Polymeric Nanoparticles in Brain Cancer Therapy: A Review of Current Approaches
Abstract
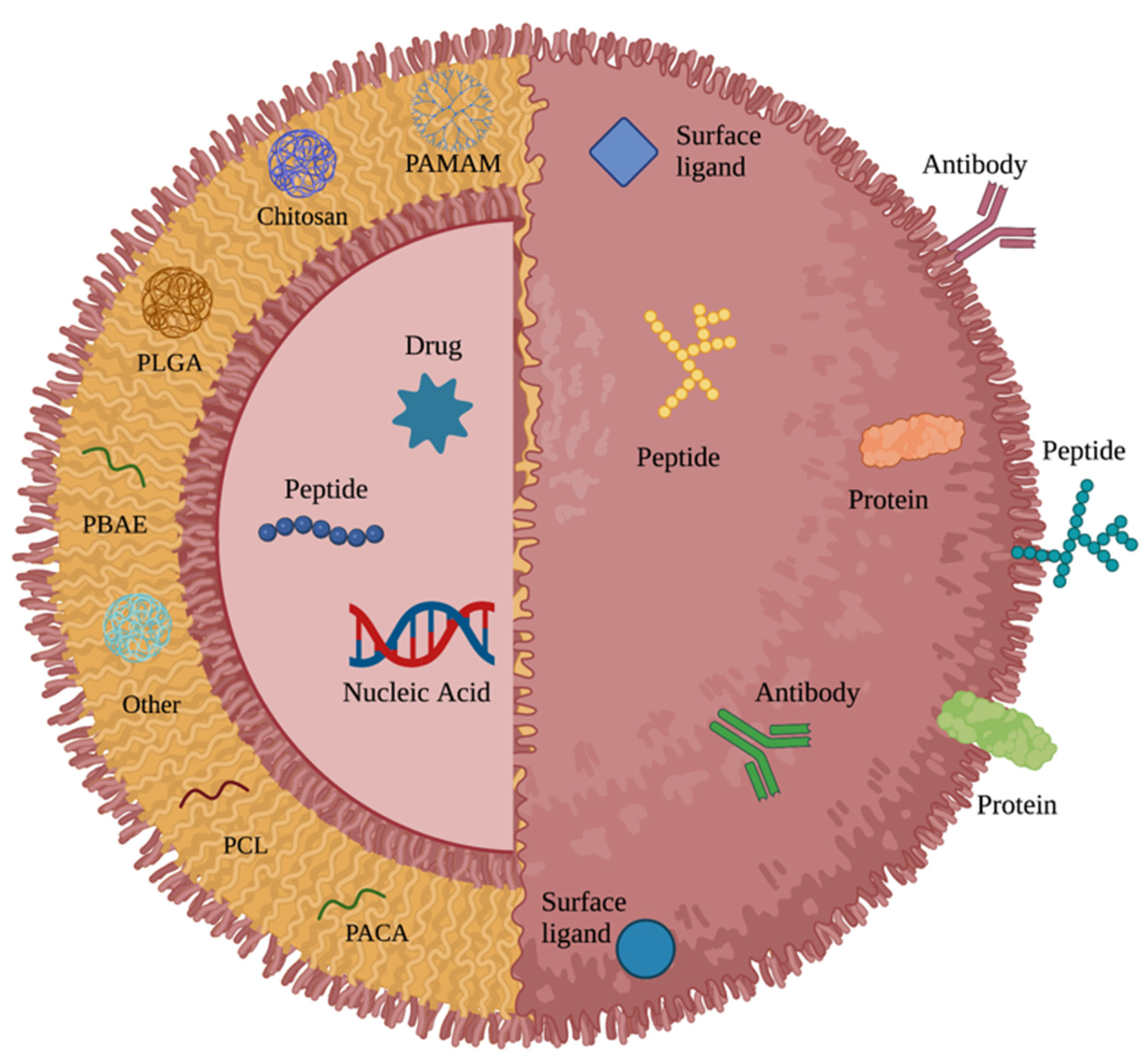
:1. Introduction
2. Major Polymers in Nanoparticle-Based Brain Cancer Research
2.1. Polyanhydride
2.2. Poly (lactic-co-glycolic acid)
2.3. Poly (β-amino ester)
2.4. Chitosan
2.5. Poly(amidoamine) Dendrimers
2.6. Poly(caprolactone)
2.7. Poly(alkyl cyanoacrylate)
3. General Modifications
3.1. Polyethylene Glycol
3.2. pH
3.3. Size
3.4. Shape
4. Receptor Targeting for Blood–Brain Barrier Penetration
5. Receptor Targeting for Delivery to Brain Cancer Cells
5.1. Vascular Endothelial Growth Factor
5.2. Epidermal Growth Factor Receptor
6. Mechanisms of Delivery
6.1. Focused Ultrasound
6.2. Convection-Enhanced Delivery
6.3. Nose-to-Brain Delivery
6.4. Intracranial Hydrogel Delivery
6.5. Cell-Based Delivery
7. Discussion and Future Directions
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro-Oncology 2020, 22 (Suppl. S2), iv1–iv96. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Visser, O.; Ardanaz, E.; Botta, L.; Sant, M.; Tavilla, A.; Minicozzi, P.; Hackl, M.; Zielonke, N.; Oberaigner, W.; Van Eycken, E.; et al. Survival of adults with primary malignant brain tumours in Europe; Results of the EUROCARE-5 study. Eur. J. Cancer 2015, 51, 2231–2241. [Google Scholar] [CrossRef]
- Tang, L.; Feng, Y.; Gao, S.; Mu, Q.; Liu, C. Nanotherapeutics Overcoming the Blood-Brain Barrier for Glioblastoma Treatment. Front. Pharmacol. 2021, 12, 786700. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Lahiri, D.; Maji, T.; Biswas, J. Recurrent Glioblastoma: Where we stand. S. Asian J. Cancer 2015, 4, 163. [Google Scholar] [CrossRef] [PubMed]
- Aldape, K.; Brindle, K.M.; Chesler, L.; Chopra, R.; Gajjar, A.; Gilbert, M.R.; Gottardo, N.; Gutmann, D.H.; Hargrave, D.; Holland, E.C.; et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 2019, 16, 509–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Mehta, A.; Tong, Z.; Esser, L.; Voelcker, N.H. Development of Polymeric Nanoparticles for Blood–Brain Barrier Transfer—Strategies and Challenges. Adv. Sci. 2021, 8, 2003937. [Google Scholar] [CrossRef]
- Fisher, D.; Price, R.J. Recent Advances in the Use of Focused Ultrasound for Magnetic Resonance Image-Guided Therapeutic Nanoparticle Delivery to the Central Nervous System. Front. Pharmacol. 2019, 10, 1348. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Bai, H.; Wang, G.; Hu, X.; Kumar, S.; Min, R. Biocompatible and Biodegradable Polymer Optical Fiber for Biomedical Application: A Review. Biosensors 2021, 11, 472. [Google Scholar] [CrossRef]
