Organic–Inorganic Double-Gel System Thermally Insulating and Hydrophobic Polyimide Aerogel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Amino-Modified SiO2@GO (SiO2@GO-NH2) Wet Gel by Sol-Gel Method
2.3. Preparation of Polyamic Acid (PAA) Precursors and Wet Gel
2.4. Preparation of Polyimide (PI) Aerogels
2.5. Characterization
3. Results and Discussion
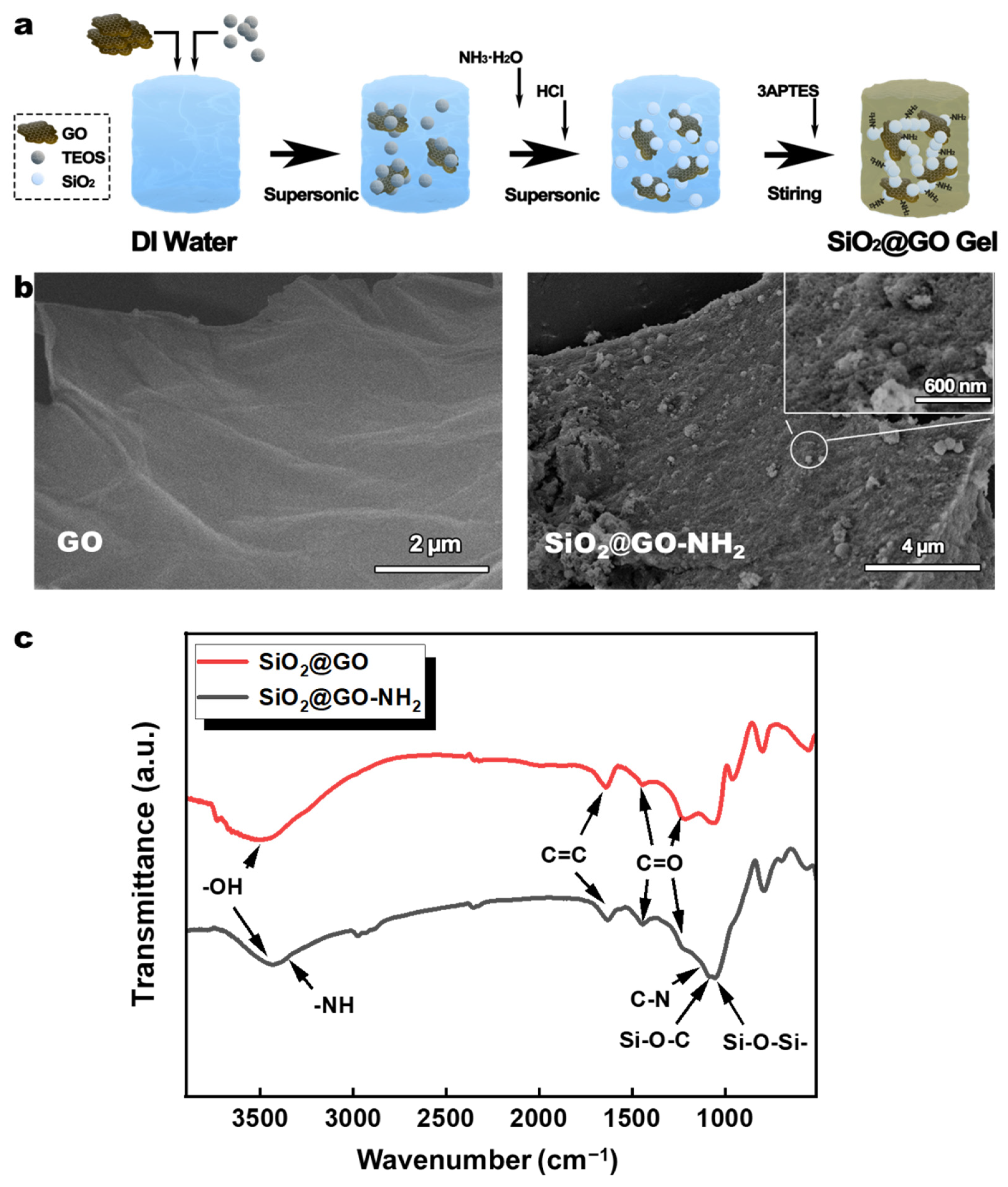
3.1. Amino-Modified SiO2@GO (SiO2@GO-NH2) Wet Gel
3.2. Preparation of PI Aerogel with Inorganic–Organic Double-Gel System
3.3. Chemical Structure of PI Aerogel
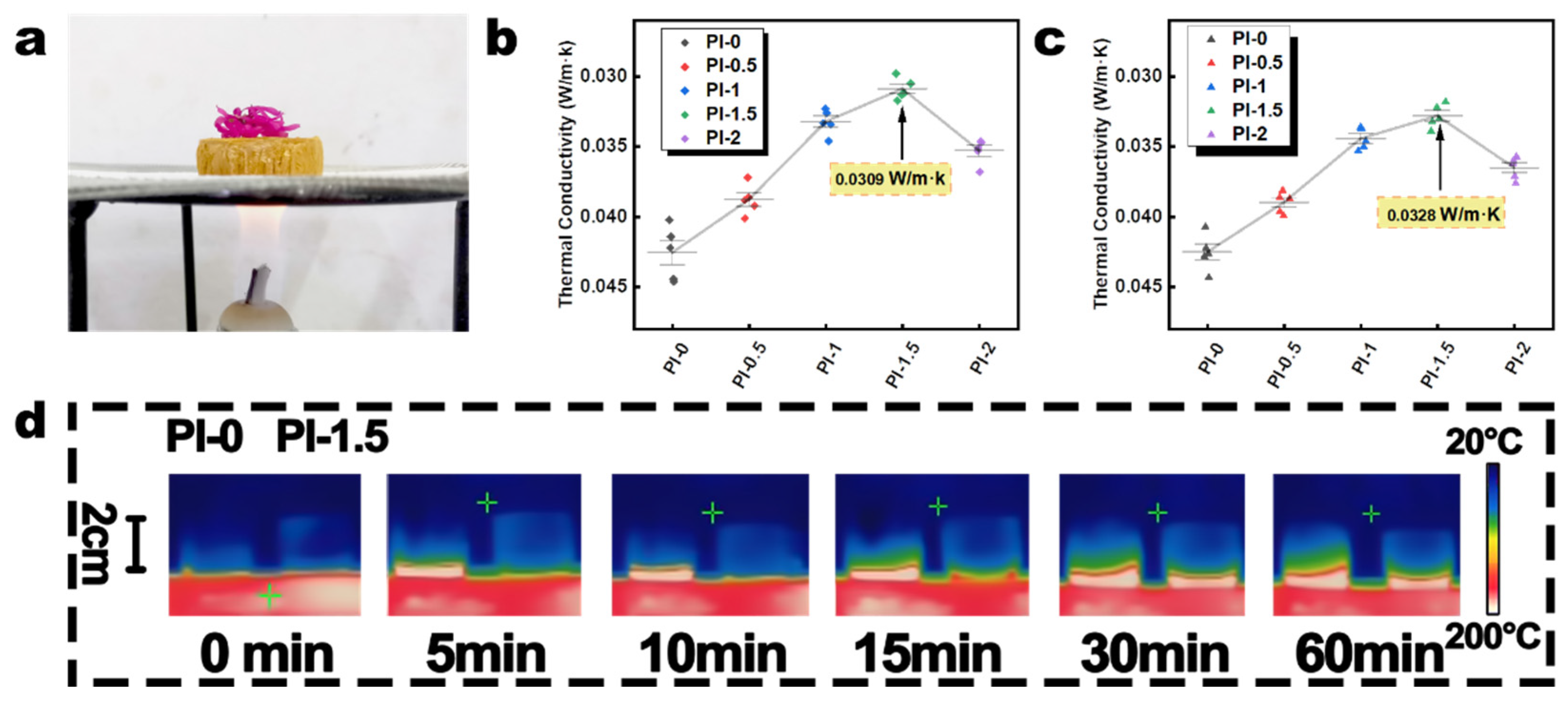
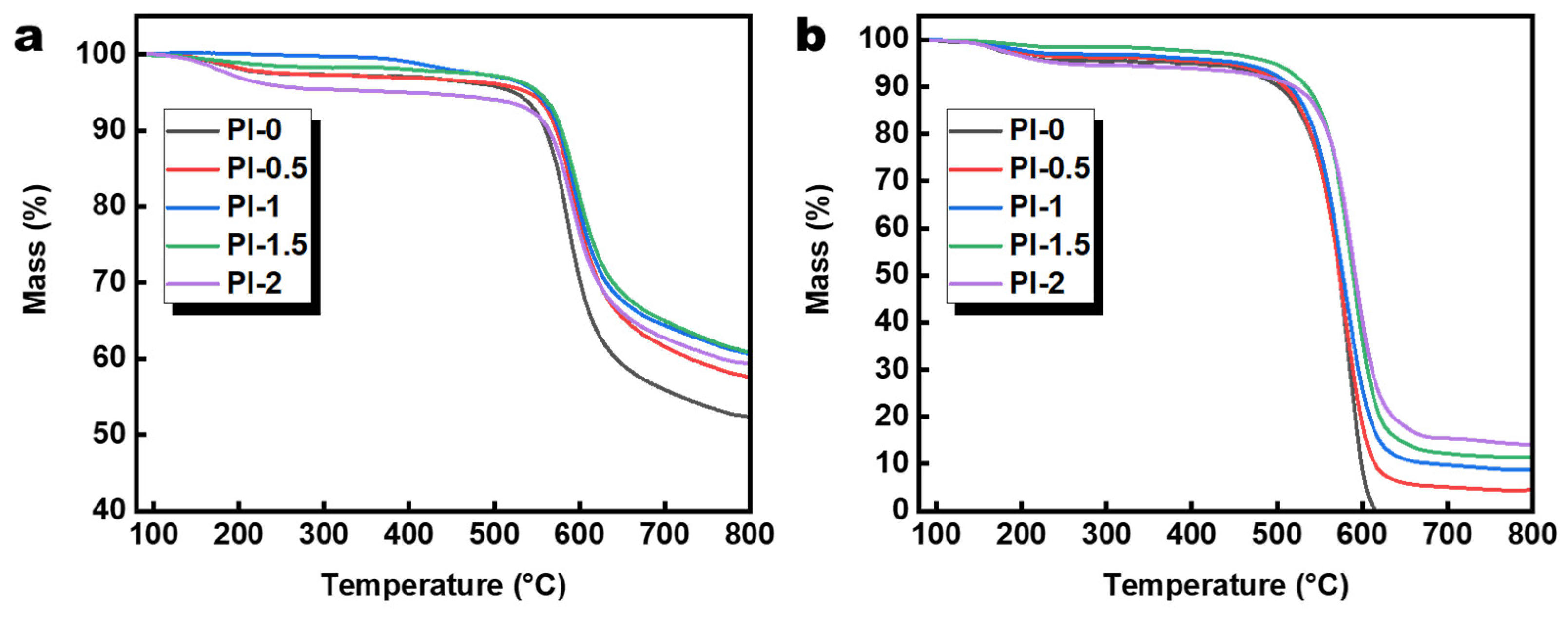
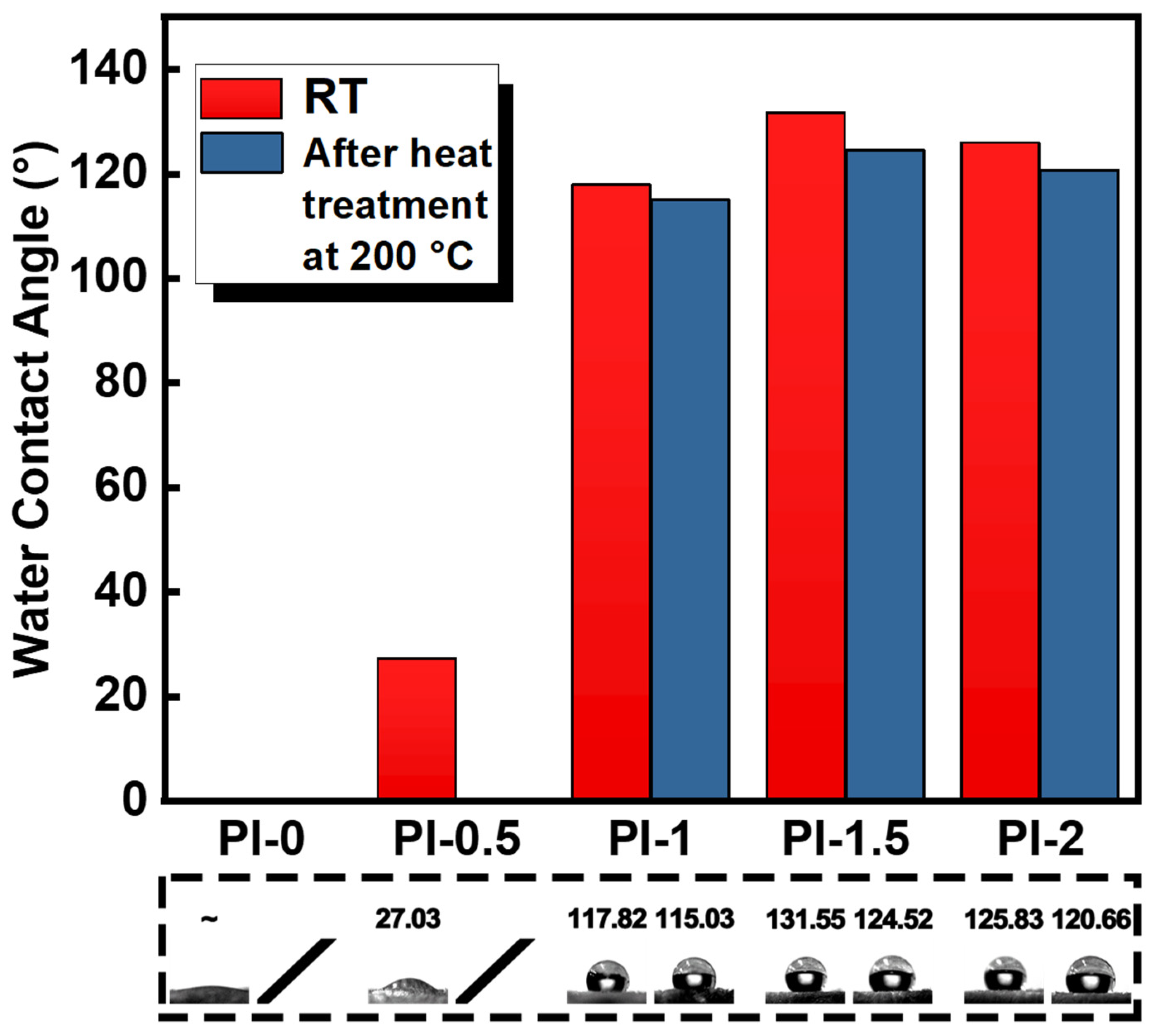
3.4. Properties of PI Aerogel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Memon, S.A. Phase change materials integrated in building walls: A state of the art review. Renew. Sustain. Energy Rev. 2014, 31, 870–906. [Google Scholar] [CrossRef]
- Akeiber, H.; Nejat, P.; Majid, M.Z.A.; Wahid, M.A.; Jomehzadeh, F.; Famileh, I.Z.; Calautit, J.K.; Hughes, B.R.; Zaki, S.A. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Nejat, P.; Jomehzadeh, F.; Taheri, M.M.; Gohari, M.; Majid, M.Z.A. A global review of energy consumption, CO2 emissions and policy in the residential sector (with an overview of the top ten CO2 emitting countries). Renew. Sustain. Energy Rev. 2015, 43, 843–862. [Google Scholar] [CrossRef]
- Sikder, A.; Sikder, N. A review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications. J. Hazard. Mater. 2004, 112, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in polyimide-based materials for space appli-cations. Adv. Mater. 2019, 31, e1807738. [Google Scholar] [CrossRef]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent advances in energy-saving chemiresistive gas sensors: A review. Nano Energy 2021, 79, 105369. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, T.; Ma, A. Recent Advances in Design and Preparation of Polymer-Based Thermal Management Material. Polymers 2021, 13, 2797. [Google Scholar] [CrossRef] [PubMed]
