Melting Point Depression of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide) Triblock Polymers in Supercritical Carbon Dioxide in the Presence of Menthol as a Solid Co-Plasticiser
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
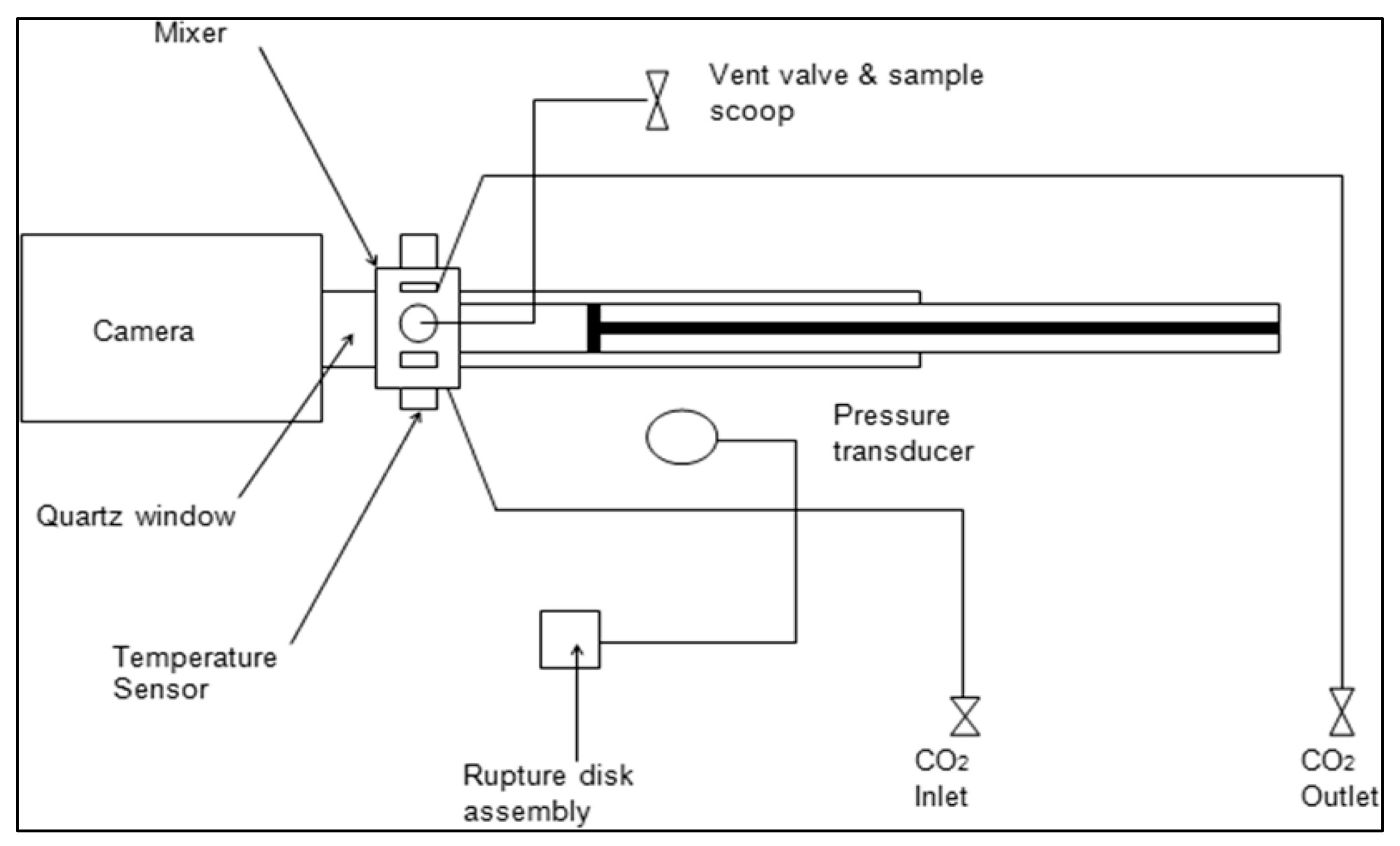
2.2.1. Determination of Melting Temperature of Pluronics and Mixtures in scCO2
2.2.2. Differential Scanning Calorimetry (DSC)
2.2.3. X-ray Diffraction
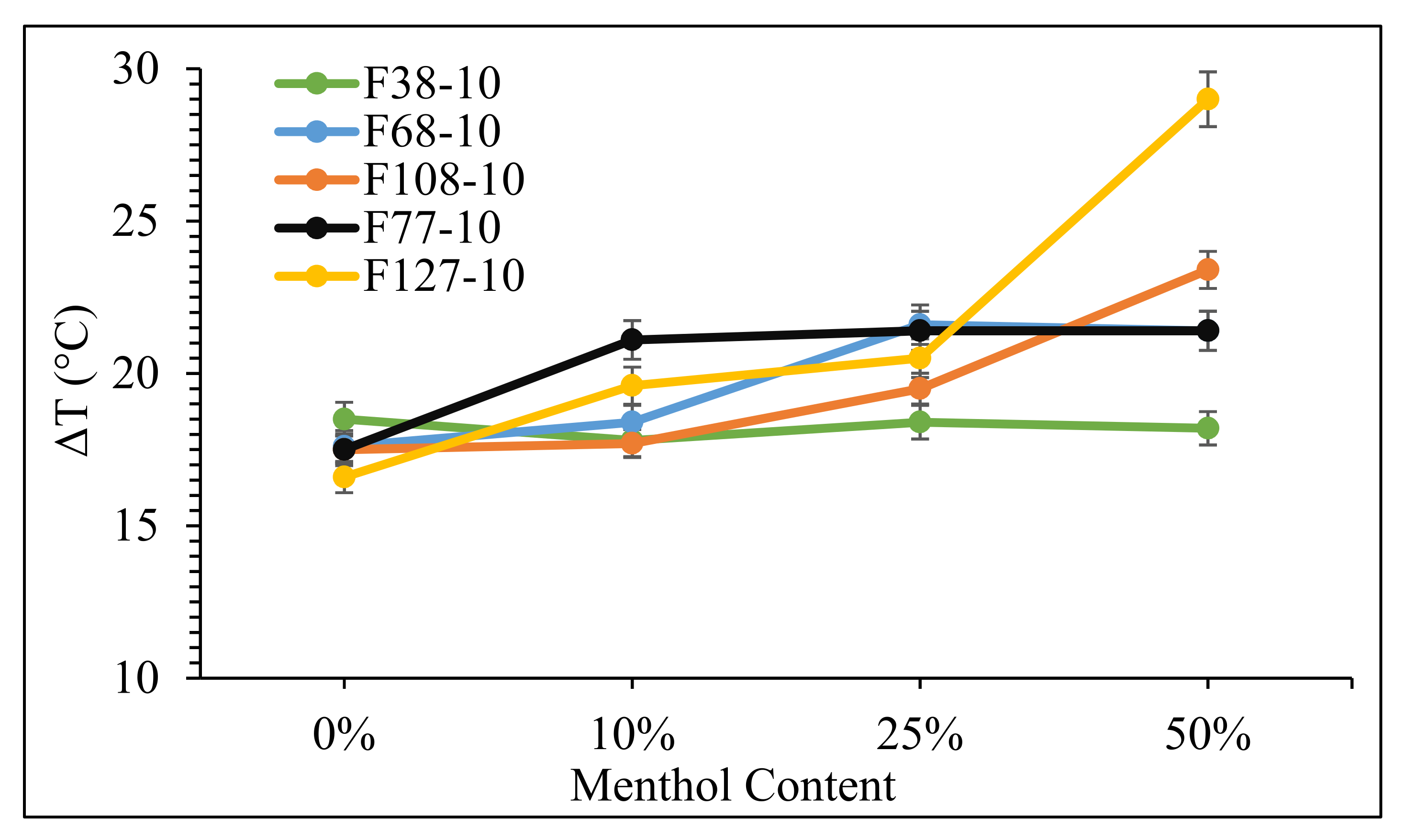
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexandridis, P.; Hatton, T.A. Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 7757, 1–46. [Google Scholar] [CrossRef]
- Fusco, S.; Borzacchiello, A.; Netti, A.P. Perspectives on: PEO-PPO-PEO Triblock Copolymers and their Biomedical Applications. J. Bioact. Compat. Polym. 2006, 21, 149–164. [Google Scholar] [CrossRef]
- Bhomia, R.; Trivedi, V.; Mitchell, J.C.; Coleman, N.J.; Snowden, M.J. Effect of Pressure on the Melting Point of Pluronics in Pressurized Carbon Dioxide. Ind. Eng. Chem. Res. 2014, 53, 10820–10825. [Google Scholar] [CrossRef]
- Yoganathan, R.B.; Mammucari, R.; Foster, N.R. Dense Gas Processing of Polymers. Polym. Rev. 2010, 50, 144–177. [Google Scholar] [CrossRef]
- Kirby, C.F.; McHugh, M.A. Phase Behavior of Polymers in Supercritical Fluid Solvents. Chem. Rev. 1999, 99, 565–602. [Google Scholar] [CrossRef] [PubMed]
- Conway, S.E.; Lim, J.S.; McHugh, M.A.; Wang, J.D.; Mandel, F.S. Phase behavior of semicrystalline polyester resin in supercritical fluid solvents and solvent mixtures: Implications for supercritical fluid processing. J. Appl. Polym. Sci. 2001, 81, 2642–2648. [Google Scholar] [CrossRef]
- Rindfleisch, F.; DiNoia, T.P.; McHugh, M.A. Solubility of Polymers and Copolymers in Supercritical CO2. J. Phys. Chem. 1996, 100, 15581–15587. [Google Scholar] [CrossRef]