- Yu, H.; Yang, Z.; Li, F.; Xu, L.; Sun, Y. Cell-mediated targeting drugs delivery systems. Drug Deliv. 2020, 27, 1425–1437. [Google Scholar] [CrossRef]
- Lluch, C.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cadiz, V. “Click” Synthesis of Fatty Acid Derivatives as Fast-Degrading Polyanhydride Precursors. Macromol. Rapid Commun. 2011, 32, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.M.; Panigrahi, M.; Das, P.K. Brain tumor and Gliadel wafer treatment. Indian J. Cancer 2011, 48, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Gabikian, P.; Abel, T.; Ryken, T.; Lesniak, M. Gliadel for brain metastasis. Surg. Neurol. Int. 2013, 4 (Suppl. S4), S289–S293. [Google Scholar] [CrossRef]
- Qi, Z.-Y.; Xing, W.-K.; Shao, C.; Yang, C.; Wang, Z. The role of Gliadel wafers in the treatment of newly diagnosed GBM: A meta-analysis. Drug Des. Dev. Ther. 2015, 9, 3341–3348. [Google Scholar] [CrossRef] [Green Version]
- Iuchi, T.; Inoue, A.; Hirose, Y.; Morioka, M.; Horiguchi, K.; Natsume, A.; Arakawa, Y.; Iwasaki, K.; Fujiki, M.; Kumabe, T.; et al. Long-term effectiveness of Gliadel implant for malignant glioma and prognostic factors for survival: 3-year results of a postmarketing surveillance in Japan. Neuro-Oncol. Adv. 2022, 4, vdab189. [Google Scholar] [CrossRef]
- Brenza, T.M.; Ghaisas, S.; Ramirez, J.E.V.; Harischandra, D.; Anantharam, V.; Kalyanaraman, B.; Kanthasamy, A.G.; Narasimhan, B. Neuronal protection against oxidative insult by polyanhydride nanoparticle-based mitochondria-targeted antioxidant therapy. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 809–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavez-Santoscoy, A.V.; Roychoudhury, R.; Pohl, N.L.; Wannemuehler, M.J.; Narasimhan, B.; Ramer-Tait, A. Tailoring the immune response by targeting C-type lectin receptors on alveolar macrophages using “pathogen-like” amphiphilic polyanhydride nanoparticles. Biomaterials 2012, 33, 4762–4772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binnebose, A.M.; Haughney, S.L.; Martin, R.J.; Imerman, P.M.; Narasimhan, B.; Bellaire, B.H. Polyanhydride Nanoparticle Delivery Platform Dramatically Enhances Killing of Filarial Worms. PLOS Negl. Trop. Dis. 2015, 9, e0004173. [Google Scholar] [CrossRef]
- Schlichtmann, B.W.; Kalyanaraman, B.; Schlichtmann, R.L.; Panthani, M.G.; Anantharam, V.; Kanthasamy, A.G.; Mallapragada, S.K.; Narasimhan, B. Functionalized polyanhydride nanoparticles for improved treatment of mitochondrial dysfunction. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 110, 450–459. [Google Scholar] [CrossRef]
- Geraili, A.; Mequanint, K. Systematic Studies on Surface Erosion of Photocrosslinked Polyanhydride Tablets and Data Correlation with Release Kinetic Models. Polymers 2020, 12, 1105. [Google Scholar] [CrossRef]
