A Green Resin Wood Adhesive from Synthetic Polyamide Crosslinking with Glyoxal
Abstract
:1. Introduction
2. Materials and Methods
2.1. CHG Adhesive Resins Preparation
2.2. FTIR Spectrometry
2.3. Characterization by ESI-MS (Electrospray Ionization Mass Spectrometry)
2.4. Differential Scanning Calorimetry
2.5. Dynamic Thermomechanical Analysis (DMA)
2.6. Thermogravimetric Analysis (TGA)
2.7. Preparation and Testing of Plywood
3. Results and Discussion
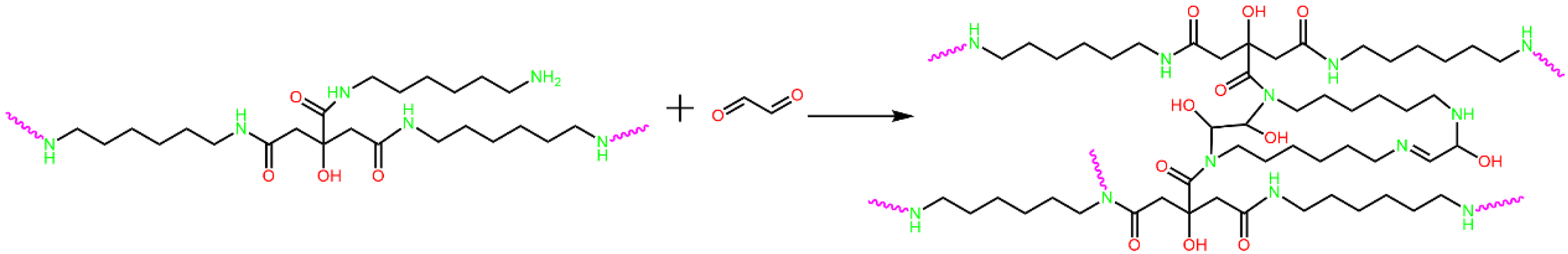
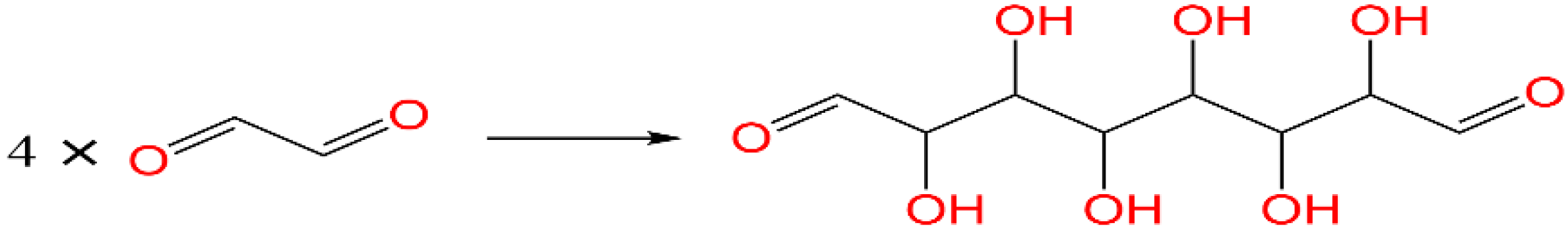
3.1. LC-MS Analysis of CHG Resin
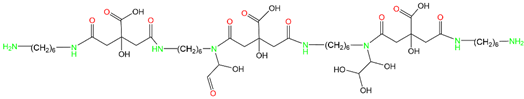
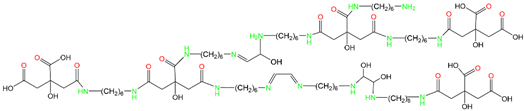
3.2. FTIR Analysis of CHGs
3.3. Bonding Performance of Resins
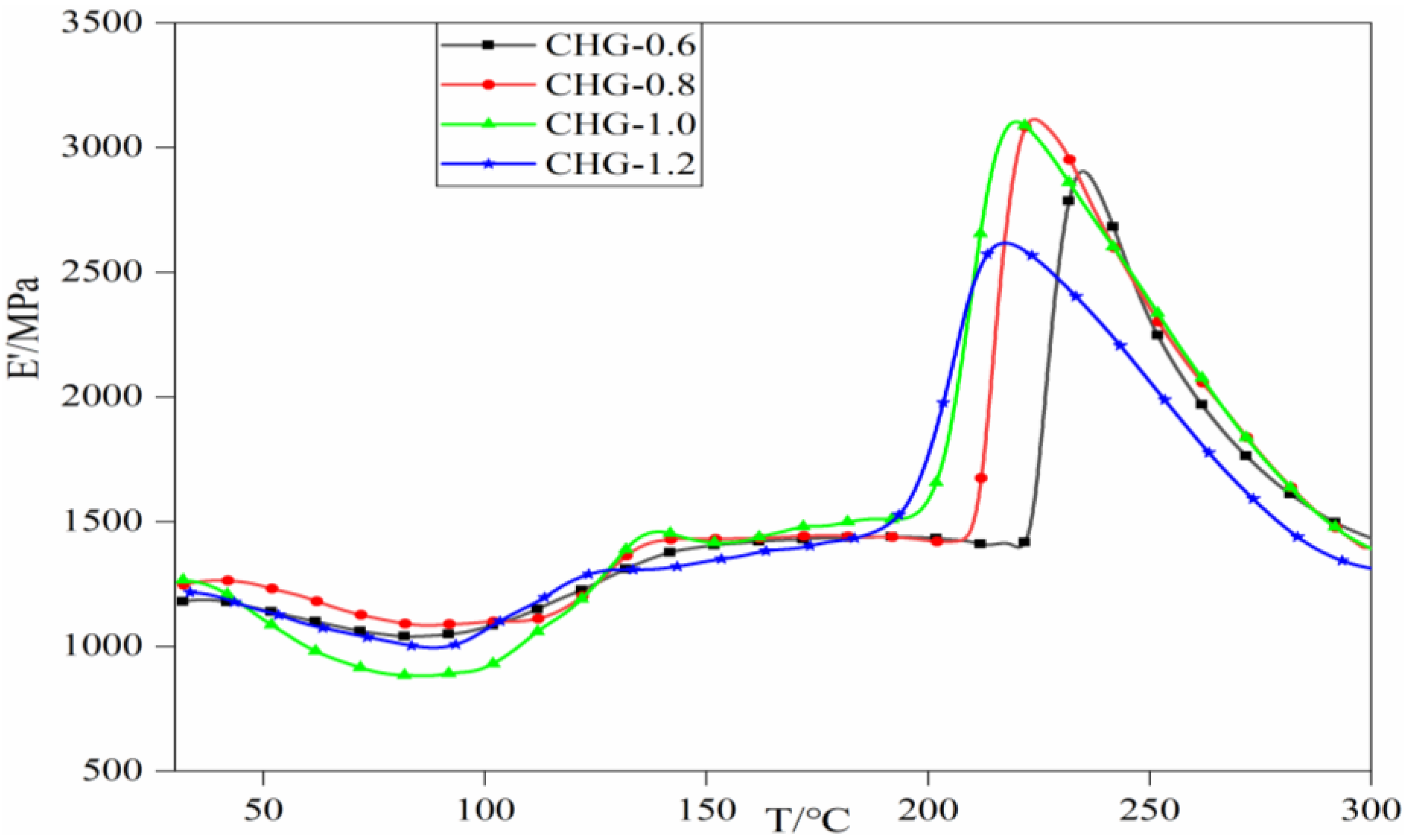
3.4. DMA Analysis of CHGs
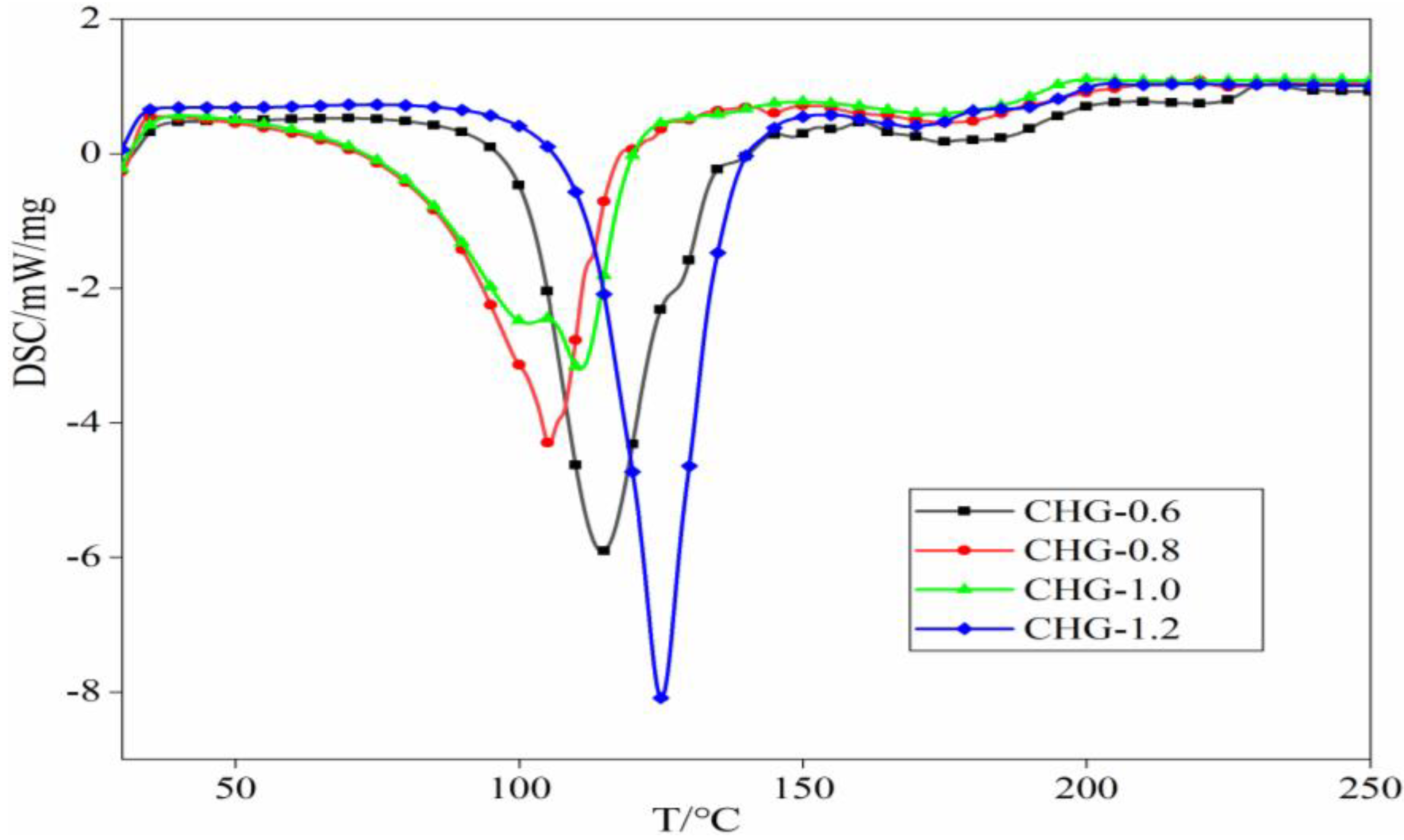
3.5. DSC Analysis CHGs
3.6. TG Analysis CHG Resins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pizzi, A.; Papadopoulos, A.N.; Policardi, F. Wood Composites and Their Polymer Binders. Polymers 2020, 12, 1115. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jeong, B.; Park, B.D. A Comparison of Adhesion Behavior of Urea-Formaldehyde Resins with Melamine-Urea-Formaldehyde Resins in Bonding Wood. Forests 2021, 12, 1037. [Google Scholar] [CrossRef]
- Pizzi, A. Wood products and green chemistry. Ann. For. Sci. 2016, 73, 185–203. [Google Scholar] [CrossRef] [Green Version]
