Oxidation Behavior of Carbon Fibers in Ceramizable Phenolic Resin Matrix Composites at Elevated Temperatures
Abstract
:1. Introduction
2. Experimental
2.1. Raw Materials
2.2. Preparation of Composites
2.3. Thermal Oxidation Treatment of Carbon Fibers and Composites
2.4. Characterizations
3. Results and Discussion
3.1. Thermal Stability of Carbon Fibers and Composites
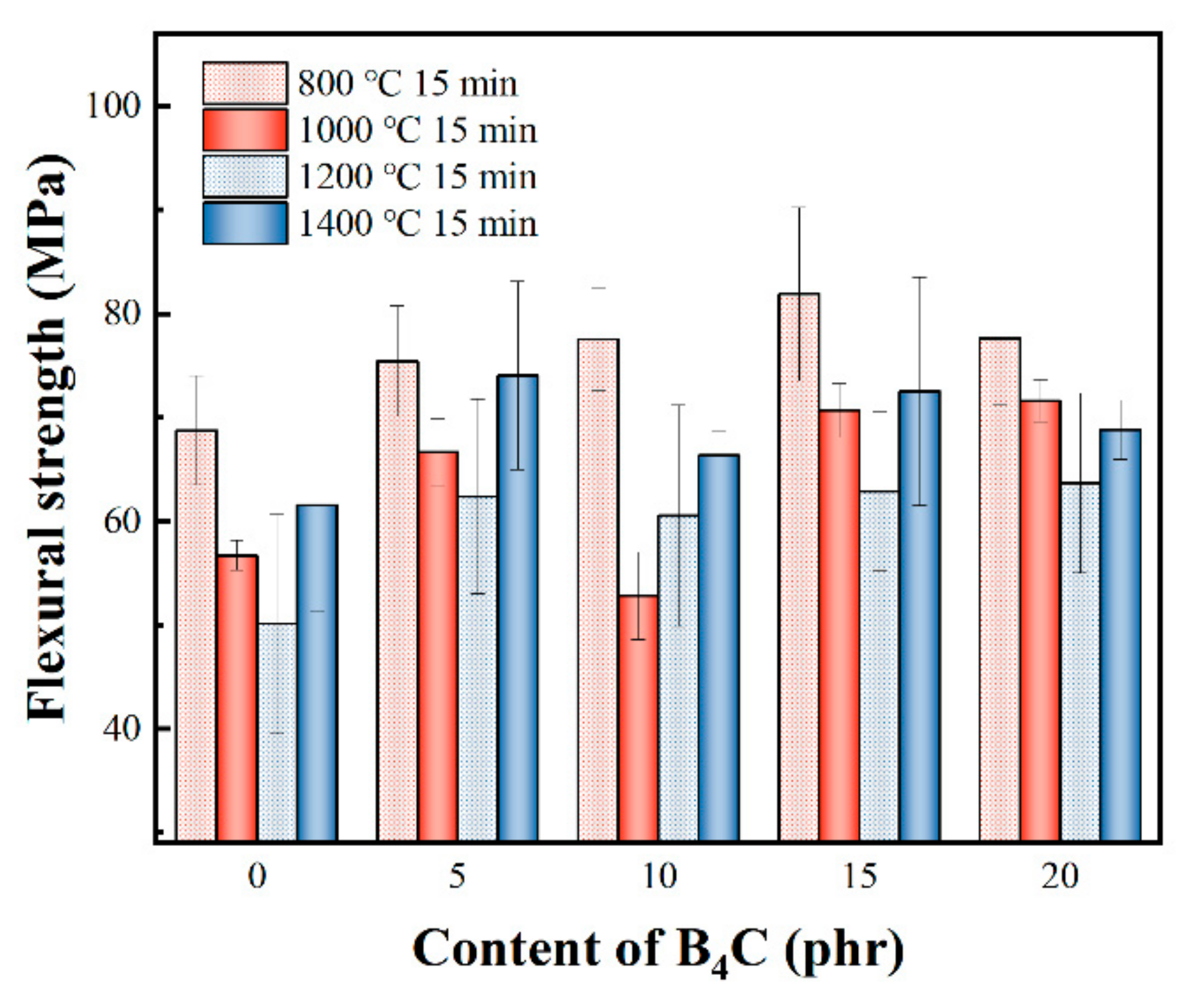
3.2. Flexural Strength of Composites Treated at Different Temperatures
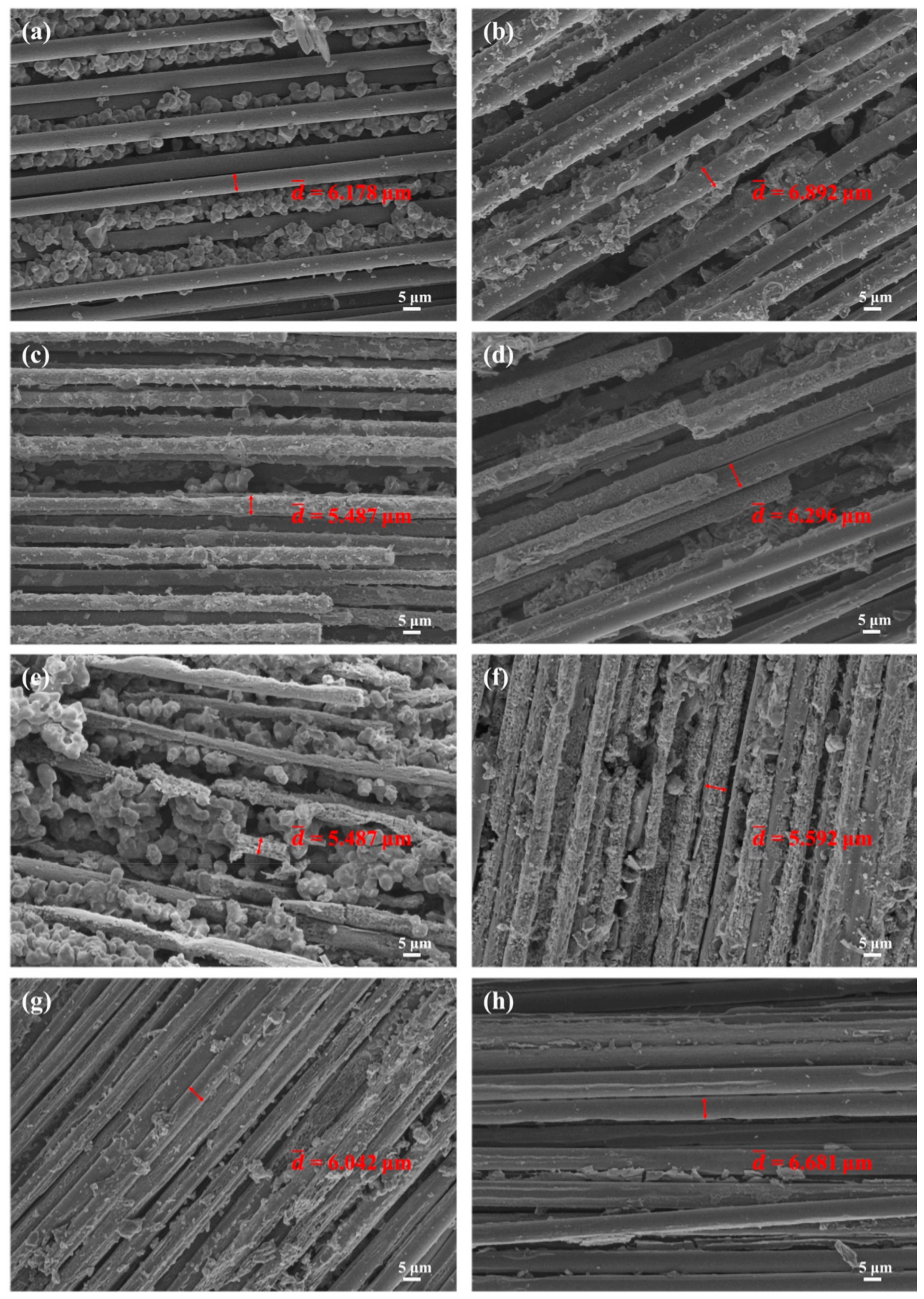
3.3. Micromorphology Changes of Composites with Different Contents of B4C
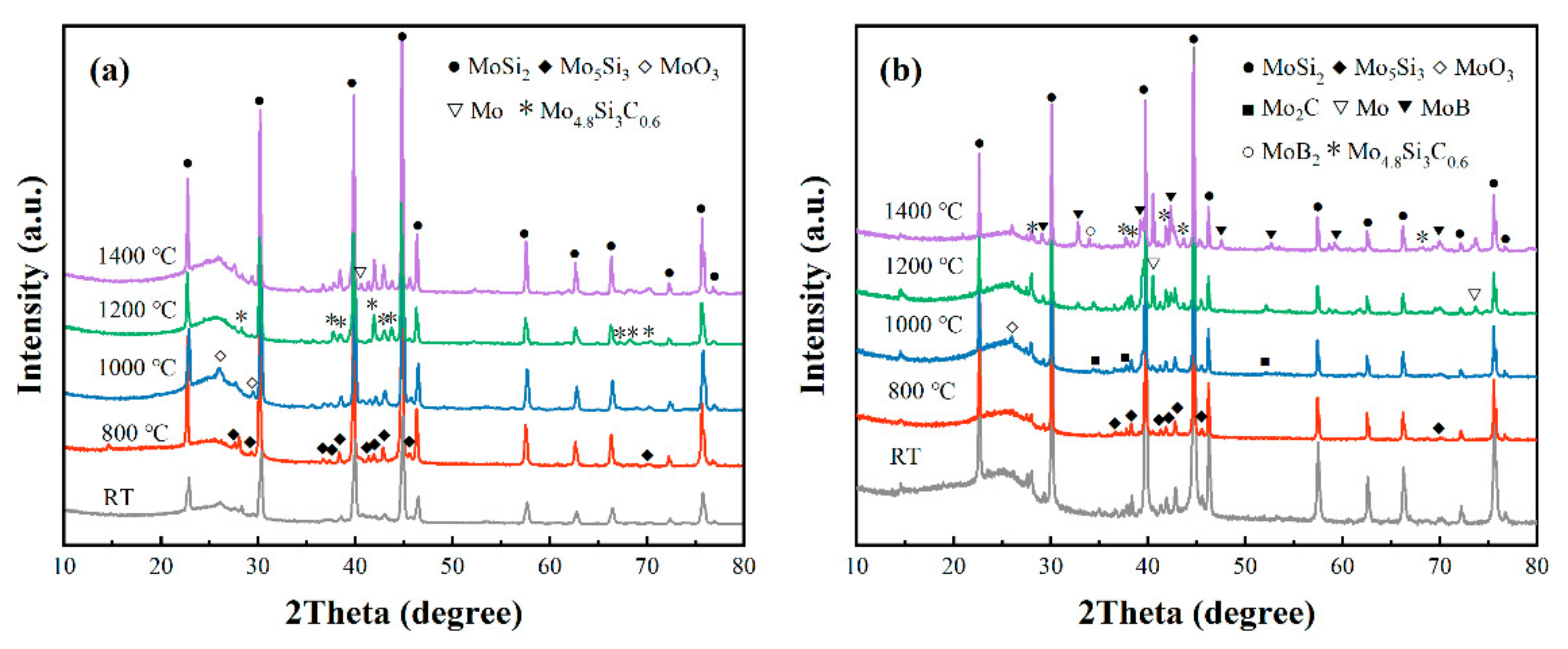
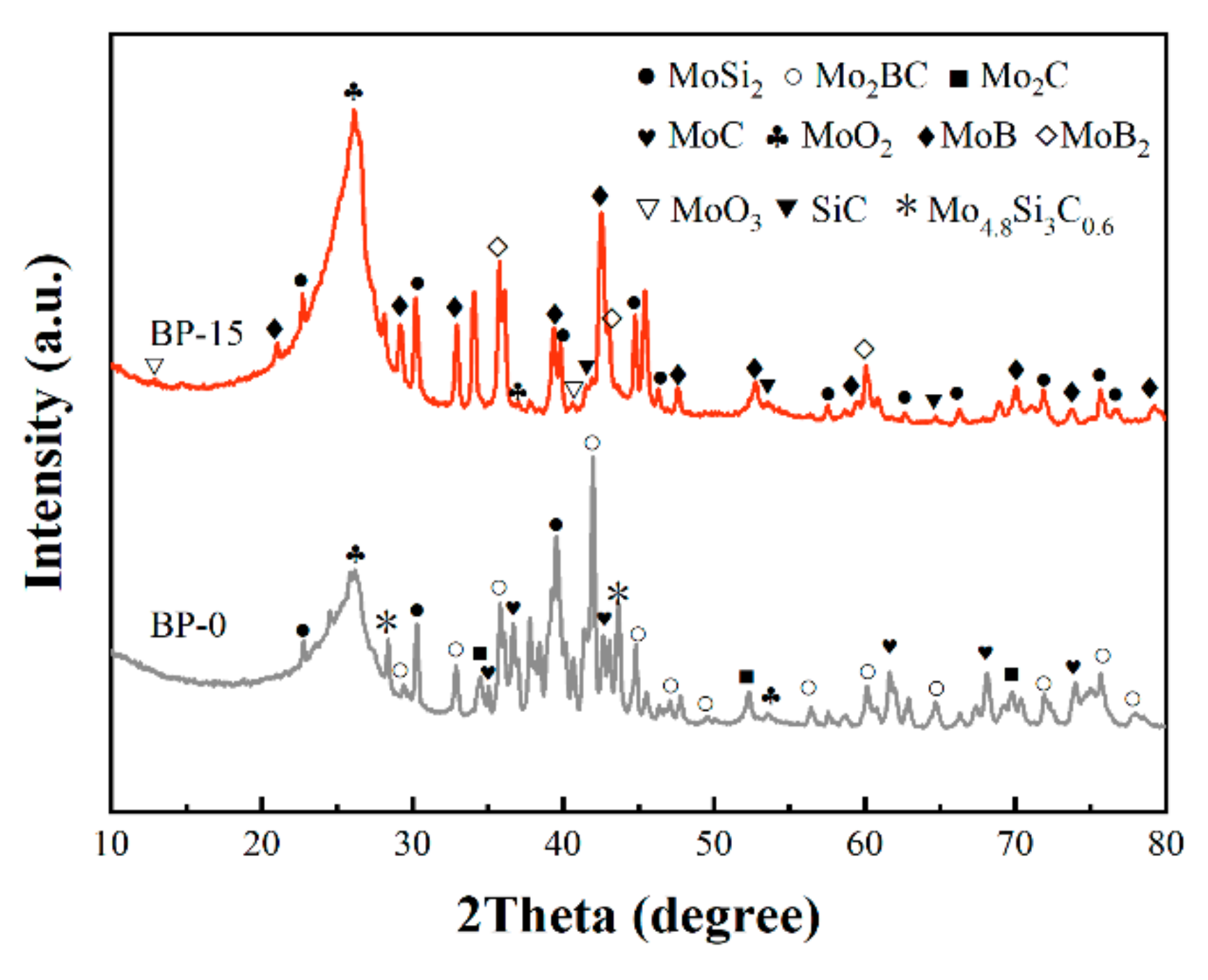
3.4. Phase Evolution of Composites at Elevated Temperatures
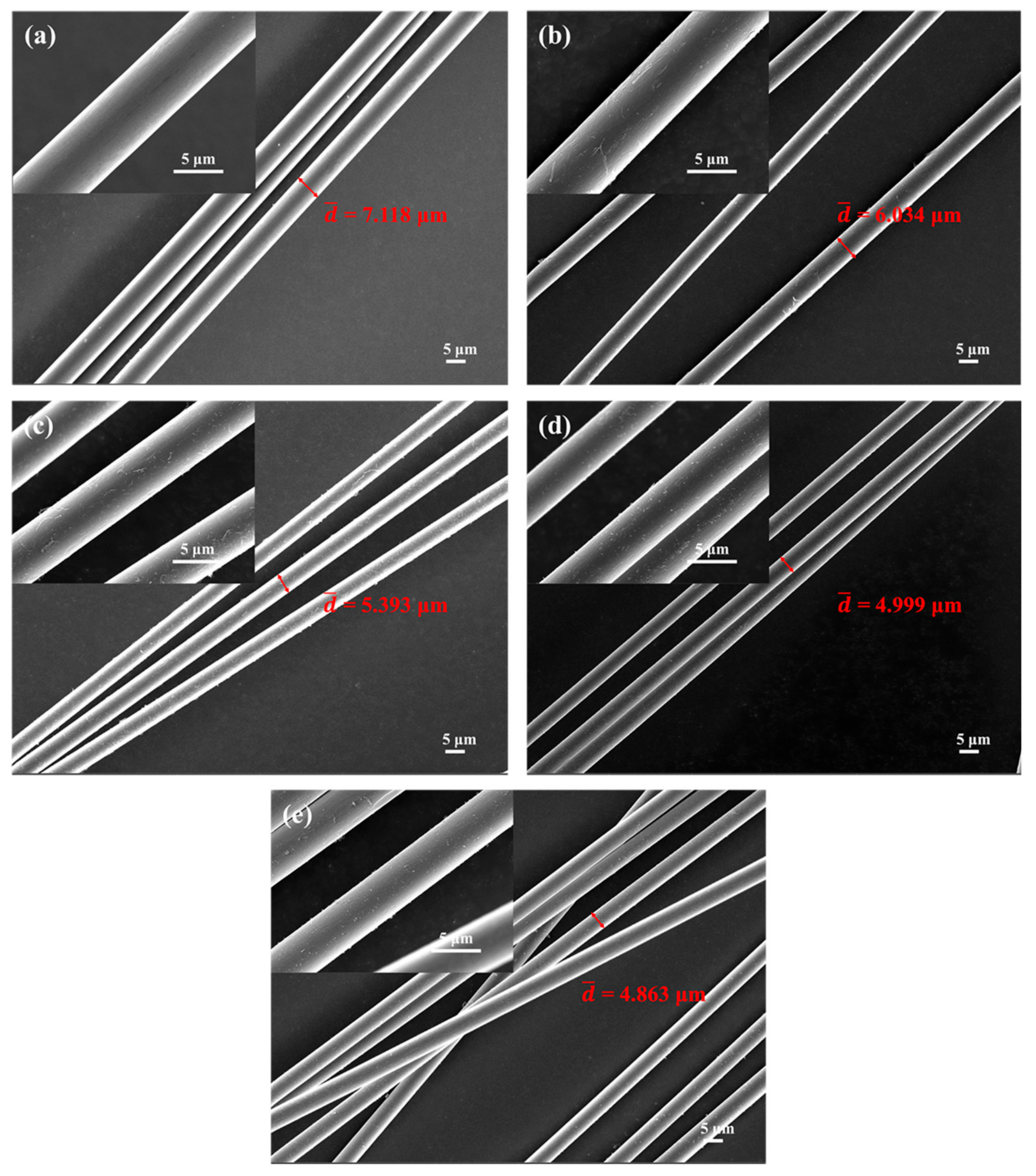
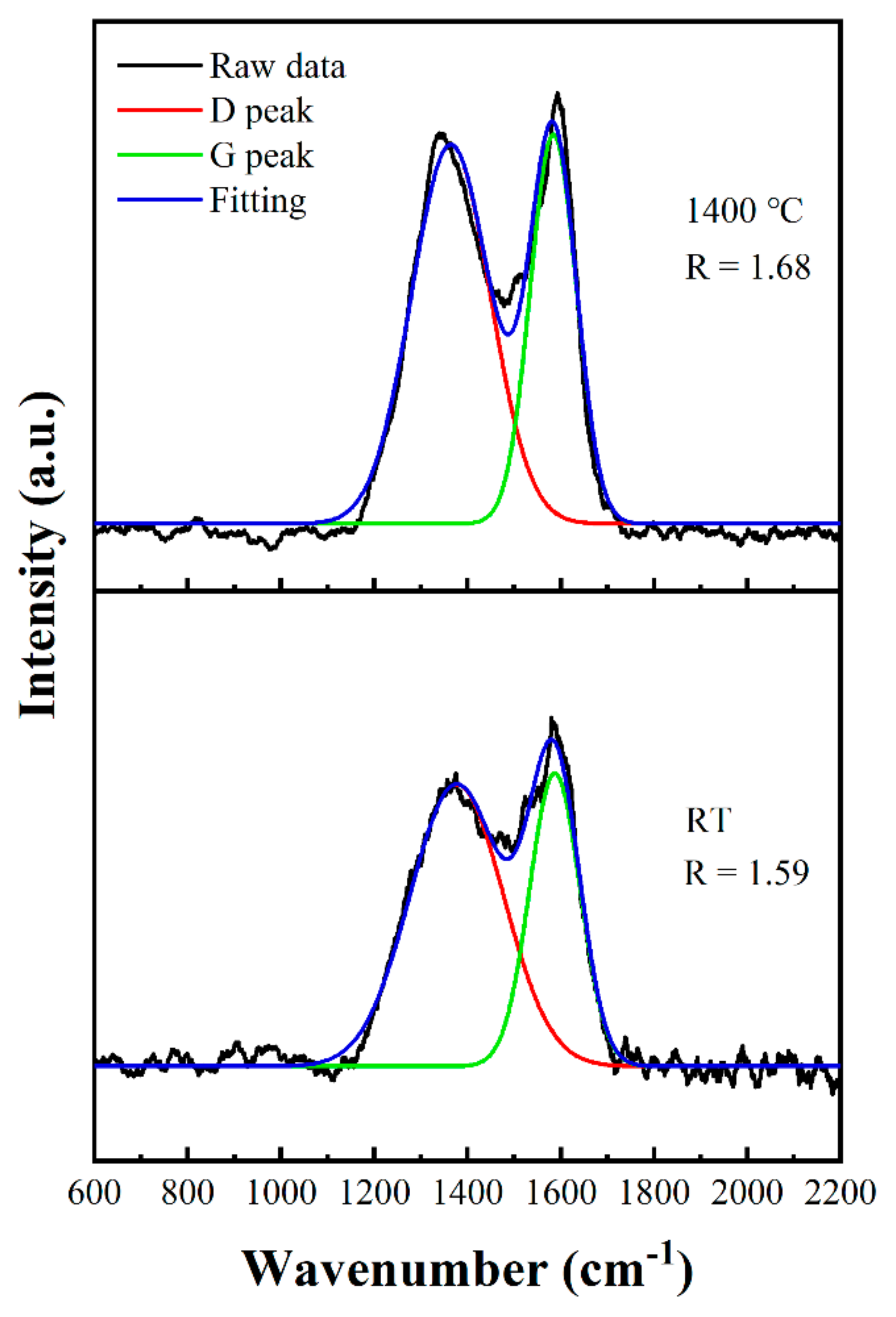
3.5. Micromorphology Changes and Phase Evolution of Carbon Fibers Treated at Different Temperatures
3.6. The Ablation Behavior of Composites
3.6.1. The Ablation Properties of Composites under an Oxygen–Acetylene Torch
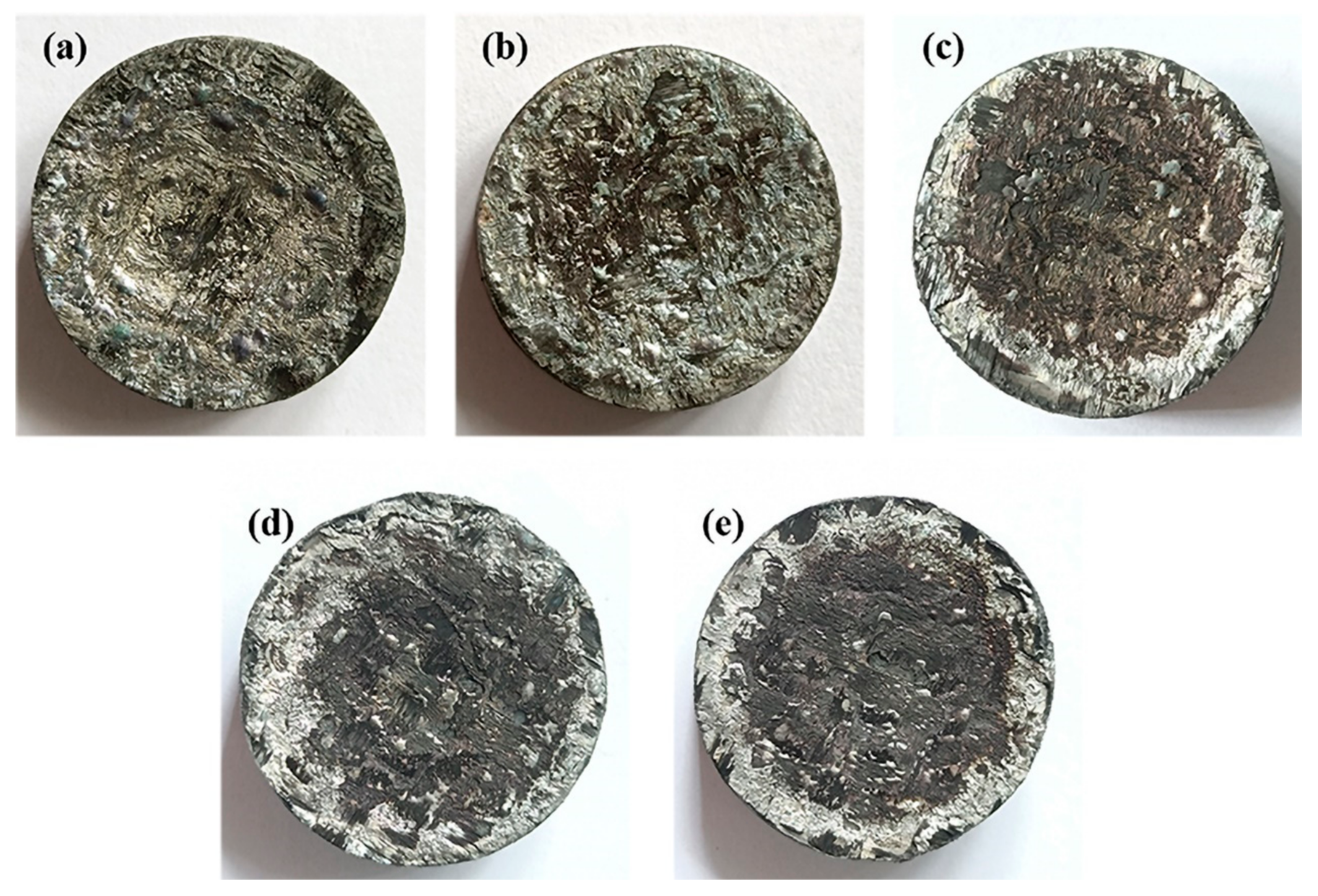
3.6.2. Morphology of Ablated Surface
3.6.3. Phase Composition of Ablated Surface
3.7. Effect of MoSi2 and B4C on Carbon Fiber Oxidation in Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Reaction | ∆G (kJ/mol) | |||||||
| 800 °C | 1000 °C | 1200 °C | 1400 °C | 1600 °C | 1800 °C | 2000 °C | 2200 °C | |
| (3) | −4708.499 | −4478.956 | −4252.360 | −4028.161 | −3805.981 | −3588.251 | −3377.260 | −3168.831 |
| (4) | −1781.391 | −1675.767 | −1572.219 | −1470.295 | −1369.734 | −1271.108 | −1174.976 | −1080.064 |
| (5) | −344.272 | −525.977 | −703.651 | −878.027 | −1049.531 | −1218.490 | −1385.158 | −1549.733 |
| (7) | −144.394 | −234.337 | −322.212 | −408.403 | −493.150 | −576.646 | −659.053 | −740.511 |
| (8) | −242.457 | −330.223 | −415.840 | −499.675 | −581.943 | −662.808 | −742.401 | −820.826 |