- Kaya, G.G.; Deveci, H. Synergistic effects of silica aerogels/xerogels on properties of polymer composites: A review. J. Ind. Eng. Chem. 2020, 89, 13–27. [Google Scholar] [CrossRef]
- Peng, F.; Jiang, Y.; Feng, J.; Cai, H.; Feng, J.; Li, L. Thermally insulating, fiber-reinforced alumina–silica aerogel composites with ultra-low shrinkage up to 1500 °C. Chem. Eng. J. 2021, 411, 128402. [Google Scholar] [CrossRef]
- Su, L.; Wang, H.; Niu, M.; Fan, X.; Ma, M.; Shi, Z.; Guo, S.-W. Ultralight, Recoverable, and High-Temperature-Resistant SiC Nanowire Aerogel. ACS Nano 2018, 12, 3103–3111. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, N.; Ma, Y.; Wang, S.; Liu, W.; Luo, C.; Zhang, H.; Cheng, F.; Rao, J.; Hu, X.; et al. Highly Self-Healable 3D Mi-crosupercapacitor with MXene-Graphene Composite Aerogel. ACS Nano 2018, 12, 4224–4232. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.-B.; Sun, R.; Liu, Y.; Liu, Z.; Zhou, A.; Yu, Z.-Z. Hydrophobic, Flexible, and Lightweight MXene Foams for High-Performance Electromagnetic-Interference Shielding. Adv. Mater. 2017, 29, 1702367. [Google Scholar] [CrossRef]
- An, L.; Wang, J.; Petit, D.; Armstrong, J.; Hanson, K.; Hamilton, J.; Souza, M.; Zhao, D.; Li, C.; Liu, Y.; et al. An All-Ceramic, Anisotropic, and Flexible Aerogel Insulation Material. Nano Lett. 2020, 20, 3828–3835. [Google Scholar] [CrossRef] [PubMed]
- Karamikamkar, S.; Naguib, H.E.; Park, C.B. Advances in precursor system for silica-based aerogel production toward improved mechanical properties, customized morphology, and multifunctionality: A review. Adv. Colloid Interface Sci. 2020, 276, 102101. [Google Scholar] [CrossRef]
- Ahankari, S.; Paliwal, P.; Subhedar, A.; Kargarzadeh, H. Recent developments in nanocellulose-based aerogels in thermal applications: A review. ACS Nano 2021, 15, 3849–3874. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chu, Y.; Wu, W.; Xiao, H. Nanocellulose-based lightweight porous materials: A review. Carbohydr. Polym. 2020, 255, 117489. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.; Duan, G.; Jiang, S.; Zhang, K. Recent progress on nanocellulose aerogels: Prepa-ration, modification, composite fabrication, applications. Adv. Mater. 2021, 33, e2005569. [Google Scholar] [CrossRef]
