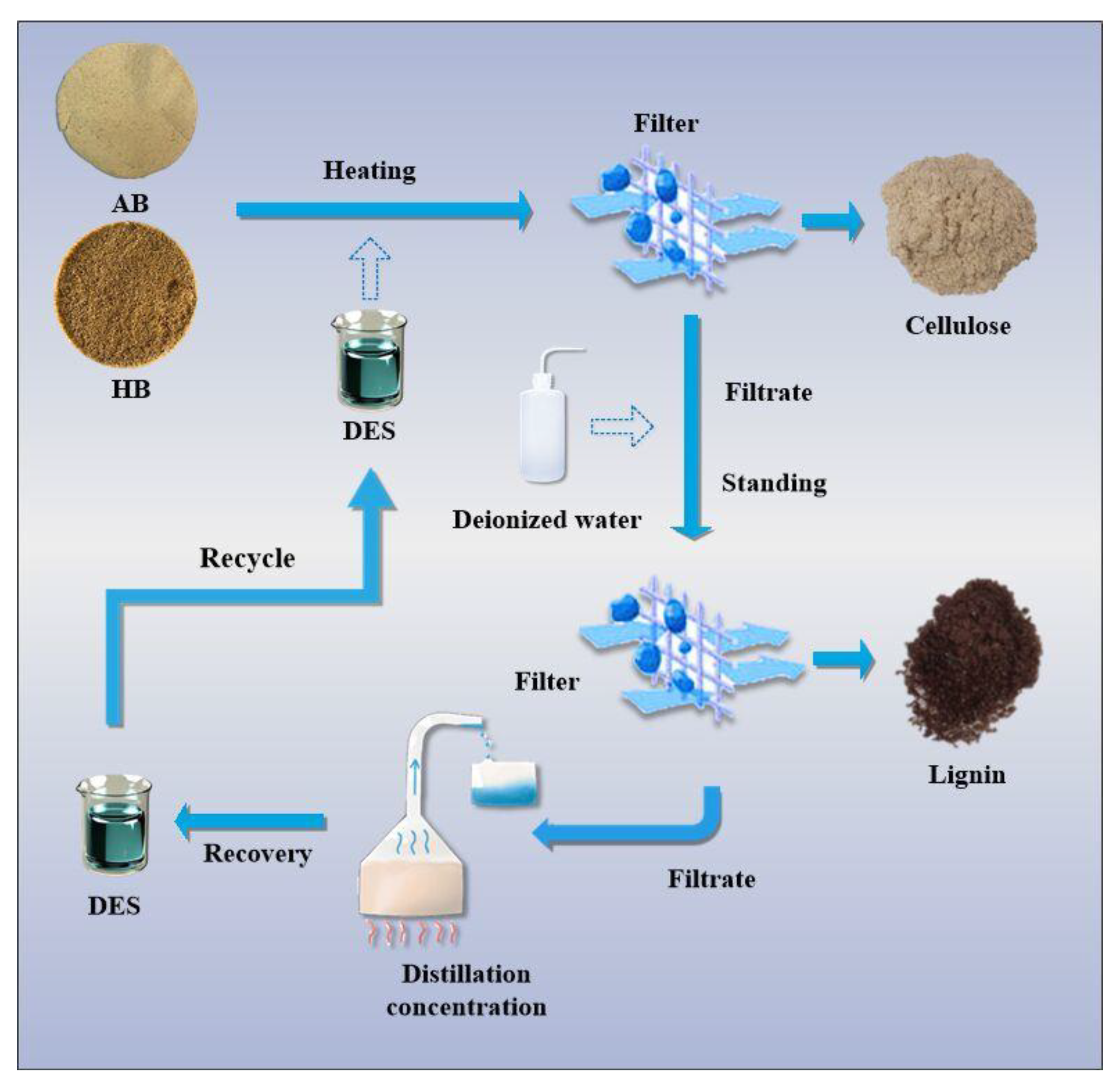
- Separation method of hemicellulose, cellulose, and lignin
- (3)
- Separation and extraction of cellulolytic enzyme lignin (CEL)
2.3. Characterization of Cellulose and Lignin
3. Results and Discussion
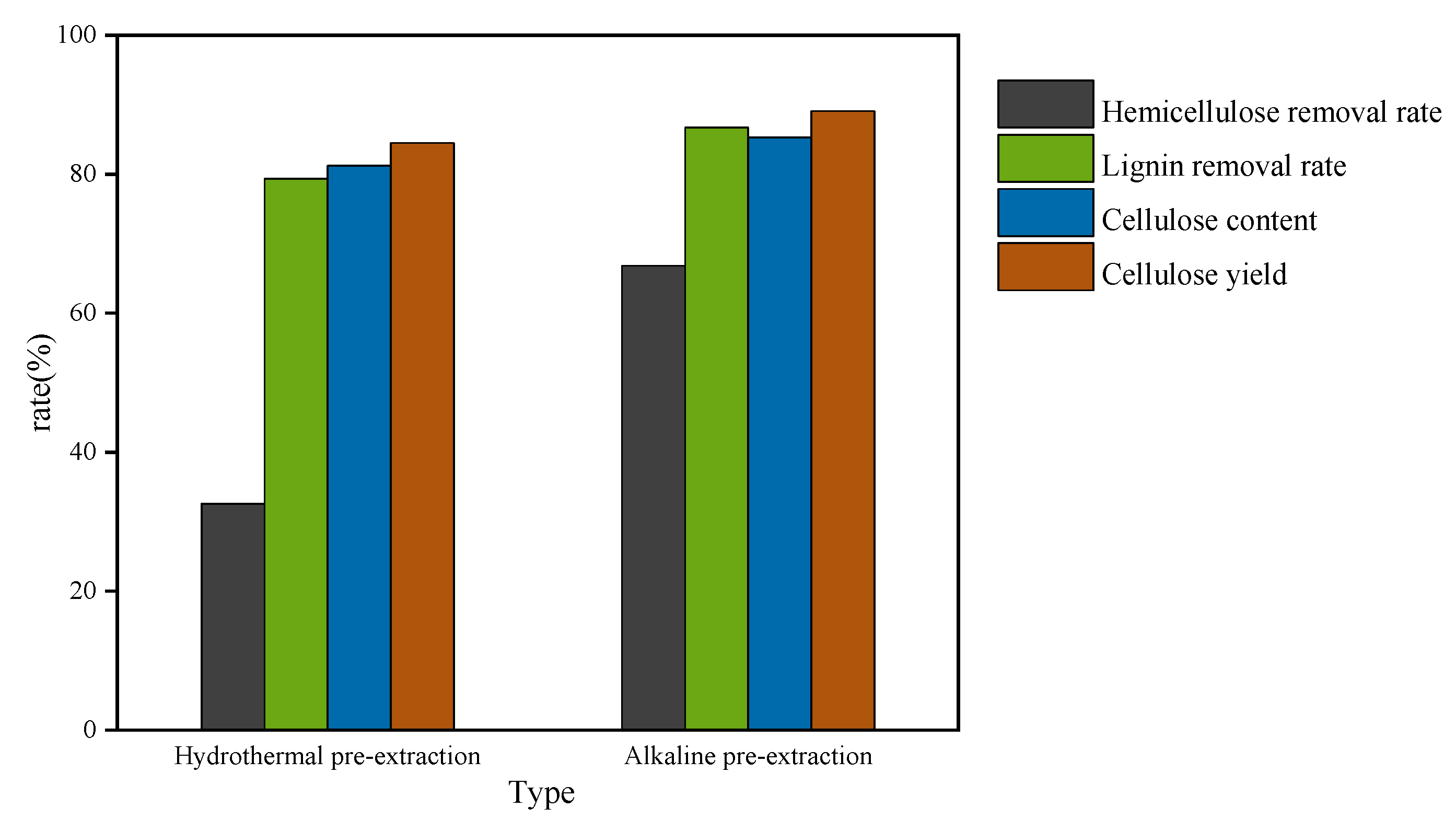
3.1. Comparison of Lignin Removal Rates by Hydrothermal Extraction Method and Alkali Extraction Method
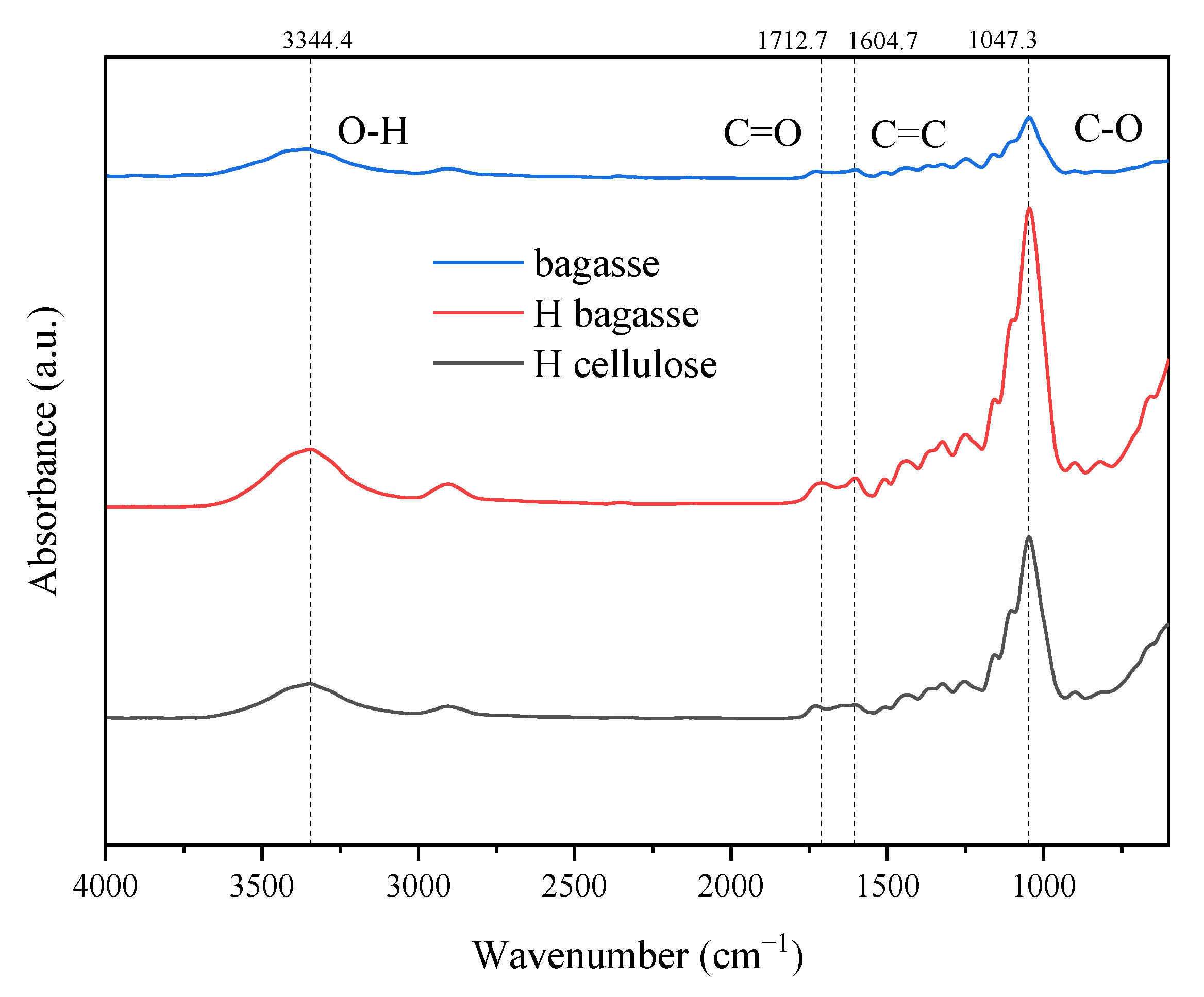
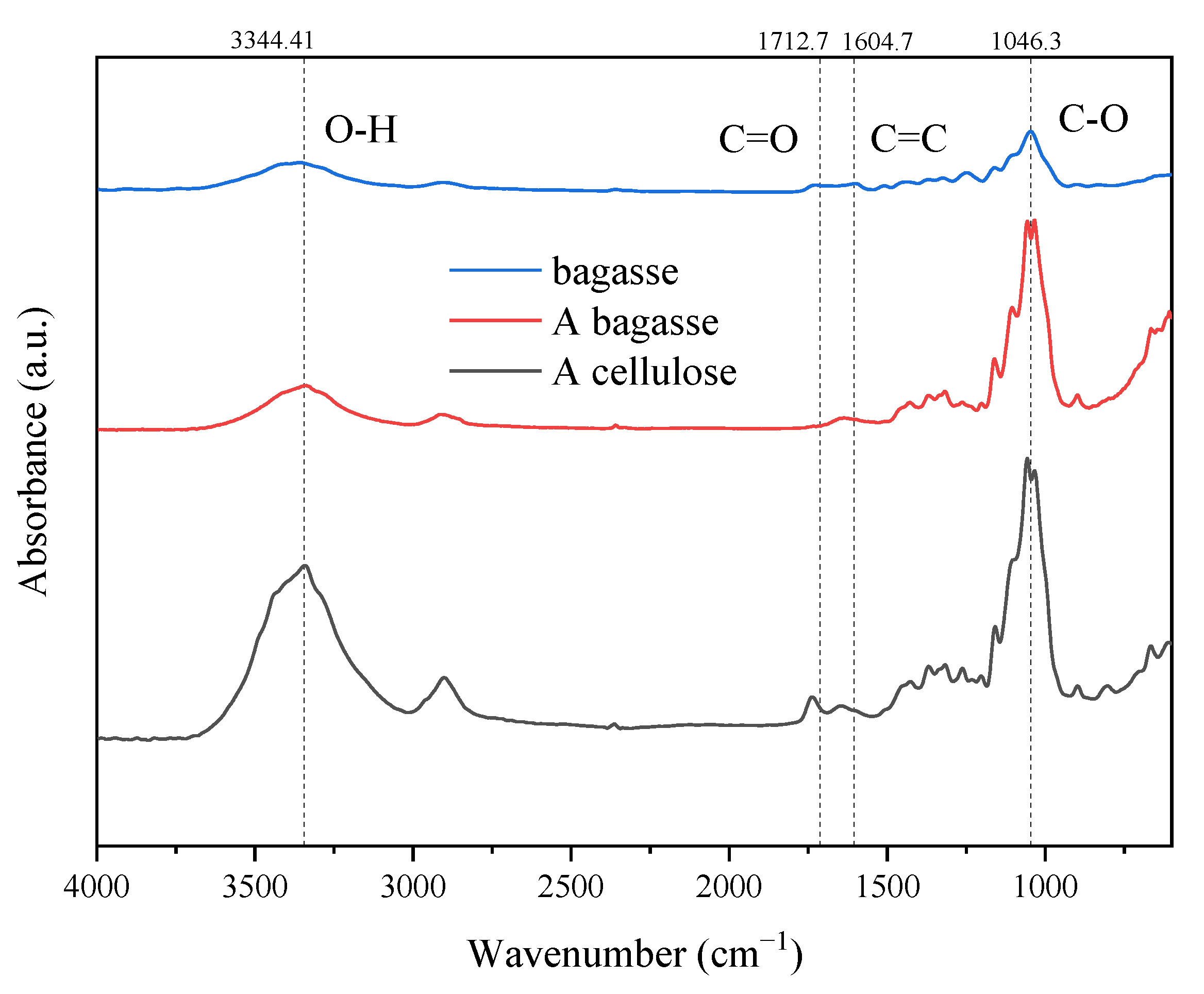
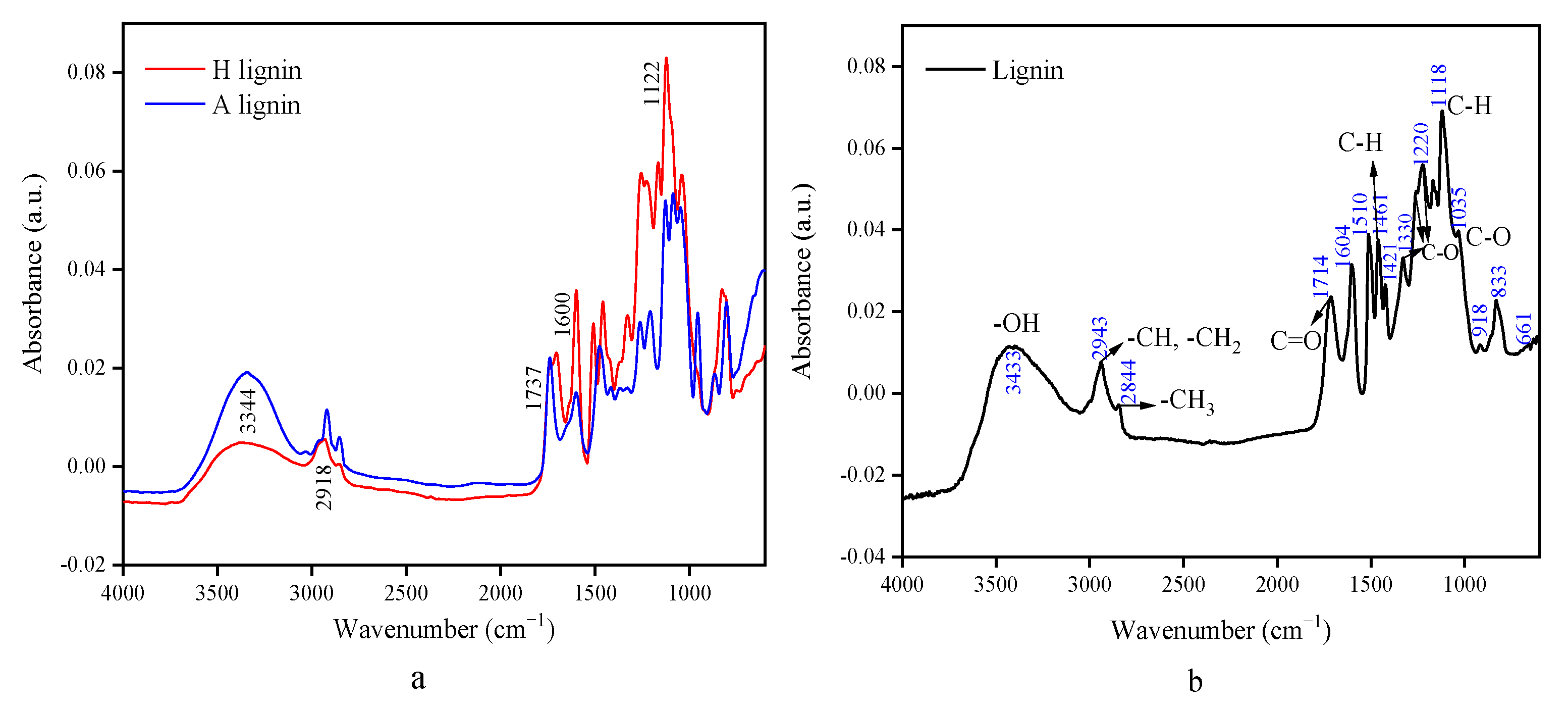
3.2. Structure Analysis of Crude Lignin and Cellulose by Infrared Spectroscopy
3.3. Morphology Analysis and Comparison of Crude Cellulose AC and HC
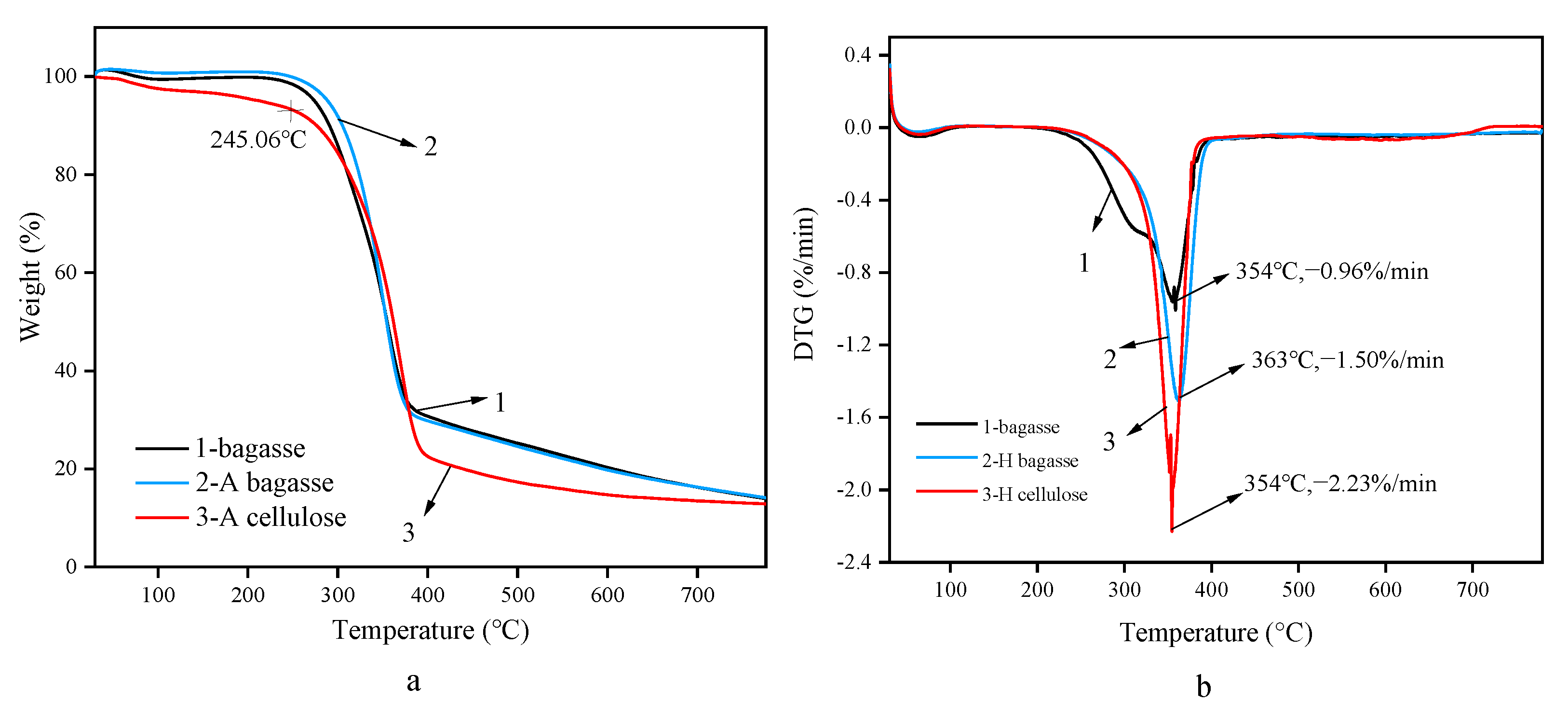
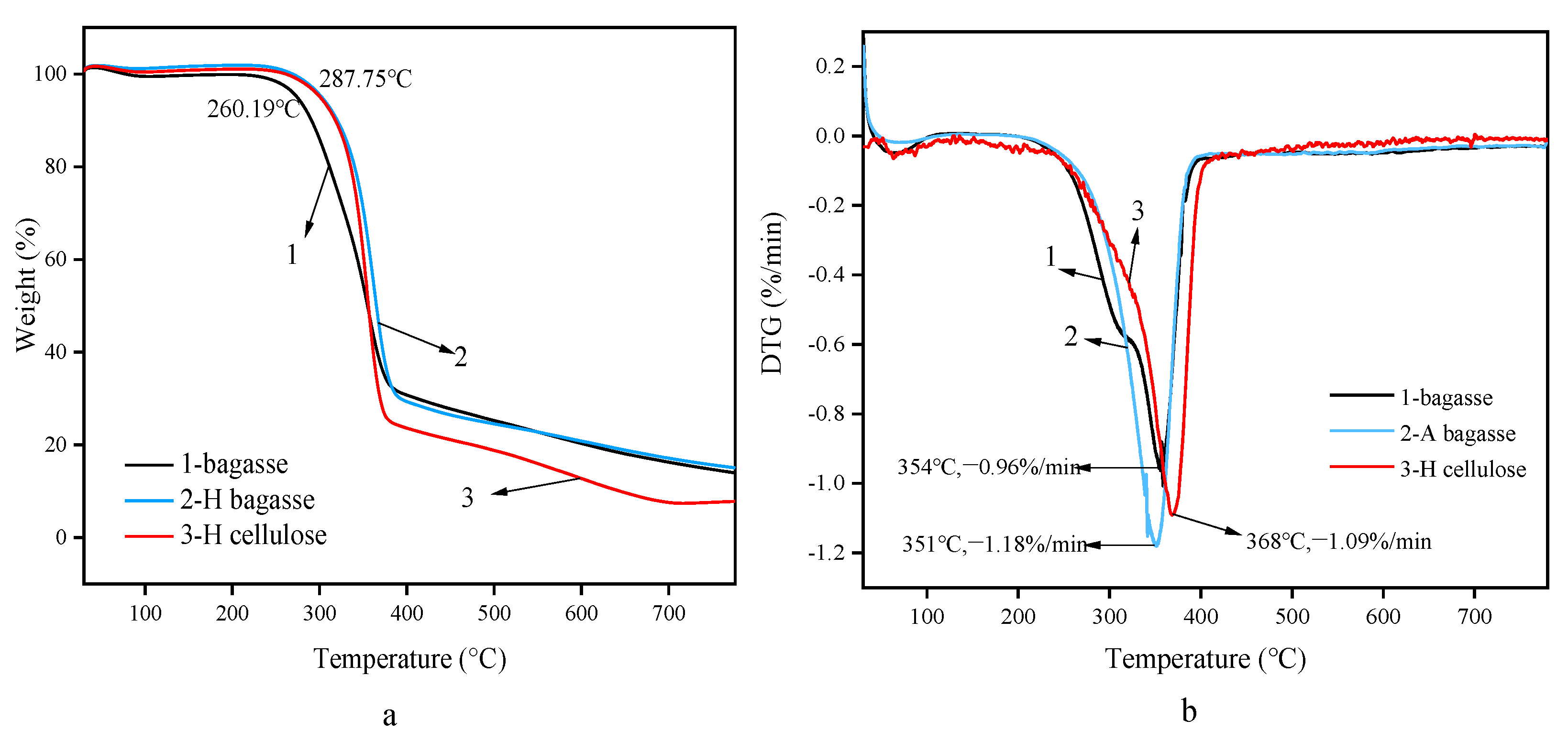
3.4. Analysis of Thermogravimetric Behavior
3.5. Analysis of Lignin Molecular Weight
3.6. Elemental Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, G.H.; Wang, X.Y.; Ren, L.T. China’s crop residues resources evaluation. Chin. J. Biotechnol. 2010, 26, 855–863. [Google Scholar]