- Brenza, T.M.; Schlichtmann, B.W.; Bhargavan, B.; Ramirez, J.E.V.; Nelson, R.D.; Panthani, M.G.; McMillan, J.M.; Kalyanaraman, B.; Gendelman, H.E.; Anantharam, V.; et al. Biodegradable polyanhydride-based nanomedicines for blood to brain drug delivery. J. Biomed. Mater. Res. Part A 2018, 106, 2881–2890. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [PubMed]
- Mirakabad, F.S.T.; Nejati-Koshki, K.; Akbarzadeh, A.; Yamchi, M.R.; Milani, M.; Zarghami, N.; Zeighamian, V.; Rahimzadeh, A.; Alimohammadi, S.; Hanifehpour, Y.; et al. PLGA-Based Nanoparticles as Cancer Drug Delivery Systems. Asian Pac. J. Cancer Prev. 2014, 15, 517–535. [Google Scholar] [CrossRef] [Green Version]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) As biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Maksimenko, O.; Malinovskaya, J.; Shipulo, E.; Osipova, N.; Razzhivina, V.; Arantseva, D.; Yarovaya, O.; Mostovaya, U.; Khalansky, A.; Fedoseeva, V.; et al. Doxorubicin-loaded PLGA nanoparticles for the chemotherapy of glioblastoma: Towards the pharmaceutical development. Int. J. Pharm. 2019, 572, 118733. [Google Scholar] [CrossRef]
- Agarwal, S.; Mohamed, M.S.; Mizuki, T.; Maekawa, T.; Kumar, D.S. Chlorotoxin modified morusin–PLGA nanoparticles for targeted glioblastoma therapy. J. Mater. Chem. B 2019, 7, 5896–5919. [Google Scholar] [CrossRef]
- Banstola, A.; Duwa, R.; Emami, F.; Jeong, J.-H.; Yook, S. Enhanced Caspase-Mediated Abrogation of Autophagy by Temozolomide-Loaded and Panitumumab-Conjugated Poly(lactic-co-glycolic acid) Nanoparticles in Epidermal Growth Factor Receptor Overexpressing Glioblastoma Cells. Mol. Pharm. 2020, 17, 4386–4400. [Google Scholar] [CrossRef]
- Eivazi, N.; Rahmani, R.; Paknejad, M. Specific cellular internalization and pH-responsive behavior of doxorubicin loaded PLGA-PEG nanoparticles targeted with anti EGFRvIII antibody. Life Sci. 2020, 261, 118361. [Google Scholar]
- Younis, M.; Faming, W.; Hongyan, Z.; Mengmeng, T.; Hang, S.; Liudi, Y. Iguratimod encapsulated PLGA-NPs improves therapeutic outcome in glioma, glioma stem-like cells and temozolomide resistant glioma cells. Nanomed. Nanotechnol. Biol. Med. 2019, 22, 102101. [Google Scholar] [CrossRef]
- Mao, J.; Meng, X.; Zhao, C.; Yang, Y.; Liu, G. Development of transferrin-modified poly(lactic-co-glycolic acid) nanoparticles for glioma therapy. Anti-Cancer Drugs 2019, 30, 604–610. [Google Scholar] [CrossRef]
