Efficient Removal of Methylene Blue by Bio-Based Sodium Alginate/Lignin Composite Hydrogel Beads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SA/Lig Composite Hydrogel Beads
2.3. Characterization of SA/Lig Composite Beads
2.4. Removal of Methylene Blue
3. Results and Discussion
3.1. Fabrication and Characterization of SA/Lig Composite Beads
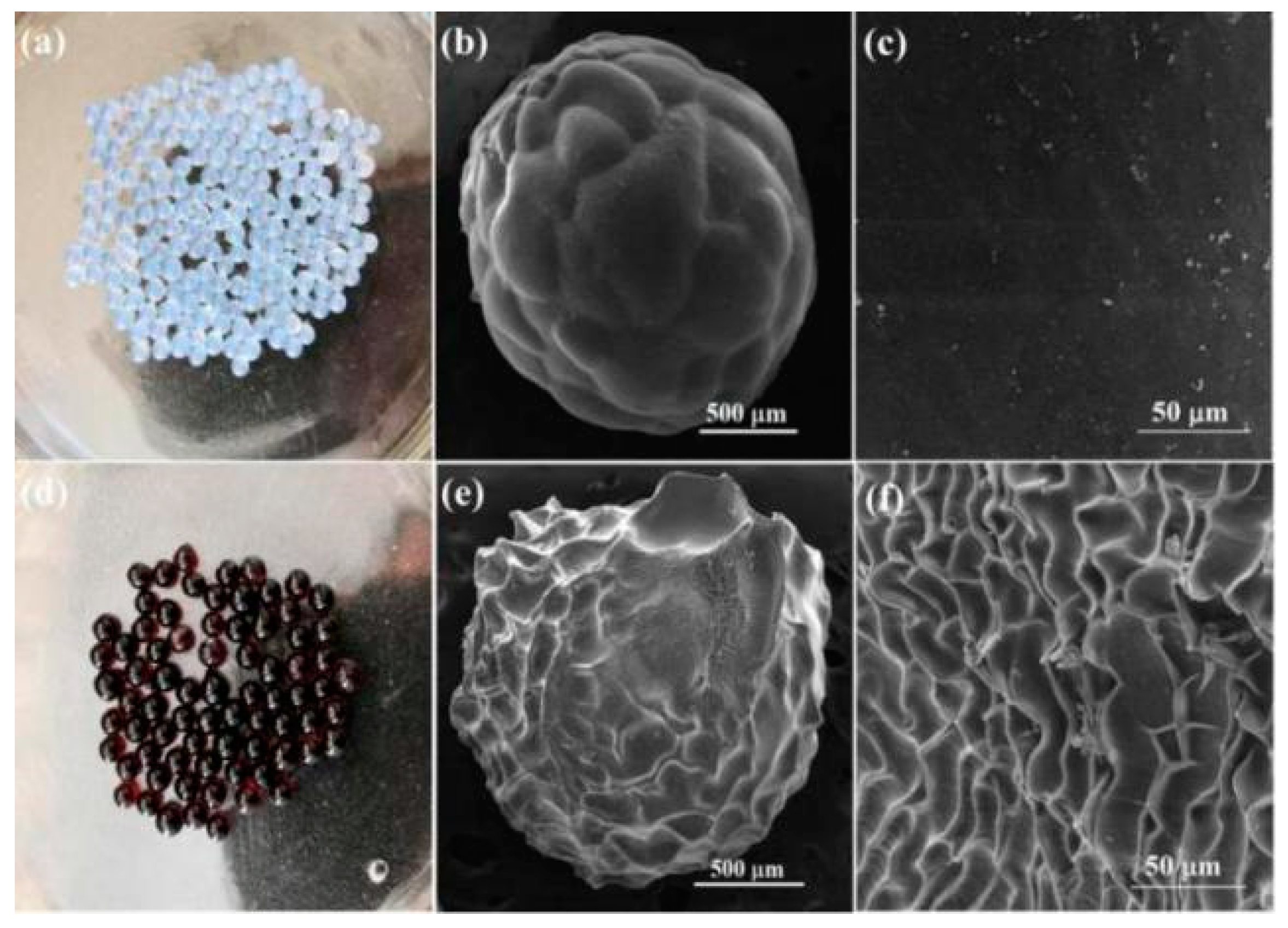
3.1.1. Surface Morphology
3.1.2. Composition Analysis
3.1.3. Thermal Stability
3.2. Removal of MB with SA/Lig Beads
3.2.1. Effect of Lignin Content
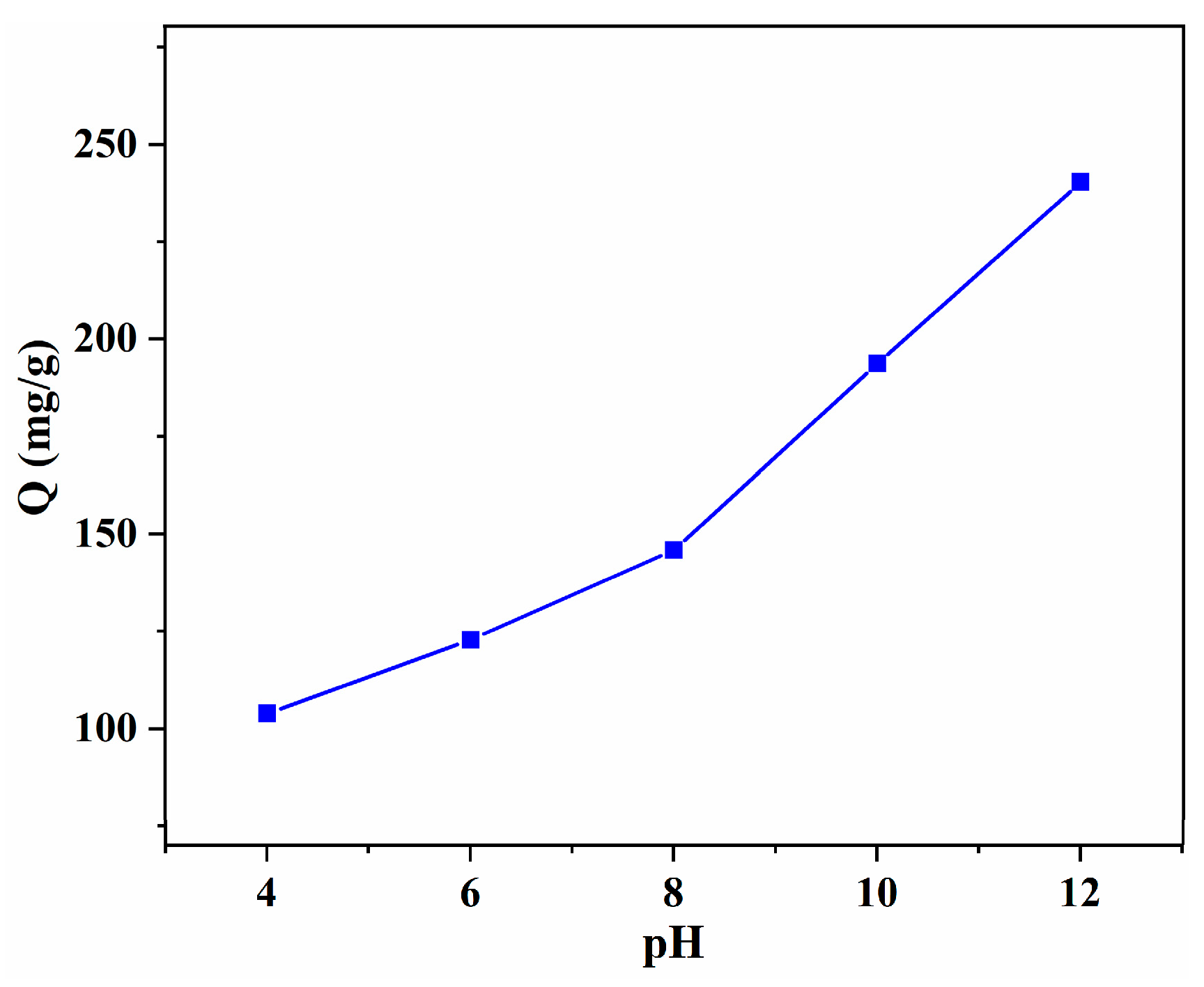
3.2.2. Effect of pH Value
3.3. Adsorption Thermodynamic
3.4. Adsorption Kinetic
3.5. Regeneration Performance
3.6. Adsorption Performance Comparison
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sala, E.; Mayorga, J.; Bradley, D.; Cabral, R.B.; Atwood, T.B.; Auber, A.; Cheung, W.; Costello, C.; Ferretti, F.; Friedlander, A.M.; et al. Protecting the global ocean for biodiversity, food and climate. Nature 2021, 7854, 397–402. [Google Scholar] [CrossRef]
- Sullivan, S.M.P.; Rains, M.C.; Rodemald, A.D.; Buzbee, W.W.; Rosemond, A.D. Distorting science, putting water at risk. Science 2020, 6505, 766–768. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Gupta, S.; Singh, A.K.; Sinha, S. Optimizing adsorption of crystal violet dye from water by magnetic nano composite using response surface modeling approach. J. Hazard. Mater. 2011, 186, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Roosta, M.; Ghaedi, M.; Daneshfar, A.; Sahraei, R.; Asghari, A. Optimization of the ultrasonic assisted removal of methylene blue by gold nanoparticles loaded on activated carbon using experimental design methodology. Ultrason. Sonochem. 2014, 21, 242–252. [Google Scholar] [CrossRef]
- Ma, M.S.; Liu, Z.; Hui, L.F.; Shang, Z.; Yuan, S.Y.; Dai, L.; Liu, P.T.; Liu, X.L.; Ni, Y.H. Lignin-containing cellulose nanocrystals/sodium alginate beads as highly effective adsorbents for cationic organic dyes. Int. J. Biol. Macromol. 2019, 139, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Mohammadabadi, S.I.; Javanbakht, V. Lignin extraction from barley straw using ultrasound-assisted treatment method for a lignin-based biocomposite preparation with remarkable adsorption capacity for heavy metal. Int. J. Biol. Macromol. 2020, 164, 1133–1148. [Google Scholar] [CrossRef] [PubMed]