| (9) | −206.397 | −296.627 | −385.521 | −473.544 | −560.980 | −648.041 | −734.887 | −821.643 |
| (10) | −1699.199 | −1597.309 | −1496.734 | −1397.354 | −1299.107 | −1202.741 | −1108.966 | −1016.649 |
| (13) | −1128.581 | −1042.883 | −957.937 | −873.603 | −789.778 | −707.155 | −626.376 | −546.231 |
| (14) | −1335.313 | −1267.079 | −1199.388 | −1132.122 | −1065.191 | −999.301 | −935.104 | −871.398 |
| (15) | −117.916 | −184.486 | −249.874 | −314.098 | −377.157 | −439.029 | −499.683 | −559.075 |
| (16) | −81.856 | −150.890 | −219.554 | −287.967 | −356.194 | −424.262 | −492.169 | −559.892 |
| (17) | 240.864 | 173.222 | 106.285 | 39.969 | −25.786 | −90.633 | −154.249 | −217.232 |
| 2: Thermodynamic parameters come from the HSC Chemistry software. | ||||||||
References
- Gnoffo, P.A. Planetary-Entry Gas Dynamics. Annu. Rev. Fluid Mech. 1999, 31, 459–494. [Google Scholar] [CrossRef]
- Laub, B.; Venkatapathy, E. Thermal Protection System Technology and Facility Needs for Demanding Future Planetary Missions. In Proceedings of the International Workshop Planetary Probe Atmospheric Entry and Descent Trajectory Analysis and Science, Lisbon, Portugal, 6–9 October 2003; ESA Publications Division: Noordwijk, The Netherlands, 2004; Volume 544, pp. 239–247. [Google Scholar]
- Davis, J.B.; Marshall, D.B.; Oka, K.S.; Housley, R.M.; Morgan, P.E.D. Ceramic Composites for Thermal Protection Systems. Compos. Part A Appl. Sci. Manuf. 1999, 30, 483–488. [Google Scholar] [CrossRef]
- Deng, Z.; Yue, J.; Huang, Z. Solvothermal Degradation and Reuse of Carbon Fiber Reinforced Boron Phenolic Resin Composites. Compos. Part B Eng. 2021, 221, 109011. [Google Scholar] [CrossRef]
- Mansouri, J.; Burford, R.P.; Cheng, Y.B. Pyrolysis Behaviour of Silicone-Based Ceramifying Composites. Mater. Sci. Eng. A 2006, 425, 7–14. [Google Scholar] [CrossRef]
- Saghar, A.; Khan, M.; Sadiq, I.; Subhani, T. Effect of Carbon Nanotubes and Silicon Carbide Particles on Ablative Properties of Carbon Fiber Phenolic Matrix Composites. Vacuum 2018, 148, 124–126. [Google Scholar] [CrossRef]