- Ayrilmis, N.; Kaptı, T.; Gürel, A.; Ohlmeyer, M. Reducing formaldehyde emission from wood-based panels by modification of UF and MUF resins with condensates obtained from kiln-drying of wood. Holzforschung 2018, 72, 753–757. [Google Scholar] [CrossRef]
- Song, J.; Chen, S.; Yi, X.; Zhao, X.; Zhang, J.; Liu, X.; Liu, B. Preparation and Properties of the Urea-Formaldehyde Res-In/Reactive Halloysite Nanocomposites Adhesive with Low-Formaldehyde Emission and Good Water Resistance. Polymers 2021, 13, 2224. [Google Scholar] [CrossRef]
- Cao, J.; Jin, S.; Li, C.; Li, J. Bioinspired mineral–organic hybridization strategy to produce a green high performance soybean meal based adhesive. J. Clean. Prod. 2021, 299, 126939. [Google Scholar] [CrossRef]
- Ding, X.; Dai, R.; Chen, H.; Shan, Z. Gelatin as green adhesive for the preparation of a multifunctional biobased cryogel derived from bamboo industrial waste. Carbohydr. Polym. 2020, 255, 117340. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Kasbe, P.S.; Mahanwar, P.A.; Gadekar, P.T. Synthesis and characterization of lignin-polyurethane based wood adhesive. Int. J. Adhes. Adhes. 2019, 95, 102427. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Y.; Di, M. Green Modification of Corn Stalk Lignin and Preparation of Environmentally Friendly Lignin-Based Wood Adhesive. Polymers 2018, 10, 631. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Din, Z.; Yang, D.; Hu, C.; Cai, J.; Xiong, H. Functional nanoparticle reinforced starch-based adhesive emulsion: Toward robust stability and high bonding performance. Carbohydr. Polym. 2021, 269, 118270. [Google Scholar] [CrossRef]
- Frihart, C.R.; Pizzi, A.; Xi, X.; Lorenz, L.F. Reactions of Soy Flour and Soy Protein by Non-Volatile Aldehydes Generation by Specific Oxidation. Polymers 2019, 11, 1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Technical Information Service (NTIS). Formerly U.S. Clearinghouse for Scientific and Technical Information; AD-A125-539; National Technical Information Service (NTIS): Alexandria, VA, USA, 2005.
- Deng, S.; Du, G.; Li, X.; Pizzi, A. Performance and reaction mechanism of zero formaldehyde-emission urea-glyoxal (UG) resin. J. Taiwan Inst. Chem. Eng. 2014, 45, 2029–2038. [Google Scholar] [CrossRef]
- Deng, S.; Pizzi, A.; Du, G.; Zhang, J.; Zhang, J. Synthesis, Structure, and Characterization of Glyoxal-Urea- Formaldehyde Cocondensed Resins. J. Appl. Polym. Sci. 2014, 131, 41009. [Google Scholar] [CrossRef]
- Deng, S.; Pizzi, A.; Du, G.; Lagel, M.C.; Delmotte, L.; Abdalla, S. Synthesis, structure characterization and application of melamine–glyoxal adhesive resins. Eur. J. Wood Wood Prod. 2018, 76, 283–296. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Amirou, S. Melamine–Glyoxal–Glutaraldehyde Wood Panel Adhesives without Formaldehyde. Polymers 2017, 10, 22. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Yuan, L.; Sheng, N.; Gu, Z.; Feng, W.; Yin, H.; Morsi, Y.; Mo, X. A soft tissue adhesive based on aldehyde-sodium alginate and amino-carboxymethyl chitosan preparation through the Schiff reaction. Front. Mater. Sci. 2017, 11, 215–222. [Google Scholar] [CrossRef]
- Pervaiz, M.; Faruq, M.; Jawaid, M.; Sain, M. Polyamides: Developments and Applications Towards Next-Generation Engineered Plastics. Curr. Org. Synth. 2017, 14, 146–155. [Google Scholar] [CrossRef]
- Kazuma, Y.; Yunfan, Z.; Yukiko, E.; Tadahisa, I. Synthesis of highly thermally stable divanillic acid-derived polyamides and their mechanical properties. Polymer 2021, 228, 123907. [Google Scholar]
- Griffiths, K. Polyamides in aerospace industry. Trans. IMF 2013, 85, 235–236. [Google Scholar] [CrossRef]
- Long, C.; Antonio, P.; Qianyu, Z.; Heng, T.; Hong, L.; Xuedong, X.; Guanben, D. Preparation and characterization of a novel environment-friendly urea-glyoxal resin of improved bonding performance. Eur. Polym. J. 2022, 162, 110915. [Google Scholar]
- China National Standard GB/T 14074; Testing Methods for Wood Adhesives and Their Resins. Standardization Administration of China: Beijing, China, 2006.