- Albadarin, A.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Marrakchi, F.; Khanday, W.A.; Asif, M.; Hameed, B.H. Cross-linked chitosan/sepiolite composite for the adsorption of methylene blue and reactive orange 16. Int. J. Biol. Macromol. 2016, 93, 1231–1239. [Google Scholar] [CrossRef]
- Viscusi, G.; Lamberti, E.; Gorrasi, G. Design of sodium alginate/soybean extract beads loaded with hemp hurd and halloysite as novel and sustainable systems for methylene blue adsorption. Polym. Eng. Sci. 2022, 62, 129–144. [Google Scholar] [CrossRef]
- Li, C.D.; Lu, J.J.; Li, S.M.; Tong, Y.B.; Ye, B.C. Synthesis of magnetic microspheres with sodium alginate and activated carbon for removal of methylene blue. Materials 2017, 10, 84. [Google Scholar] [CrossRef]
- Sezen, S.; Thakur, V.K.; Ozmen, M.M. Highly effective covalently crosslinked composite alginate cryogels for cationic dye removal. Gels 2021, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, I.A.; Arriagada, D.C.; Mayorga, C.K.; Urbina, K.P.; Valencia, R.M.; Gonzalez, J. Importance of the interaction adsorbent-adsorbate in the dyes adsorption process and DFT modeling. J. Mol. Struct. 2020, 1203, 127398. [Google Scholar] [CrossRef]
- Han, X.B.; Li, R.; Miao, P.P.; Gao, J.; Hu, G.W.; Zhao, Y.; Chen, T. Design, synthesis and adsorption evaluation of bio-based lignin/chitosan beads for congo red removal. Materials 2022, 15, 2310. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Liu, Q.Z.; Gao, T.T.; Dong, K.J.; Wei, G.; Yao, J.S. Facile preparation of tannic acid-poly(vinyl alcohol)/sodium alginate hydrogel beads for methylene blue removal from simulated solution. ACS Omega 2018, 3, 7523–7531. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, Y.; Sang, Y.N.; Tang, M.; Hu, G.W.; Han, X.B.; Gao, J.; Ma, R. Facile synthesis of magnetic CS-g-SPSS microspheres via electron beam radiation for efficient removal of methylene blue. J. Saudi Chem. Soc. 2021, 25, 101299. [Google Scholar] [CrossRef]
- Wu, M.; Chen, W.J.; Mao, Q.H.; Bai, Y.S.; Ma, H.Z. Facile Synthesis of chitosan/gelatin filled with graphene bead adsorbent for orange II removal. Chem. Eng. Res. Des. 2019, 144, 35–46. [Google Scholar] [CrossRef]