- Rajeshkumar, U.; Hariahran, V.; Sivakumar, G. Synthesis and characterization of activated carbon from unburned carbon bagasse fly ash. Int. J. Phys. 2016, 2, 25–28. [Google Scholar]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; Da Silva, S.S. The path forward for lignocellulose biorefineries: Bottlenecks, solutions, and perspective on commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef]
- Hou, X.-D.; Smith, T.J.; Li, N.; Zong, M.-H. Novel Renewable Ionic Liquids as Highly Effective Solvents for Pretreatment of Rice Straw Biomass by Selective Removal of Lignin. Biotechnol. Bioeng. 2012, 109, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Satlewal, A.; Agrawal, R.; Das, P.; Bhagia, S.; Pu, Y.; Puri, S.K.; Ramakumar, S.S.V.; Ragauskas, A.J. Assessing the Facile Pretreatments of Bagasse for Efficient Enzymatic Conversion and Their Impacts on Structural and Chemical Properties. ACS Sustain. Chem. Eng. 2018, 7, 1095–1104. [Google Scholar] [CrossRef]
- Huang, Z.J.; Yang, L.M.; Lin, K.P.; Feng, G.J.; Zhang, Y.H.; Pu, F.L.; Gu, R.R.; Hou, X.D. The difference for enzymatic hydrolysis of sugarcane bagasse pre-treated by different deep eutectic solvents. Mod. Food Sci. Technol. 2020, 36, 60–67. [Google Scholar]
- Kumar, N.; Gautam, R.; Stallings, J.D.; Coty, G.G.; Lynam, J.G. Secondary Agriculture Residues Pretreatment Using Deep Eutectic Solvents. Waste Biomass- Valorization 2020, 12, 2259–2269. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.-C.; Chen, W.; Huang, R.; Hong, S.; Wu, Q.; Mei, C. Production of lignin-containing cellulose nanofibers using deep eutectic solvents for UV-absorbing polymer reinforcement. Carbohydr. Polym. 2020, 246, 116548. [Google Scholar] [CrossRef]
- Li, C.; Huang, C.; Zhao, Y.; Zheng, C.; Su, H.; Zhang, L.; Luo, W.; Zhao, H.; Wang, S.; Huang, L.-J. Effect of Choline-Based Deep Eutectic Solvent Pretreatment on the Structure of Cellulose and Lignin in Bagasse. Processes 2021, 9, 384. [Google Scholar] [CrossRef]