- Wang, S.; Huang, H.; Tian, Y.; Huang, J. Effects of SiC Content on Mechanical, Thermal and Ablative Properties of Carbon/Phenolic Composites. Ceram. Int. 2020, 46, 16151–16156. [Google Scholar] [CrossRef]
- Song, J.; Huang, Z.; Qin, Y.; Wang, H.; Shi, M. Effects of Zirconium Silicide on the Vulcanization, Mechanical and Ablation Resistance Properties of Ceramifiable Silicone Rubber Composites. Polymers 2020, 12, 496. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Chen, P.; Hong, C.; Zhang, B.; Hui, D. Improved Ablation Resistance of Carbon-Phenolic Composites by Introducing Zirconium Diboride Particles. Compos. Part B Eng. 2013, 47, 320–325. [Google Scholar] [CrossRef]
- Ding, J.; Sun, J.; Huang, Z.; Wang, Y. Improved High-Temperature Mechanical Property of Carbon-Phenolic Composites by Introducing Titanium Diboride Particles. Compos. Part B Eng. 2019, 157, 289–294. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, S.; Ma, Z.; Liu, H.; Chen, Y.; Wu, T. Effect of TaSi2/ZrSi2 on Ablation Properties of Carbon-Phenolic Composite Irradiated by High-Intensity Continuous Laser. Ceram. Int. 2020, 46, 28443–28450. [Google Scholar] [CrossRef]
- Yang, W.; Xu, B.; Qi, M.; Chen, D.; Ding, J.; Huang, Z.; Wang, Y. Improving Ablation Properties of Ceramifiable Vitreous Silica Fabric Reinforced Boron Phenolic Resin Composites via an Incorporation of MoSi2. Plast. Rubber Compos. 2020, 49, 456–469. [Google Scholar] [CrossRef]
- Yan, J.H.; Wang, Y.; Liu, L.F.; Wang, Y.; Chen, F. Preparation of Protective MoSi2 Coating on Niobium Substrate. J. Therm. Spray Technol. 2015, 24, 1093–1099. [Google Scholar] [CrossRef]