- Duan, Y.Q.; Freyburger, A.; Kunz, W.; Zollfrank, C. Lignin/chitin films and their adsorption characteristics for heavy metal ions. ACS Sustain. Chem. Eng. 2018, 6, 6965–6973. [Google Scholar] [CrossRef]
- Larosa, C.; Salerno, M.; Lima, J.S.; Meri, R.M.; Silva, M.F.; Carvalho, L.B.; Converti, A. Characterisation of bare and tannase-loaded calcium alginate beads by microscopic, thermogravimetric, FTIR and XRD analyses. Int. J. Biol. Macromol. 2018, 115, 900–906. [Google Scholar] [CrossRef]
- Abulateefeh, S.R.; Taha, M.O. Enhanced drug encapsulation and extended release profiles of calcium-alginate nanoparticles by using tannic acid as a bridging cross-linking agent. J. Microencapsul. 2015, 32, 96–105. [Google Scholar] [CrossRef]
- Schneider, M.; Finimundi, N.; Podzorova, M.; Pantyukhov, P.; Poletto, M. Assessment of morphological, physical, thermal, and thermal conductivity properties of polypropylene/lignosulfonate blends. Materials 2021, 14, 543. [Google Scholar] [CrossRef]
- Abboud, M.; Sahlabji, T.; Haija, M.A.; Zahhar, A.A.; Bondock, S.; Ismail, I.; Keshk, S. Synthesis and characterization of lignosulfonate/amino-functionalized SBA-15 nanocomposites for the adsorption of methylene blue from wastewater. New J. Chem. 2020, 44, 2291–2302. [Google Scholar] [CrossRef]
- Chupin, L.; Charrier, B.; Pizzi, A.; Perdomo, A.; Bouhtoury, F. Study of thermal durability properties of tannin-lignosulfonate adhesives. J. Therm. Anal. Calorim. 2015, 119, 1577–1585. [Google Scholar] [CrossRef]
- Bai, H.J.; Chen, J.H.; Zhou, X.Y.; Hu, C.Z. Single and binary adsorption of dyes from aqueous solutions using functionalized microcrystalline cellulose from cotton fiber. Korean J. Chem. Eng. 2020, 37, 1926–1932. [Google Scholar] [CrossRef]
- Du, Q.; Li, Y.; Li, J.; Zhang, Z.; Qiao, B.; Sui, K.; Wang, D.; Wang, C.; Li, H.; Xia, Y.; et al. Preparation of graphene oxide/chitosan pellets and their adsorption properties for congo red. Int. J. Nanosci. 2019, 18, 1850030. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.L.; Chen, Z.Z.; Guo, W.; Zhu, C.W.; Zou, Y.J. Simple fabrication of chitosan/graphene nanoplates composite spheres for efficient adsorption of acid dyes from aqueous solution. Int. J. Biol. Macromol. 