- Li, Y.H. Study on Alkali Pre-Extraction of Bagasse Hemicellulose and Its Effect on Subsequent Pulping Performance. Master’s Thesis, South China University of Technology, Guangzhou, China, 2019. [Google Scholar]
- Sun, S.-L.; Wen, J.-L.; Ma, M.-G.; Song, X.-L.; Sun, R.-C. Integrated biorefinery based on hydrothermal and alkaline treatments: Investigation of sorghum hemicelluloses. Carbohydr. Polym. 2014, 111, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-N.; Cao, X.-F.; Li, H.-Y.; Xu, F.; Sun, R.-C. Structural characterization of residual hemicelluloses from hydrothermal pretreated Eucalyptus fiber. Int. J. Biol. Macromol. 2014, 69, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Cao, T.; Gu, F.; Wu, W.; Jin, Y. Comparison of the Structural Characteristics of Cellulolytic Enzyme Lignin Preparations Isolated from Wheat Straw Stem and Leaf. ACS Sustain. Chem. Eng. 2016, 5, 342–349. [Google Scholar] [CrossRef]
- Nakagame, S.; Chandra, R.P.; Saddler, J.N. The effect of isolated lignins, obtained from a range of pretreated lignocellulosic substrates, on enzymatic hydrolysis. Biotechnol. Bioeng. 2010, 105, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yeh, T.-F.; Chang, H.-M.; Matsumoto, Y.; Kadla, J.F. Elucidation of the structure of cellulolytic enzyme lignin. Holzforschung 2006, 60, 389–397. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Derkacheva, O.; Sukhov, D. Investigation of Lignins by FTIR Spectroscopy. Macromol. Symp. 2008, 265, 61–68. [Google Scholar] [CrossRef]
- Xiong, L.; Yu, W.D. Analysis of the cellulose macromolecule structure after acid treatment by FITIR microspectroscopy. J. Cellul. Sci. Technol. 2013, 21, 59–62. [Google Scholar]
- Li, L.; Wu, Z.; Xi, X.; Liu, B.; Cao, Y.; Xu, H.; Hu, Y. A Bifunctional Brønsted Acidic Deep Eutectic Solvent to Dissolve and Catalyze the Depolymerization of Alkali Lignin. J. Renew. Mater. 2021, 9, 219–235. [Google Scholar] [CrossRef]