- Lohfeld, S.; Schütze, M. Oxidation Behaviour of Particle Reinforced MoSi2 Composites at Temperatures up to 1700 °C Part I: Literature Review. Mater. Corros. 2005, 56, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Samadzadeh, M.; Oprea, C.; Karimi Sharif, H.; Troczynski, T. Comparative Studies of the Oxidation of MoSi2 Based Materials: Low-Temperature Oxidation (300–900 °C). Int. J. Refract. Met. Hard Mater. 2017, 66, 11–20. [Google Scholar] [CrossRef]
- Wang, L.; Fu, Q.G.; Liu, N.K.; Shan, Y.C. Supersonic Plasma Sprayed MoSi2–ZrB2 Antioxidation Coating for SiC–C/C Composites. Surf. Eng. 2016, 32, 508–513. [Google Scholar] [CrossRef]
- Yong, X.; Cao, L.; Huang, J.; Kong, W.; Su, J.; Li, C.; Ouyang, H.; Zhou, L.; Liu, J. Microstructure and Oxidation Protection of a MoSi2/SiO2-B2O3-Al2O3 Coating for SiC-Coated Carbon/Carbon Composites. Surf. Coatings Technol. 2017, 311, 63–69. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, C.; Chen, Z.; Ren, X.; Shen, C.; Feng, P. Reaction Synthesis of Spark Plasma Sintered MoSi2-B4C Coatings for Oxidation Protection of Nb Alloy. Ceram. Int. 2019, 45, 4290–4297. [Google Scholar] [CrossRef]
- Chung, D.D.L. Carbon Fiber Composites [M]; Butterworth-Heinemann: Oxford, UK, 1994; ISBN 978-0-08-050073-7. [Google Scholar]
- Logesh, G.; Lodhe, M.; Balasubramanian, M. Effect of Temperature and Gaseous Medium on the Evolved Microstructures of Carbon Fiber Reinforced Reaction Bonded Silicon Nitride Composites. Ceram. Int. 2017, 43, 6110–6116. [Google Scholar] [CrossRef]
- Che, D.; Saxena, I.; Han, P.; Guo, P.; Ehmann, K. Machining of Carbon Fiber Reinforced Plastics/Polymers: A Literature Review. J. Manuf. Sci. Eng. 2014, 136, 34001. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, Z.; Dong, C.; Fan, J.; Ren, Y. A Closed-Loop Recycling Process for Carbon Fiber Reinforced Vinyl Ester Resin Composite. Chem. Eng. J. 2022, 446, 137254. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, X.; Du, A.; Wu, S.; Shen, J.; Zhou, B. Timing of Polyethylene Glycol Addition for the Control of SiO2 Sol Structure and Sol–Gel Coating Properties. J. Coat. Technol. Res. 2017, 14, 447–454. [Google Scholar] [CrossRef]