2018, 112, 1048–1054. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Ho, Y.S. Equilibrium sorption of anionic dye from aqueous solution by palm kernel fibre as sorbent. Dye Pigment. 2007, 74, 60–66. [Google Scholar] [CrossRef]
- Zhang, L.J.; Zhao, D.Q.; Lu, Y.; Chen, J.H.; Li, H.T.; Xie, J.H.; Xu, Y.; Yuan, H.K.; Liu, X.J.; Zhu, X.Y.; et al. A Graphene oxide modified cellulose nanocrystal/PNIPAAm IPN hydrogel for the adsorption of congo red and methylene blue. New J. Chem. 2021, 45, 16679–16688. [Google Scholar] [CrossRef]
- Sartape, A.S.; Mandhare, A.M.; Salvi, P.P.; Pawar, D.K.; Kolekar, S.S. Kinetic and equilibrium studies of the adsorption of Cd(II) from aqueous solutions by wood apple shell activated carbon. Desalin. Water Treat. 2013, 51, 4638–4650. [Google Scholar] [CrossRef]
- Chen, T.; Yan, C.J.; Wang, Y.X.; Tang, C.H.; Zhou, S.; Zhao, Y.; Ma, R.; Duan, P. Synthesis of activated carbon-based amino phosphonic acid chelating resin and its adsorption properties for Ce(III) removal. Environ. Technol. 2015, 36, 2168–2176. [Google Scholar] [CrossRef]
- Eldin, M.S.M.; Soliman, E.A.; Elaztahry, A.A.F.; Elaassar, M.R.; Eweida, B.Y.; Elkady, M.F.; Rahman, A.M.A.; Yossef, M.E. Carboxylated alginate hydrogel beads for methylene blue removal: Formulation, kinetic and isothermal studies. Desalin. Water Treat. 2019, 168, 308–323. [Google Scholar] [CrossRef]
- Uyar, G.; Kaygusuz, H.; Erim, F.B. Methylene blue removal by alginate–clay quasi-cryogel beads. React. Funct. Polym. 2016, 106, 1–7. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of methylene blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Liu, X.J.; Li, M.F.; Singh, S.K. Manganese-modified lignin biochar as adsorbent for removal of methylene blue. J. Mater. Res. Technol. 2021, 12, 1434–1445. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I.; Pa’e, N.; Hashim, Z. Strategies in improving properties of cellulose-based hydrogels for smart applications. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer: Cham, Switzerland, 2019; pp. 887–908. [Google Scholar]