- Qian, H.; Wang, J. Synthesis of lignin-poly(N-methylaniline)-reduced graphene oxide hydrogel for organic dye and lead ions removal. J. Bioresour. Bioprod. 2020, 5, 204–210. [Google Scholar] [CrossRef]
- Shi, W.; Ching, Y.C.; Chuah, C.H. Preparation of aerogel beads and microspheres based on chitosan and cellulose for drug delivery: A review. Int. J. Biol. Macromol. 2021, 170, 751–767. [Google Scholar] [CrossRef]
- Lin, D.; Yuen, P.Y.; Liu, Y.; Liu, W.; Liu, N.; Dauskardt, R.H.; Cui, Y. A Silica-Aerogel-Reinforced Composite Polymer Electrolyte with High Ionic Conductivity and High Modulus. Adv. Mater. 2018, 30, e1802661. [Google Scholar] [CrossRef]
- Gao, L.; Li, C.; Huang, W.; Mei, S.; Lin, H.; Ou, Q.; Zhang, Y.; Guo, J.; Zhang, F.; Xu, S.; et al. MXene/Polymer membranes: Synthesis, properties, and emerging applications. Chem. Mater. 2020, 32, 1703–1747. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.; Yang, W.; Xiao, C.; Wei, M. Effects of different amine-functionalized graphene on the mechanical, thermal, and tribological properties of polyimide nanocomposites synthesized by in situ polymerization. Polymer 2018, 140, 56–72. [Google Scholar] [CrossRef]
- Li, X.; Dong, G.; Liu, Z.; Zhang, X. Polyimide Aerogel Fibers with Superior Flame Resistance, Strength, Hydrophobicity, and Flexibility Made via a Universal Sol-Gel Confined Transition Strategy. ACS Nano 2021, 15, 4759–4768. [Google Scholar] [CrossRef]
- Wang, N.-N.; Wang, H.; Wang, Y.-Y.; Wei, Y.-H.; Si, J.-Y.; Yuen, A.C.Y.; Xie, J.-S.; Yu, B.; Zhu, S.-E.; Lu, H.-D.; et al. Robust, Lightweight, Hydrophobic, and Fire-Retarded Polyimide/MXene Aerogels for Effective Oil/Water Separation. ACS Appl. Mater. Interfaces 2019, 11, 40512–40523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lin, Y.; Wang, W.; Li, Y.; Wu, G. Mechanically strong polyimide aerogels cross-linked with dopa-mine-functionalized carbon nanotubes for oil absorption. Appl. Surf. Sci. 2021, 543, 148833. [Google Scholar] [CrossRef]