- Xia, K.; Lu, C.; Yang, Y. Preparation of Anti-Oxidative SiC/SiO2 Coating on Carbon Fibers from Vinyltriethoxysilane by Sol–Gel Method. Appl. Surf. Sci. 2013, 265, 603–609. [Google Scholar] [CrossRef]
- Kim, G.; Lee, H.; Kim, K.; Kim, D.U. Effects of Heat Treatment Atmosphere and Temperature on the Properties of Carbon Fibers. Polymers 2022, 12, 2412. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Kim, Y.; Jeon, H.; Cho, S.; Lee, W.; Lee, J.U. Graphene/Carbon Nanotube Hybrid as a Multi-Functional Interfacial Reinforcement for Carbon Fiber-Reinforced Composites. Compos. Part B Eng. 2017, 122, 23–30. [Google Scholar] [CrossRef]
- Ribeiro, L.; Flores, O.; Furtat, P.; GERVAIS, C.; Kempe, R.; Machado, R.; Motz, G. A Novel PAN/Silazane Hybrid Polymer for Processing of Carbon-Based Fibres with Extraordinary Oxidation Resistance. J. Mater. Chem. A 2016, 5, 720–729. [Google Scholar] [CrossRef]
- Wang, J.; Lin, W.; Wu, X.; Yang, Y.; Wu, F.; Yan, X. Preparation and Oxidation Behavior of a Double-Layer Coating for Three-Dimensional Braided Carbon Fiber. Surf. Coat. Technol. 2016, 298, 58–63. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, S.; Ma, Z.; Liu, Y.; Li, H.; Hu, J. Improved Interfacial Strength and Ablation Resistance of Carbon Fabric Reinforced Phenolic Composites Modified with Functionalized ZrSiO4 Sol. Mater. Des. 2020, 191, 108623. [Google Scholar] [CrossRef]
- Duan, L.; Zhao, X.; Wang, Y. Effects of Polycarbosilane Interface on Oxidation, Mechanical, and Ablation Properties of Carbon Fiber-Reinforced Composites. Ceram. Int. 2018, 44, 22919–22926. [Google Scholar] [CrossRef]
- Zou, Z.; Qin, Y.; Fu, H.; Zhu, D.; Li, Z.; Huang, Z. ZrO2f-Coated Cf Hybrid Fibrous Reinforcements and Properties of Their Reinforced Ceramicizable Phenolic Resin Matrix Composites. J. Eur. Ceram. Soc. 2021, 41, 1810–1816. [Google Scholar] [CrossRef]