- Trivedi, V.; Bhomia, R.; Mitchell, J.C.; Coleman, N.; Douroumis, D.; Snowden, M. Study of the Effect of Pressure on Melting Behavior of Saturated Fatty Acids in Liquid or Supercritical Carbon Dioxide. J. Chem. Eng. Data 2013, 58, 1861–1866. [Google Scholar] [CrossRef]
- Ciftci, O.N.; Temelli, F. Melting point depression of solid lipids in pressurized carbon dioxide. J. Supercrit. Fluids 2014, 92, 208–214. [Google Scholar] [CrossRef]
- Nalawade, S.P.; Picchioni, F.; Janssen, L.P.B.M. Supercritical carbon dioxide as a green solvent for processing polymer melts: Processing aspects and applications. Prog. Polym. Sci. 2006, 31, 19–43. [Google Scholar] [CrossRef] [Green Version]
- Lian, Z.; Epstein, S.A.; Blenk, C.W.; Shine, A.D. Carbon dioxide-induced melting point depression of biodegradable semicrystalline polymers. J. Supercrit. Fluids 2006, 39, 107–117. [Google Scholar] [CrossRef]
- Kelly, C.; Harrison, K.L.; Leeke, G.; Jenkins, M. Detection of melting point depression and crystallization of polycaprolactone (PCL) in scCO2 by infrared spectroscopy. Polym. J. 2013, 45, 188–192. [Google Scholar] [CrossRef] [Green Version]
- Shenoy, S.L.; Fujiwara, T.; Wynne, K.J. Quantifying supercritical CO2 dilation of poly(vinylidene fluoride) and poly(vinylidene fluoride-co-hexafluoro-propylene) utilizing a linear variable differential transducer: Plasticization and melting behavior. Macromol. Symp. 2003, 201, 171–178. [Google Scholar] [CrossRef]
- Pasquali, I.; Comi, L.; Pucciarelli, F.; Bettini, R. Swelling, melting point reduction and solubility of PEG 1500 in supercritical CO2. Int. J. Pharm. 2008, 356, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Gupta, R.B. Rapid Expansion of Supercritical Solution with Solid Cosolvent (RESS−SC) Process: Formation of Griseofulvin Nanoparticles. Ind. Eng. Chem. Res. 2005, 44, 7380–7387. [Google Scholar] [CrossRef]