- Wang, D.; Peng, H.; Yu, B.; Zhou, K.; Pan, H.; Zhang, L.; Li, M.; Liu, M.; Tian, A.; Fu, S. Biomimetic structural cellulose nanofiber aerogels with exceptional mechanical, flame-retardant and thermal-insulating properties. Chem. Eng. J. 2020, 389, 124449. [Google Scholar] [CrossRef]
- Zhu, J.; Xiong, R.; Zhao, F.; Peng, T.; Hu, J.; Xie, L.; Xie, H.; Wang, K.; Jiang, C. Lightweight, High-Strength, and Anisotropic Structure Composite Aerogel Based on Hydroxyapatite Nanocrystal and Chitosan with Thermal Insulation and Flame Retardant Properties. ACS Sustain. Chem. Eng. 2019, 8, 71–83. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Nie, X.; Yang, W.; Wang, Y.; Yu, R.; Shui, J. Multifunctional organic–inorganic hybrid aerogel for self-cleaning, heat-insulating, and highly efficient microwave absorbing material. Adv. Funct. Mater. 2019, 29, 1807624. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.-J.; Li, D.-S.; Jiang, L. Lightweight and recoverable ANF/rGO/PI composite aerogels for broad and high-performance microwave absorption. Compos. Part B Eng. 2021, 213, 108701. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, H.; Xu, J.; Du, X.; Cheng, X.; Du, Z.; Wang, H. Fabrication of MXene/PEI functionalized sodium alginate aerogel and its excellent adsorption behavior for Cr(VI) and Congo Red from aqueous solution. J. Hazard. Mater. 2021, 416, 125777. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Muhetaer, M.; Wang, K. Silver nanoparticles cross-linked polyimide aerogels with improved high tem-perature microstructure stabilities and high mechanical performances. Microporous Mesoporous Mater. 2020, 297, 110035. [Google Scholar] [CrossRef]
- Iglesias-Mejuto, A.; García-González, C.A. 3D-printed alginate-hydroxyapatite aerogel scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2021, 131, 112525. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, X.; Zheng, Y.; Yue, Y. “Oxynitride trap” over N/S co-doped graphene-supported catalysts promoting low temperature NH3-SCR performance: Insight into the structure and mechanisms. J. Hazard. Mater. 2021, 423, 127187. [Google Scholar] [CrossRef]