- China National Standard GB/T17657; Test Methods for Evaluating the Properties of Wood-Based Panels and Surface Decorated Wood-Based Panels. Standardization Administration of China: Beijing, China, 2013.
- Li, T.; Zhang, B.; Jiang, S.; Zhou, X.; Du, G.; Wu, Z.; Cao, M.; Yang, L. Novel Highly Branched Polymer Wood Adhesive Resin. ACS Sustain. Chem. Eng. 2020, 8, 5209–5216. [Google Scholar] [CrossRef]
- Okuda, K.; Urabe, I.; Yamada, Y.; Okada, H. Reaction of glutaraldehyde with amino and thiol compounds. J. Ferment. Bioeng. 1991, 71, 100–105. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Gerardin, C.; Lei, H.; Chen, X.; Amirou, S. Preparation and Evaluation of Glucose Based Non-Isocyanate Polyurethane Self-Blowing Rigid Foams. Polymers 2019, 11, 1802. [Google Scholar] [CrossRef] [Green Version]
- Marques, M. Catalytic enantioselective cross-Mannich reaction of aldehydes. Angew. Chem. 2006, 37, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Theodor, S. Side-reactions in diisocyanate-derived bulk polyurea synthesis. J. Appl. Polym. Sci. 2020, 137, 49034. [Google Scholar]
- Xi, X.; Pizzi, A.; Gerardin, C.; Chen, X.; Amirou, S. Soy protein isolate-based polyamides as wood adhesives. Wood Sci. Technol. 2020, 54, 89–102. [Google Scholar] [CrossRef]
- Kamoun, C.; Pizzi, A. Particleboard IB forecast by TMA bending in MUF adhesives curing. Eur. J. Wood Prod. 2000, 58, 288–289. [Google Scholar] [CrossRef]
- Lecourt, M.; Pizzi, A.; Humphrey, P. Comparison of TMA and ABES as forecasting systems of wood bonding efectiveness. Eur. J. Wood Prod. 2003, 61, 75–76. [Google Scholar] [CrossRef]
- Lei, H.; Frazier, C.E. Curing behavior of melamine-urea-formaldehyde (MUF) resin adhesive. Int. J. Adhes. Adhes. 2015, 62, 40–44. [Google Scholar] [CrossRef]
- Simon, C.; George, B.; Pizzi, A. Copolymerization in UF/pMDI adhesives networks. J. Appl. Polym. Sci. 2002, 86, 3681–3688. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, Y.; Han, Y.; Chen, M.; Zhang, W.; Gao, Q.; Li, J.; Drasar, P.B.; Khripach, V.A. The Effect of Enzymolysis on Performance of Soy Protein-Based Adhesive. Molecules 2018, 23, 2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Luo, J.; Bai, Y.; Gao, Q.; Li, J. A high performance soy protein-based bio-adhesive enhanced with a melamine/epichlorohydrin prepolymer and its application on plywood. RSC Adv. 2016, 6, 67669–67676. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Xu, G.; Pizzi, A.; Lei, H.; Xi, X.; Du, G. A Green Resin Wood Adhesive from Synthetic Polyamide Crosslinking with Glyoxal. Polymers 2022, 14, 2819. https://doi.org/10.3390/polym14142819
Zhang Q, Xu G, Pizzi A, Lei H, Xi X, Du G. A Green Resin Wood Adhesive from Synthetic Polyamide Crosslinking with Glyoxal. Polymers. 2022; 14(14):2819. https://doi.org/10.3390/polym14142819
Chicago/Turabian StyleZhang, Qianyu, Gaoxiang Xu, Antonio Pizzi, Hong Lei, Xuedong Xi, and Guanben Du. 2022. "A Green Resin Wood Adhesive from Synthetic Polyamide Crosslinking with Glyoxal" Polymers 14, no. 14: 2819. https://doi.org/10.3390/polym14142819