- Samei, M.; Vatanara, A.; Fatemi, S.; Najafabadi, A.R. Process variables in the formation of nanoparticles of megestrol acetate through rapid expansion of supercritical CO2. J. Supercrit. Fluids 2012, 70, 1–7. [Google Scholar] [CrossRef]
- Keshmiri, K.; Vatanara, A.; Tavakoli, O.; Manafi, N. Production of ultrafine clobetasol propionate via rapid expansion of supercritical solution (RESS): Full factorial approach. J. Supercrit. Fluids 2015, 101, 176–183. [Google Scholar] [CrossRef]
- Thakur, R.; Gupta, R.B. Formation of phenytoin nanoparticles using rapid expansion of supercritical solution with solid cosolvent (RESS-SC) process. Int. J. Pharm. 2006, 308, 190–199. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Vatanara, A.; Zarghami, R. Formation and Characterization of Beclomethasone Dipropionate Nanoparticles Using Rapid Expansion of Supercritical Solution. Adv. Pharm. Bull. 2015, 5, 343–349. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A. Solubility measurement and preparation of nanoparticles of an anticancer drug (Letrozole) using rapid expansion of supercritical solutions with solid cosolvent (RESS-SC). J. Supercrit. Fluids 2018, 133, 239–252. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A.; Daneshyan, S. Preparation of Aprepitant nanoparticles (efficient drug for coping with the effects of cancer treatment) by rapid expansion of supercritical solution with solid cosolvent (RESS-SC). J. Supercrit. Fluids 2018, 140, 72–84. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A. Experimental measurement of solubilities of sertraline hydrochloride in supercriticalcarbon dioxide with/without menthol: Data correlation. J. Supercrit. Fluids 2019, 149, 79–87. [Google Scholar] [CrossRef]