- Sun, R.; Fang, J.M.; Rowlands, P.; Bolton, J. Physicochemical and Thermal Characterization of Wheat Straw Hemicelluloses and Cellulose. J. Agric. Food Chem. 1998, 46, 2804–2809. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhai, S.-C.; Zhang, Y.-M.; Zhang, Y.-L. Simple evaluation of the degradation state of archaeological wood based on the infrared spectroscopy combined with thermogravimatery. Spectrosc. Spectr. Anal. 2020, 40, 2943–2950. [Google Scholar]
- Van De Vyver, S.; Geboers, J.; Jacobs, P.A.; Sels, B.F. Recent Advances in the Catalytic Conversion of Cellulose. ChemCatChem 2010, 3, 82–94. [Google Scholar] [CrossRef]
- Francisco, M.; Bruinhorst, A.V.D.; Kroon, M.C. New natural and renewable low transition temperature mixtures (LTTMs): Screening as solvents for lignocellulosic biomass processing. Green Chem. 2012, 14, 2153–2157. [Google Scholar] [CrossRef]
- Loow, Y.-L.; New, E.K.; Yang, G.H.; Ang, L.Y.; Foo, L.Y.W.; Wu, T.Y. Potential use of deep eutectic solvents to facilitate lignocellulosic biomass utilization and conversion. Cellulose 2017, 24, 3591–3618. [Google Scholar] [CrossRef]