- Zhuang, Y.; Seong, J.G.; Lee, Y.M. Polyimides containing aliphatic/alicyclic segments in the main chains. Prog. Polym. Sci. 2019, 92, 35–88. [Google Scholar] [CrossRef]
- Ma, P.; Dai, C.; Wang, H.; Li, Z.; Liu, H.; Li, W.; Yang, C. A review on high temperature resistant polyimide films: Heterocyclic structures and nanocomposites. Compos. Commun. 2019, 16, 84–93. [Google Scholar] [CrossRef]
- He, X.; Zhang, L.; Meng, D.; Wu, J. From hydrogel to aerogel: A green fabrication of multifunctional polyimide absorbents. Eur. Polym. J. 2017, 89, 461–467. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Haeri, Z.; Ramezanzadeh, M. A facile route of making silica nanoparticles-covered graphene oxide na-nohybrids (SiO2-GO); fabrication of SiO2-GO/epoxy composite coating with superior barrier and corrosion protection per-formance. Chem. Eng. J. 2016, 303, 511–528. [Google Scholar] [CrossRef]
- Xu, Q.; Chang, X.; Zhu, Z.; Xu, L.; Chen, X.; Luo, L.; Liu, X.; Qin, J. Flexible pressure sensors with high pressure sensitivity and low detection limit using a unique honeycomb-designed polyimide/reduced graphene oxide composite aerogel. RSC Adv. 2021, 11, 11760–11770. [Google Scholar] [CrossRef]
- Xue, T.; Fan, W.; Zhang, X.; Zhao, X.; Yang, F.; Liu, T. Layered double hydroxide/graphene oxide synergistically enhanced pol-yimide aerogels for thermal insulation and fire-retardancy. Compos. Part B Eng. 2021, 219, 108963. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Song, X.; Su, B.; Mandal, B.; Prasad, B.; Gao, X.; Gao, C. Graphene quantum dots-doped thin film nanocomposite polyimide membranes with enhanced solvent resistance for solvent-resistant nanofiltration. ACS Appl. Mater. Interfaces 2019, 11, 6527–6540. [Google Scholar] [CrossRef]
- Zhou, X.; Ding, C.; Cheng, C.; Liu, S.; Duan, G.; Xu, W.; Liu, K.; Hou, H. Mechanical and thermal properties of electrospun pol-yimide/rGO composite nanofibers via in-situ polymerization and in-situ thermal conversion. Eur. Polym. J. 2020, 141, 110083. [Google Scholar] [CrossRef]