- Lin, P.C.; Su, C.S.; Tang, M.; Chen, Y.P. Micronization of tolbutamide using rapid expansion of supercritical solution with solid co-solvent (RESS-SC) process. Res. Chem. Intermed. 2011, 37, 153–163. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A.; Razmimanesh, F.; Hazaveie, S.M. Solubility of Ketoconazole (antifungal drug) in SC-CO2 for binary and ternary systems: Measurements and empirical correlations. Sci. Rep. 2021, 11, 7546. [Google Scholar] [CrossRef] [PubMed]
- Sabet, J.K.; Ghotbi, C.; Dorkoosh, F.; Striolo, A. Solubilities of acetaminophen in supercritical carbon dioxide with and without menthol cosolvent: Measurement and correlation. Sci. Iran. 2012, 19, 619–625. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, M.H.; Alizadeh, N.; Khanchi, A.R. Effect of menthol as solid cosolvent on the solubility enhancement of clozapine and lamorigine in supercritical CO2. J. Supercrit. Fluids 2010, 55, 14–22. [Google Scholar] [CrossRef]
- Rostamian, H.; Lotfollahi, M.N. Production and characterization of ultrafine aspirin particles by rapid expansion of supercritical solution with solid co-solvent (RESS-SC): Expansion parameters effects. Part. Sci. Technol. 2020, 38, 617–625. [Google Scholar] [CrossRef]
- Gupta, R.B.; Shims, J.-J. Solubility in Supercritical Carbon Dioxide; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Keating, L.; Harris, H.H.; Chickos, J.S. Vapor pressures and vaporization enthalpy of (−) α-bisabolol and (dl) menthol by correlation gas chromatography. J. Chem. Thermodyn. 2017, 107, 18–25. [Google Scholar] [CrossRef]
- Singla, P.; Singh, O.; Chabba, S.; Mahajan, R.K. Pluronic-SAILs (surface active ionic liquids) mixed micelles as efficient hydrophobic quercetin drug carriers. J. Mol. Liq. 2018, 249, 294–303. [Google Scholar] [CrossRef]
- Singla, P.; Garg, S.; McClements, J.; Jamieson, O.; Peeters, M.; Mahajan, R.K. Advances in the therapeutic delivery and applications of functionalized Pluronics: A critical review. Adv. Colloid Interface Sci. 2022, 299, 102563. [Google Scholar] [CrossRef]