- Meng, X.; Parikh, A.; Seemala, B.; Kumar, R.; Pu, Y.; Wyman, C.E.; Cai, C.; Ragauskas, A.J. Characterization of fractional cuts of co-solvent enhanced lignocellulosic fractionation lignin isolated by sequential precipitation. Bioresour. Technol. 2019, 272, 202–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Sample | Mn | Mw | Mz | Mz1 | Mw/Mn | Mz/Mw |
|---|---|---|---|---|---|---|
| CEL | 3793 | 5319 | 6819 | 7942 | 1.40232 | 1.28200 |
| AL | 1904 | 2347 | 2782 | 3279 | 1.23267 | 1.18534 |
| HL | 3448 | 3973 | 4620 | 5346 | 1.15207 | 1.16294 |
| Sample | C/% | H/% | O/% | N/% |
|---|---|---|---|---|
| Bagasse | 52.32 | 8.51 | 33.71 | 1.26 |
| CEL | 62.10 | 6.32 | 27.94 | 1.15 |
| AL | 65.47 | 6.45 | 25.83 | 1.17 |
| HL | 63.53 | 6.09 | 27.41 | 1.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Xu, B.; Wang, X.; Lang, J.; Zhang, H. Chemical and Structural Elucidation of Lignin and Cellulose Isolated Using DES from Bagasse Based on Alkaline and Hydrothermal Pretreatment. Polymers 2022, 14, 2756. https://doi.org/10.3390/polym14142756
Wang N, Xu B, Wang X, Lang J, Zhang H. Chemical and Structural Elucidation of Lignin and Cellulose Isolated Using DES from Bagasse Based on Alkaline and Hydrothermal Pretreatment. Polymers. 2022; 14(14):2756. https://doi.org/10.3390/polym14142756
Chicago/Turabian StyleWang, Na, Baoming Xu, Xinhui Wang, Jinyan Lang, and Heng Zhang. 2022. "Chemical and Structural Elucidation of Lignin and Cellulose Isolated Using DES from Bagasse Based on Alkaline and Hydrothermal Pretreatment" Polymers 14, no. 14: 2756. https://doi.org/10.3390/polym14142756
APA StyleWang, N., Xu, B., Wang, X., Lang, J., & Zhang, H. (2022). Chemical and Structural Elucidation of Lignin and Cellulose Isolated Using DES from Bagasse Based on Alkaline and Hydrothermal Pretreatment. Polymers, 14(14), 2756. https://doi.org/10.3390/polym14142756