- Yang, G.; Yang, G.; Wang, W.; Peng, S.; Huang, Z. The Research on Oxidation Resistance Ability and Mechanical Properties of Carbon Fiber Reinforced Phenolic Resin Composites. Mater. Res. Express 2020, 7, 065604. [Google Scholar] [CrossRef]
- Ding, J.; Yang, T.; Huang, Z.; Qin, Y.; Wang, Y. Thermal Stability and Ablation Resistance, and Ablation Mechanism of Carbon–Phenolic Composites with Different Zirconium Silicide Particle Loadings. Compos. Part B Eng. 2018, 154, 313–320. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Bian, C.; Zhong, Y.; Jing, X. The Thermal Stability and Pyrolysis Mechanism of Boron-Containing Phenolic Resins: The Effect of Phenyl Borates on the Char Formation. Appl. Surf. Sci. 2015, 331, 519–529. [Google Scholar] [CrossRef]
- Trick, K.A.; Saliba, T.E. Mechanisms of the Pyrolysis of Phenolic Resin in a Carbon/Phenolic Composite. Carbon N. Y. 1995, 33, 1509–1515. [Google Scholar] [CrossRef]
- Chen, X.; Li, S.; Yan, L.; Yuan, Y. Curing and Pyrolysis of Boron-Modified Phenolic Resin. Fuhe Cailiao Xuebao/Acta Mater. Compos. Sin. 2011, 28, 89–95. [Google Scholar]
- Hatami Ramsheh, H.; Faghihi Sani, M.A.; Kokabi, A.H. Microstructure and Mechanical Properties of MoSi2–MoSi2 Joints Brazed by Ag–Cu–Zr Interlayer. Mater. Des. 2013, 49, 197–202. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, X.; Ren, X.; Kang, X.; Akhtar, F.; Feng, P. Preparation and High-Temperature Oxidation Resistance of Multilayer MoSi2/MoB Coating by Spent MoSi2-Based Materials. J. Am. Ceram. Soc. 2021, 104, 3682–3694. [Google Scholar] [CrossRef]
- Gnesin, B.A.; Gnesin, I.B.; Nekrasov, A.N. The Interaction of Carbon with Mo5Si3 and W5Si3 Silicides. Nowotny Phase Synthesis. Intermetallics 2013, 41, 82–95. [Google Scholar] [CrossRef]
- Gao, P.; Xu, M.; Xie, W.; Lü, H.; Li, Q.; Peng, S.; Xiao, H. Study on Formation Mechanism of Mo4.8Si3C0.6 Nanopowders Prepared by Solid-Reaction Method. Hunan Daxue Xuebao/J. Hunan Univ. Nat. Sci. 2018, 45, 85–89. [Google Scholar] [CrossRef]