- Kamboj, V.K.; Verma, P.K. Poloxamers based nanocarriers for drug delivery system. Pharm. Lett. 2015, 7, 264–269. [Google Scholar]
- Abdeltawab, H.; Svirskis, D.; Sharma, M. Formulation strategies to modulate drug release from poloxamer based in situ gelling systems. Expert Opin. Drug Deliv. 2020, 17, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Mehanna, M.M.; Motawaa, A.M.; Samaha, M.W. In sight into tadalafil–block copolymer binary solid dispersion: Mechanistic investigation of dissolution enhancement. Int. J. Pharm. 2010, 402, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.B.; Shete, D.K.; Narade, S.B.; Surve, S.S.; Khan, Z.K.; Bhise, S.B.; Pore, Y.V. Improvement in the dissolution profile of diacerein using a surfactant-based solid dispersion technique. Drug Discov. Ther. 2010, 4, 435–441. [Google Scholar]
- Pore, Y.V.; Sancheti, P.P.; Karekar, P. Preparation and physicochemical characterization of surfactant based solid dispersions of ezetimibe. Pharmazie. 2009, 64, 227–231. [Google Scholar] [CrossRef]
- Karolewicz, B.; Gajda, M.; Owczarek, A.; Pluta, J.; Górniak, A. Physicochemical Characterization and Dissolution Studies of Solid Dispersions of Clotrimazole with Pluronic F127. Trop. J. Pharm. Res. 2014, 13, 1225–1232. [Google Scholar] [CrossRef] [Green Version]
- Karolewicz, B.; Górniak, A.; Owczarek, A.; Żurawska-Płaksej, E.; Piwowar, A.; Pluta, J. Thermal, spectroscopic, and dissolution studies of ketoconazole–Pluronic F127 system. J. Therm. Anal. 2014, 115, 2487–2493. [Google Scholar] [CrossRef] [Green Version]
- Shaker, M.A.; Elbadawy, H.M.; Shaker, M.A. Improved solubility, dissolution, and oral bioavailability for atorvastatin-Pluronic® solid dispersions. Int. J. Pharm. 2020, 574, 118891. [Google Scholar] [CrossRef]