- Sabbagh, F.; Muhamad, I.I.; Nazari, Z.; Mobini, P.; Khatir, N.M. Investigation of acyclovir-loaded, acrylamide-based hydrogels for potential use as vaginal ring. Mater. Today Commun. 2018, 16, 274–280. [Google Scholar] [CrossRef]







| Temperature (K) | lnK0 | ΔG0 (kJ/mol) | ΔH0 (kJ/mol) | ΔS0 (kJ/mol·k) |
|---|---|---|---|---|
| 288 | −0.805 | 1.93 | 64.19 | 0.22 |
| 298 | 0.398 | −0.99 | - | - |
| 308 | 1.166 | 2.98 | - | - |
| 318 | 1.77 | 4.68 | - | - |
| Kinetic Models | Coefficients | 60 mg/L | 80 mg/L | 100 mg/L |
|---|---|---|---|---|
| Pseudo-first-order | qe,cal (mg/g) | 13.82 | 15.06 | 24.05 |
| k1 (min−1) | 0.0154 | 0.0163 | 0.0144 | |
| R2 | 0.9711 | 0.9846 | 0.9871 | |
| Pseudo-second-order | qe,cal (mg/g) | 87.03 | 116.28 | 145.35 |
| k2 (×10−3) (g/mg min) | 2.34 | 2.21 | 1.32 | |
| R2 | 0.9999 | 0.9999 | 0.9999 |
| Adsorbent | Experimental Conditions (C0/(mg/L), pH, T/°C, t/h) | Qmax (mg/g) | Removal (%) | Ref. |
|---|---|---|---|---|
| SA/Lig | 200, 12, 45, 24 | 254.3 | 84.8 | This work |
| Lig/CS | 82, 7, 20, 40 | 36.25 | 88.4 | [7] |
| CS/SP | 300, 9, 30, 24 | 40.986 | 27.3 | [8] |
| SA/SB/HNT | 100, 7, 25, 24 | 49 | 94 | [9] |
| Fe3O4/AC/SA | 700, 7, 25, 4 | 222.3 | 31.8 | [10] |
| SA/MMT | 1000, 7, 25, 24 | 559.94 | 56 | [11] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Liu, H.; Gao, J.; Hu, G.; Zhao, Y.; Tang, X.; Han, X. Efficient Removal of Methylene Blue by Bio-Based Sodium Alginate/Lignin Composite Hydrogel Beads. Polymers 2022, 14, 2917. https://doi.org/10.3390/polym14142917
Chen T, Liu H, Gao J, Hu G, Zhao Y, Tang X, Han X. Efficient Removal of Methylene Blue by Bio-Based Sodium Alginate/Lignin Composite Hydrogel Beads. Polymers. 2022; 14(14):2917. https://doi.org/10.3390/polym14142917
Chicago/Turabian StyleChen, Tao, Haochen Liu, Jie Gao, Guowen Hu, Yuan Zhao, Xiuqin Tang, and Xiaobing Han. 2022. "Efficient Removal of Methylene Blue by Bio-Based Sodium Alginate/Lignin Composite Hydrogel Beads" Polymers 14, no. 14: 2917. https://doi.org/10.3390/polym14142917
APA StyleChen, T., Liu, H., Gao, J., Hu, G., Zhao, Y., Tang, X., & Han, X. (2022). Efficient Removal of Methylene Blue by Bio-Based Sodium Alginate/Lignin Composite Hydrogel Beads. Polymers, 14(14), 2917. https://doi.org/10.3390/polym14142917