- Lee, D.B.; Woo, S.W. Oxidation of SiOC Composite Having Dispersoids of Mo4.8Si3C0.6 and MoSi2. Mater. Sci. Forum 2005, 486–487, 165–168. [Google Scholar] [CrossRef]
- Kumar, S.; Sairam, K.; Sonber, J.K.; Murthy, T.S.R.C.; Reddy, V.; Nageswara Rao, G.V.S.; Srinivasa Rao, T. Hot-Pressing of MoSi2 Reinforced B4C Composites. Ceram. Int. 2014, 40, 16099–16105. [Google Scholar] [CrossRef]
- Paul, A.; Venugopal, S.; Binner, J.G.P.; Vaidhyanathan, B.; Heaton, A.C.J.; Brown, P.M. UHTC-Carbon Fibre Composites: Preparation, Oxyacetylene Torch Testing and Characterisation. J. Eur. Ceram. Soc. 2013, 33, 423–432. [Google Scholar] [CrossRef]
- Feng, T.; Li, H.; Hu, M.; Lin, H.; Li, L. Oxidation and Ablation Resistance of the ZrB2-CrSi2-Si/SiC Coating for C/C Composites at High Temperature. J. Alloys Compd. 2016, 662, 302–307. [Google Scholar] [CrossRef]
- Tang, S.; Deng, J.; Wang, S.; Liu, W.; Yang, K. Ablation Behaviors of Ultra-High Temperature Ceramic Composites. Mater. Sci. Eng. A 2007, 465, 1–7. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, C.; Hu, H.; Zhang, Y. Ablation Behavior and Mechanism of 3D C/ZrC Composite in Oxyacetylene Torch Environment. Compos. Sci. Technol. 2011, 71, 1392–1396. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Y.; Wen, Q.; Riedel, R.; Yu, Z. Dielectric Properties and Electromagnetic Wave Absorbing Performance of Single-Source-Precursor Synthesized Mo4.8Si3C0.6/SiC/Cfree Nanocomposites with an in Situ Formed Nowotny Phase. ACS Appl. Mater. Interfaces 2020, 12, 16912–16921. [Google Scholar] [CrossRef]
- Tian, X.D.; Guo, X.P.; Sun, Z.P.; Qu, J.L.; Wang, L.J. Oxidation Resistance Comparison of MoSi2 and B-Modified MoSi2 Coatings on Pure Mo Prepared through Pack Cementation. Mater. Corros. 2015, 66, 681–687. [Google Scholar] [CrossRef]
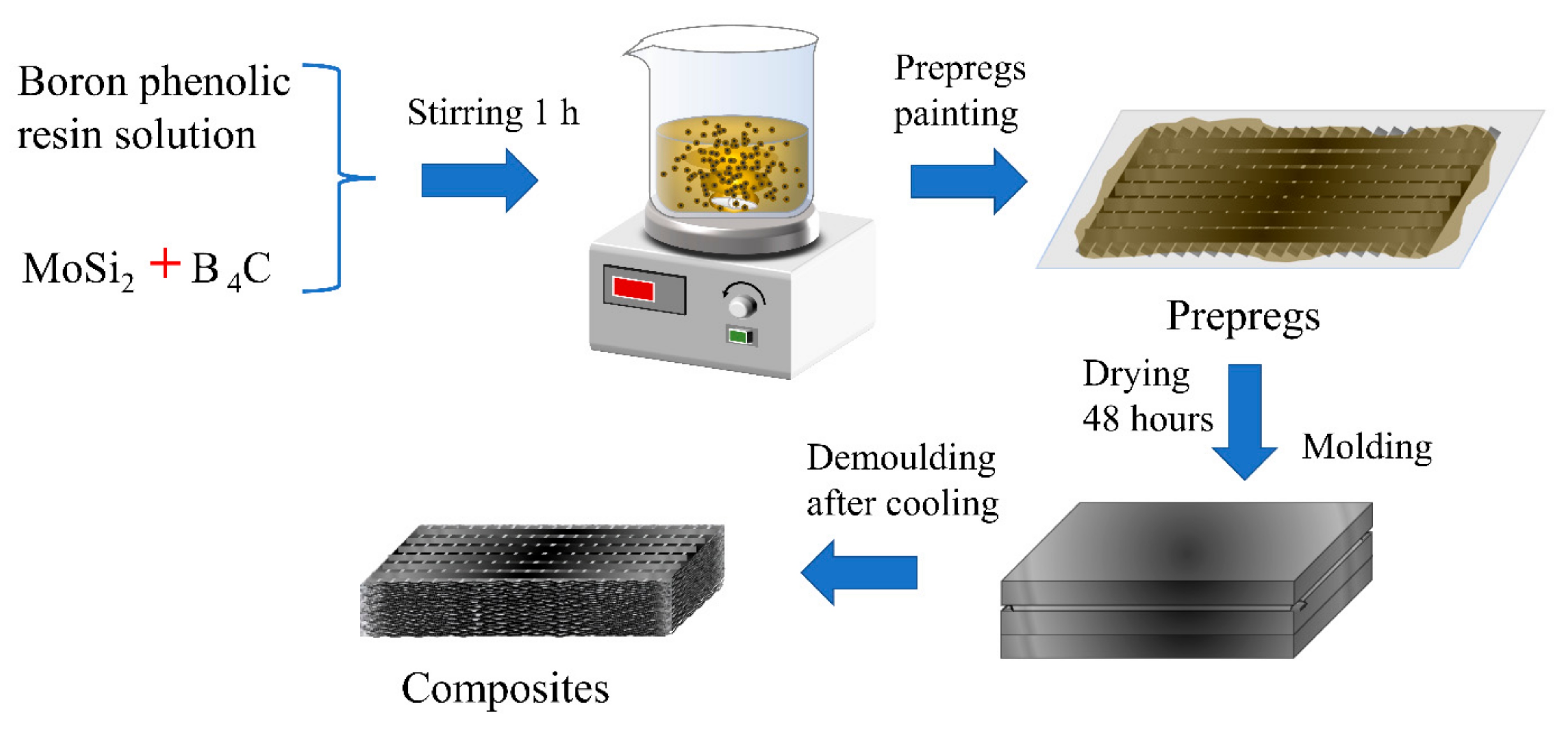