- Feng, H.; Fang, X.; Liu, X.; Pei, Q.; Cui, Z.-K.; Deng, S.; Gu, J.; Zhuang, Q. Reduced polyaniline decorated reduced graphene ox-ide/polyimide nanocomposite films with enhanced dielectric properties and thermostability. Compos. Part A Appl. Sci. Manuf. 2018, 109, 578–584. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Song, P.; You, B.; Sun, G. Double-cross-linking strategy for preparing flexible, robust, and multifunctional polyimide aerogel. Chem. Eng. J. 2019, 381, 122784. [Google Scholar] [CrossRef]
- Zhong, A.; Li, J.; Zhang, Y.; Zhang, F.; Wang, T.; Zhang, G.; Sun, R.; Wong, C.-P. Low temperature microwave fabrication of three-dimensional graphene/polyimide foams with flexibility strain responsivity. Compos. Part A Appl. Sci. Manuf. 2020, 137, 105995. [Google Scholar] [CrossRef]
- Wu, Y.; Ju, D.; Wang, H.; Zhao, H.; Sun, C.; Wu, Y.; Guo, B.; Wang, Y. Modification of surface structure and mechanical properties in polyimide aerogel by low-energy proton implantation. Surf. Coat. Technol. 2020, 403, 126364. [Google Scholar] [CrossRef]
- Hu, C.; Qi, H.; Song, J.; Zhao, G.; Yu, J.; Zhang, Y.; He, H.; Lai, J. Exploration on the tribological mechanisms of polyimide with different molecular structures in different temperatures. Appl. Surf. Sci. 2021, 560, 150051. [Google Scholar] [CrossRef]
- Jiang, X.; Pan, W.; Xiong, Z.; Zhang, Y.; Zhao, L. Facile synthesis of layer-by-layer decorated graphene oxide based magnetic nanocomposites for β-agonists/dyes adsorption removal and bacterial inactivation in wastewater. J. Alloys Compd. 2021, 870, 159414. [Google Scholar] [CrossRef]
- Sasikumar, B.; Bisht, S.; Arthanareeswaran, G.; Ismail, A.F.; Othman, M.H.D. Performance of polysulfone hollow fiber membranes encompassing ZIF-8, SiO2/ZIF-8, and amine-modified SiO2/ZIF-8 nanofillers for CO2/CH4 and CO2/N2 gas separation. Sep. Purif. Technol. 2021, 264, 118471. [Google Scholar] [CrossRef]
- Abbas, S.S.; Rees, G.J.; Kelly, N.L.; Dancer, C.E.J.; Hanna, J.V.; McNally, T. Facile silane functionalization of graphene oxide. Nanoscale 2018, 10, 16231–16242. [Google Scholar] [CrossRef] [Green Version]
- Kong, P.; Tan, H.; Lei, T.; Wang, J.; Yan, W.; Wang, R.; Waclawik, E.R.; Zheng, Z.; Li, Z. Oxygen vacancies confined in conjugated polyimide for promoted visible-light photocatalytic oxidative coupling of amines. Appl. Catal. B Environ. 2020, 272, 118964. [Google Scholar] [CrossRef]