- Castro, S.G.; Bruni, S.S.; Lanusse, C.E.; Allemandi, D.; Palma, S.D. Improved Albendazole Dissolution Rate in Pluronic 188 Solid Dispersions. AAPS PharmSciTech 2010, 11, 1518–1525. [Google Scholar] [CrossRef] [Green Version]
- Karolewicz, B.; Gajda, M.; Pluta, J.; Górniak, A. Dissolution study and thermal analysis of fenofibrate–Pluronic F127 solid dispersions. J. Therm. Anal. 2016, 125, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Kazarian, S.G.; Vincent, M.F.; Bright, F.V.; Liotta, C.L.; Eckert, C.A. Specific Intermolecular Interaction of Carbon Dioxide with Polymers. J. Am. Chem. Soc. 1996, 118, 1729–1736. [Google Scholar] [CrossRef]
- Shieh, Y.T.; Su, J.H.; Manivannan, G.; Lee, P.H.; Sawan, S.P.; Dale Spall, W. Interaction of supercritical carbon dioxide with polymers. I. Crystalline polymers. J. Appl. Polym. Sci. 1996, 59, 695–705. [Google Scholar] [CrossRef]
- Shieh, Y.; Su, J. Interaction of supercritical carbon dioxide with polymers. II. Amorphous polymers. J. Appl. Polym. Sci. 1996, 59, 707–717. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Lemieux, P.; Vinogradov, S.; Alakhov, V. Pluronic® block copolymers: Novel functional molecules for gene therapy. Adv. Drug Deliv. Rev. 2002, 54, 223–233. [Google Scholar] [CrossRef]
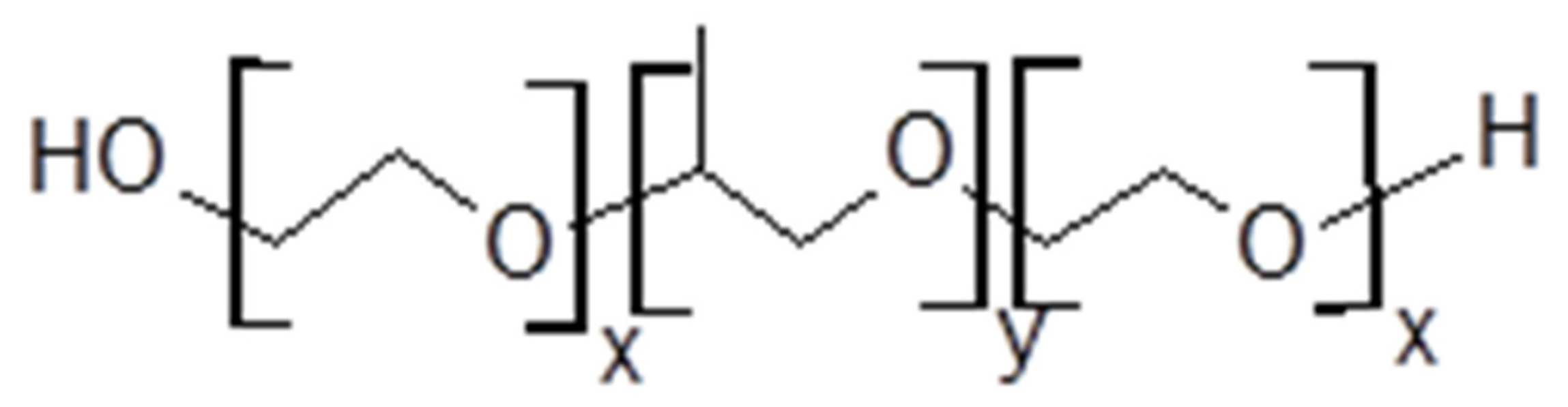
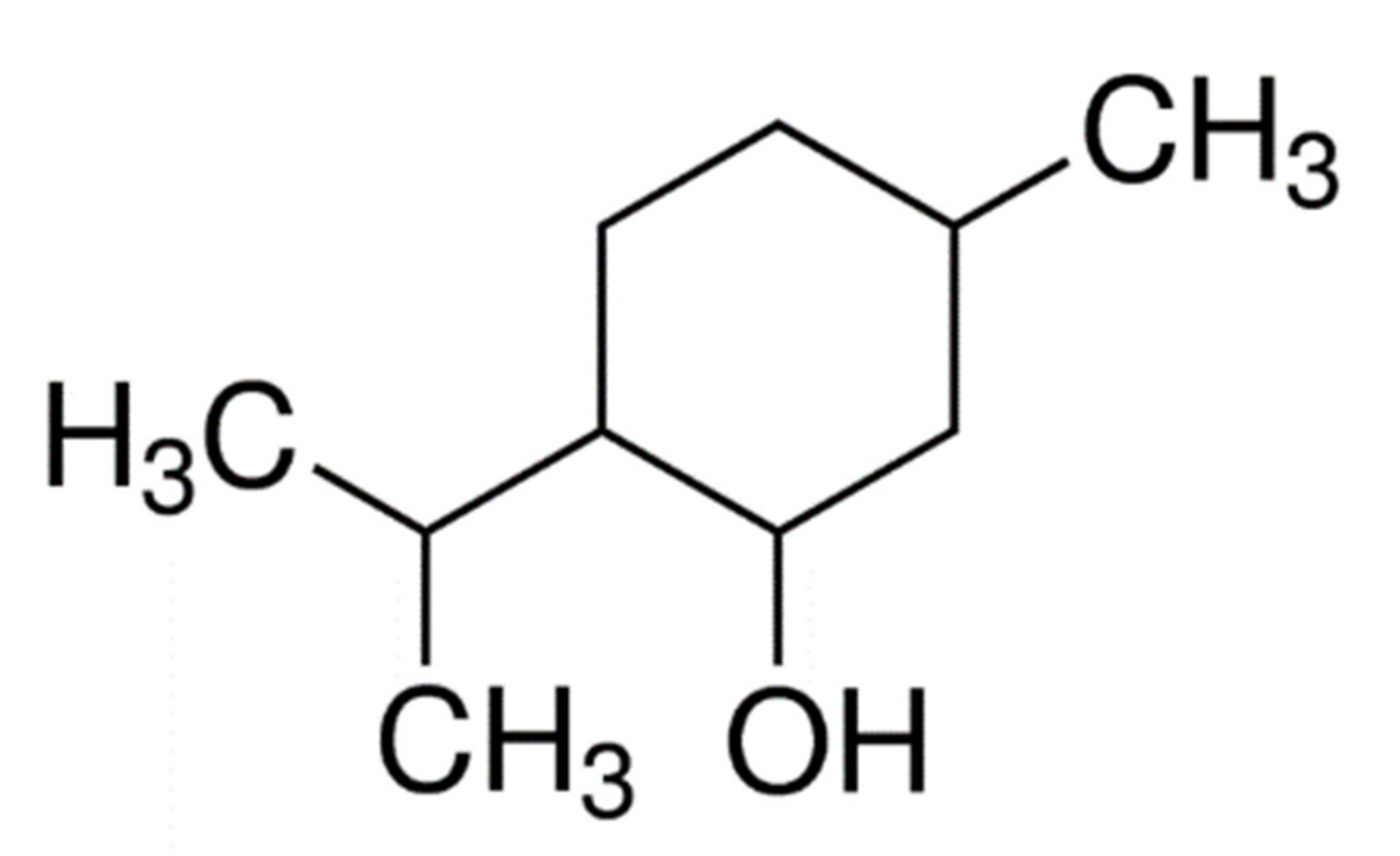



| Polymer (Pluronic®) | Molecular Weight (g·mol−1) | Tm (°C) | Weight PEO | Weight PPO | PEO Units | PPO Units | PEO:PPO (Units) |
|---|---|---|---|---|---|---|---|
| F38 | 4600 | 48 | 3680 | 920 | 84 | 16 | 5.3:1.0 |
| F68 | 8400 | 52 | 6720 | 1680 | 152 | 30 | 5.2:1.0 |
| F108 | 14,600 | 57 | 11,680 | 2920 | 266 | 50 | 5.3:1.0 |
| F77 | 6600 | 48 | 4620 | 1980 | 106 | 34 | 3.1:1.0 |
| F127 | 12,600 | 56 | 8820 | 3780 | 202 | 65 | 3.1:1.0 |
| API | P (MPa) | T (°C) | Purpose | Outcome | Ref. |
|---|---|---|---|---|---|