| Samples | Boron Phenolic Resin Solution (phr) | MoSi2 (phr) | B4C (phr) |
|---|---|---|---|
| BP-0 | 100 | 65 | 0 |
| BP-5 | 100 | 65 | 5 |
| BP-10 | 100 | 65 | 10 |
| BP-15 | 100 | 65 | 15 |
| BP-20 | 100 | 65 | 20 |
| Samples | 1 Tmax/°C | Residue Yield/% | ||
|---|---|---|---|---|
| 500 °C | 1000 °C | 1400 °C | ||
| BP-0 | 755.2 | 93.20 | 50.78 | 24.03 |
| BP-5 | 813.4 | 92.11 | 54.02 | 28.36 |
| BP-10 | 885.0 | 93.44 | 66.31 | 30.22 |
| BP-15 | 886.0 | 93.93 | 69.92 | 32.91 |
| BP-20 | 892.4 | 93.79 | 66.65 | 30.02 |
| Treatment Temperature | /μm | ||
|---|---|---|---|
| Carbon Fibers | BP-0 | BP-15 | |
| RT | 7.118 | 7.118 | 7.118 |
| 800 °C | 6.034 | 6.178 | 6.892 |
| 1000 °C | 5.393 | 5.487 | 6.296 |
| 1200 °C | 4.999 | 5.097 | 5.592 |
| 1400 °C | 4.863 | 6.042 | 6.681 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Dong, C.; Rong, Y.; Deng, Z.; Li, P.; Han, P.; Shi, M.; Huang, Z. Oxidation Behavior of Carbon Fibers in Ceramizable Phenolic Resin Matrix Composites at Elevated Temperatures. Polymers 2022, 14, 2785. https://doi.org/10.3390/polym14142785
Yang T, Dong C, Rong Y, Deng Z, Li P, Han P, Shi M, Huang Z. Oxidation Behavior of Carbon Fibers in Ceramizable Phenolic Resin Matrix Composites at Elevated Temperatures. Polymers. 2022; 14(14):2785. https://doi.org/10.3390/polym14142785
Chicago/Turabian StyleYang, Tingli, Chuang Dong, Yiyang Rong, Zongyi Deng, Pengfei Li, Pengkun Han, Minxian Shi, and Zhixiong Huang. 2022. "Oxidation Behavior of Carbon Fibers in Ceramizable Phenolic Resin Matrix Composites at Elevated Temperatures" Polymers 14, no. 14: 2785. https://doi.org/10.3390/polym14142785
APA StyleYang, T., Dong, C., Rong, Y., Deng, Z., Li, P., Han, P., Shi, M., & Huang, Z. (2022). Oxidation Behavior of Carbon Fibers in Ceramizable Phenolic Resin Matrix Composites at Elevated Temperatures. Polymers, 14(14), 2785. https://doi.org/10.3390/polym14142785