- Zhang, X.; Ni, X.; Li, C.; You, B.; Sun, G. Co-gel strategy for preparing hierarchically porous silica/polyimide nanocomposite aerogel with thermal insulation and flame retardancy. J. Mater. Chem. A 2020, 8, 9701–9712. [Google Scholar] [CrossRef]
- Hassanzadeh-Aghdam, M.; Ansari, R.; Darvizeh, A. Micromechanical modeling of thermal expansion coefficients for unidirectional glass fiber-reinforced polyimide composites containing silica nanoparticles. Compos. Part A Appl. Sci. Manuf. 2017, 96, 110–121. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Li, X.; Ma, X.; Li, S.; Sun, M.; Liu, H.; Wang, K. Ultralight and heat-insulating mesoporous polyimide aerogels cross-linked with aminated SiO2 nanoparticles. Microporous Mesoporous Mater. 2021, 319, 111074. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Wu, T.; Dong, J.; De France, K.J.; Li, M.; Zhao, X.; Zhang, Q. Fabrication of polyimide aerogels cross-linked by a cost-effective amine-functionalized hyperbranched polysiloxane (NH2–HBPSi). ACS Appl. Polym. Mater. 2020, 2, 3876–3885. [Google Scholar] [CrossRef]
- Pan, C.; Liu, L.; Chen, Q.; Zhang, Q.; Guo, G. Tough, Stretchable, Compressive Novel Polymer/Graphene Oxide Nano-composite Hydrogels with Excellent Self-Healing Performance. ACS Appl. Mater. Interfaces 2017, 9, 38052–38061. [Google Scholar] [CrossRef]
- Zou, W.; Dong, J.; Luo, Y.; Zhao, Q.; Xie, T. Dynamic covalent polymer networks: From old chemistry to modern day innovations. Adv. Mater. 2017, 29, 1606100. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Li, Z.; Wang, Y.; Dai, K.; Zheng, G.; Liu, C.; Shen, C. The effect of filler dimensionality on the electromechanical performance of polydimethylsiloxane based conductive nanocomposites for flexible strain sensors. Compos. Sci. Technol. 2017, 139, 64–73. [Google Scholar] [CrossRef]
- Jiang, S.; Cheong, J.Y.; Nam, J.S.; Kim, I.-D.; Agarwal, S.; Greiner, A. High-density Fibrous Polyimide Sponges with Superior Mechanical and Thermal Properties. ACS Appl. Mater. Interfaces 2020, 12, 19006–19014. [Google Scholar] [CrossRef]