| Megestrol acetate | 15–25 | 40–60 | Size reduction | 103–515 nm | [16] |
| Clobetasol propionate | 20–26 | 70–110 | Size reduction | 95–319 nm | [17] |
| Phenytoin | 9.6–19.6 | 45 | Size reduction | 75–120 nm | [18] |
| Beclomethasone dipropionate | 20–26 | 70–110 | Size reduction | 65–294 nm | [19] |
| Letrozole | 12–36 | 45–75 | Size reduction | 19–260 nm | [20] |
| Aprepitant | 12–33 | 35–65 | Size reduction | 85–523 nm | [21] |
| Sertraline hydrochloride | 12–30 | 35–65 | Solubility improvement in scCO2 | 59-fold increase | [22] |
| Tolbutamide | 15–20 | 35–45 | Size reduction | 2.1–2.9 μm | [23] |
| Ketoconazole | 12–30 | 35–65 | Solubility improvement in scCO2 | 62-fold increase | [24] |
| Acetaminophen | 10–25 | 40–70 | Solubility improvement in scCO2 | 8-fold increase | [25] |
| Clozapine Lamotrigine | 12.3–33.6 | 40–50 | Solubility improvement in scCO2 | 56-fold increase 8-fold increase | [26] |
| Aspirin | 7.3–8.5 | 30–70 | Size reduction | 0.17–6.61 μm | [27] |
| Griseofulvin | 19.6 | 40 | Size reduction | 150–155 nm | [15] |
| Pressure (MPa) | Menthol (wt%) | Melting Point of Pluronics (°C) | ||||
|---|---|---|---|---|---|---|
| F38 | F68 | F108 | F77 | F127 | ||
| 0.1 | 0 | 49.4 ± 0.1 | 52.1 ± 0.2 | 57.8 ± 0.1 | 46.5 ± 0.1 | 54.1 ± 0.2 |
| 10 | 47.1 ± 0.1 | 50.6 ± 0.3 | 54.1 ± 0.3 | 46.6 ± 0.2 | 53.1 ± 0.3 | |
| 25 | 44.1 ± 0.2 | 47.1 ± 0.2 | 51.1 ± 0.2 | 43.6 ± 0.4 | 48.6 ± 0.2 | |
| 50 | 43.2 ± 0.2 | 44.9 ± 0.1 | 49.1 ± 0.4 | 41.6 ± 0.2 | 46.1 ± 0.2 | |
| 10 | 0 | 30.9 ± 0.6 | 34.5 ± 0.5 | 40.3 ± 0.5 | 29.0 ± 0.3 | 37.5 ± 0.7 |
| 10 | 31.7 ± 0.8 | 33.7 ± 1.3 | 40.1 ± 0.3 | 25.4 ± 2.3 | 34.5 ± 0.3 | |
| 25 | 31.0 ± 0.7 | 30.5 ± 0.7 | 38.3 ± 0.4 | 25.0 ± 0.2 * | 33.6 ± 1.3 | |
| 50 | 31.4 ± 1.1 | 30.7 ± 0.8 | 34.4 ± 1.0 | 25.0 ± 0.3 * | 25.0 ± 0.2 * | |
| 20 | 0 | 31.2 ± 0.6 | 33.9 ± 0.2 | 38.9 ± 0.3 | 27.3 ± 0.2 | 36.6 ± 0.8 |