- Yang, X.; Fan, S.; Li, Y.; Guo, Y.; Li, Y.; Ruan, K.; Zhang, S.; Zhang, J.; Kong, J.; Gu, J. Synchronously improved electromagnetic interference shielding and thermal conductivity for epoxy nanocomposites by constructing 3D copper nanowires/thermally annealed graphene aerogel framework. Compos. Part A Appl. Sci. Manuf. 2019, 128, 105670. [Google Scholar]
- Jin, X.; Wang, J.; Dai, L.; Liu, X.; Li, L.; Yang, Y.; Cao, Y.; Wang, W.; Wu, H.; Guo, S. Flame-retardant poly(vinyl alcohol)/MXene multilayered films with outstanding electromagnetic interference shielding and thermal conductive performances. Chem. Eng. J. 2020, 380, 122475. [Google Scholar] [CrossRef]
- Zhang, Z.; Ouyang, Y.; Cheng, Y.; Chen, J.; Li, N.; Zhang, G. Size-dependent phononic thermal transport in low-dimensional nanomaterials. Phys. Rep. 2020, 860, 1–26. [Google Scholar] [CrossRef]
- Qin, Y.; Peng, Q.; Ding, Y.; Lin, Z.; Wang, C.; Li, Y.; Xu, F.; Li, J.; Yuan, Y.; He, X.; et al. Lightweight, Superelastic, and Mechanically Flexible Graphene/Polyimide Nanocomposite Foam for Strain Sensor Application. ACS Nano 2015, 9, 8933–8941. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Chen, J.; Li, P.; Duan, G.; Hou, H. Robust strong electrospun polyimide composite nanofibers from a ternary polyamic acid blend. Compos. Commun. 2019, 15, 92–95. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.-Y.; Liu, Q.; Zhu, S.-P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, L.; Zheng, W.; Cao, S.; Zheng, Y. Organic–Inorganic Double-Gel System Thermally Insulating and Hydrophobic Polyimide Aerogel. Polymers 2022, 14, 2818. https://doi.org/10.3390/polym14142818
Xiong L, Zheng W, Cao S, Zheng Y. Organic–Inorganic Double-Gel System Thermally Insulating and Hydrophobic Polyimide Aerogel. Polymers. 2022; 14(14):2818. https://doi.org/10.3390/polym14142818
Chicago/Turabian StyleXiong, Liyao, Weijie Zheng, Shenglong Cao, and Yuying Zheng. 2022. "Organic–Inorganic Double-Gel System Thermally Insulating and Hydrophobic Polyimide Aerogel" Polymers 14, no. 14: 2818. https://doi.org/10.3390/polym14142818