| 10 | 32.6 ± 1.1 | 33.9 ± 1.1 | 40.1 ± 0.4 | 27.1 ± 0.5 | 36.1 ± 0.9 | |
| 25 | 25.0 ± 0.2 * | 25.1 ± 0.2 * | 37.5 ± 1.6 | 25.0 ± 0.2 * | 32.8 ± 0.7 | |
| 50 | 25.0 ± 0.1 * | 25.0 ± 0.4 * | 33.1 ± 1.2 | 25.1 ± 0.2 * | 25.0 ± 0.2 | |
| Polymer | Pressure (MPa) | Menthol (wt%) | Melting Point (°C) |
|---|---|---|---|
| F38 | 20 | 25 | 25 |
| F68 | 20 | 25 | 25.1 |
| F108 | 20 | 50 | 33.1 |
| F77 | 10 | 25 | 25 |
| F127 | 20 | 50 | 25.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivedi, V.; Ajiboye, A.L.; Coleman, N.J.; Bhomia, R.; Bascougnano, M. Melting Point Depression of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide) Triblock Polymers in Supercritical Carbon Dioxide in the Presence of Menthol as a Solid Co-Plasticiser. Polymers 2022, 14, 2825. https://doi.org/10.3390/polym14142825
Trivedi V, Ajiboye AL, Coleman NJ, Bhomia R, Bascougnano M. Melting Point Depression of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide) Triblock Polymers in Supercritical Carbon Dioxide in the Presence of Menthol as a Solid Co-Plasticiser. Polymers. 2022; 14(14):2825. https://doi.org/10.3390/polym14142825
Chicago/Turabian StyleTrivedi, Vivek, Adejumoke Lara Ajiboye, Nichola J. Coleman, Ruchir Bhomia, and Marion Bascougnano. 2022. "Melting Point Depression of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide) Triblock Polymers in Supercritical Carbon Dioxide in the Presence of Menthol as a Solid Co-Plasticiser" Polymers 14, no. 14: 2825. https://doi.org/10.3390/polym14142825
APA StyleTrivedi, V., Ajiboye, A. L., Coleman, N. J., Bhomia, R., & Bascougnano, M. (2022). Melting Point Depression of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide) Triblock Polymers in Supercritical Carbon Dioxide in the Presence of Menthol as a Solid Co-Plasticiser. Polymers, 14(14), 2825. https://doi.org/10.3390/polym14142825