- Sousa, F.; Dhaliwal, H.K.; Gattacceca, F.; Sarmento, B.; Amiji, M.M. Enhanced anti-angiogenic effects of bevacizumab in glioblastoma treatment upon intranasal administration in polymeric nanoparticles. J. Control Release 2019, 309, 37–47. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visan, A.; Popescu-Pelin, G.; Socol, G. Degradation Behavior of Polymers Used as Coating Materials for Drug Delivery—A Basic Review. Polymers 2021, 13, 1272. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Houchin, M.; Neuenswander, S.; Topp, E. Effect of excipients on PLGA film degradation and the stability of an incorporated peptide. J. Control Release 2007, 117, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Rui, Y.; Kim, J.; Gorelick, N.; Wilson, D.R.; Kozielski, K.; Mangraviti, A.; Sankey, E.; Brem, H.; Tyler, B.; et al. Nonviral polymeric nanoparticles for gene therapy in pediatric CNS malignancies. Nanomed. Nanotechnol. Biol. Med. 2020, 23, 102115. [Google Scholar] [CrossRef]
- Sunshine, J.; Peng, D.Y.; Green, J.J. Uptake and Transfection with Polymeric Nanoparticles Are Dependent on Polymer End-Group Structure, but Largely Independent of Nanoparticle Physical and Chemical Properties. Mol. Pharm. 2012, 9, 3375–3383. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.J. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef]
- Fornaguera, C.; Guerra-Rebollo, M.; Lazaro, M.A.; Cascante, A.; Rubio, N.; Blanco, J.; Borrós, S. In Vivo Retargeting of Poly(beta aminoester) (OM-PBAE) Nanoparticles is Influenced by Protein Corona. Adv. Healthc. Mater. 2019, 8, e1900849. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Lee, J.; Kim, M.J.; Kang, T.J.; Jeong, Y.; Um, S.H.; Cho, S.W. Sonic hedgehog intradermal gene therapy using a biodegradable poly(beta-amino esters) nanoparticle to enhance wound healing. Biomaterials 2012, 33, 9148–9156. [Google Scholar] [CrossRef]
- Feng, R.; Chen, Q.; Zhou, P.; Wang, Y.; Yan, H. Nanoparticles based on disulfide-containing poly(beta-amino ester) and zwitterionic fluorocarbon surfactant as a redox-responsive drug carrier for brain tumor treatment. Nanotechnology 2018, 29, 495101. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, D.; Hu, J.; Ding, P.; Chen, M. Hyaluronic acid-coated pH sensitive poly (beta-amino ester) nanoparticles for co-delivery of embelin and TRAIL plasmid for triple negative breast cancer treatment. Int. J. Pharm. 2020, 573, 118637. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.G.; Lynn, D.M.; Langer, R. Semi-Automated Synthesis and Screening of a Large Library of Degradable Cationic Polymers for Gene Delivery. Angew. Chem. Int. Ed. Engl. 2003, 42, 3153–3158. [Google Scholar] [CrossRef]
- Guerrero-Cázares, H.; Tzeng, S.Y.; Young, N.P.; Abutaleb, A.O.; Quiñones-Hinojosa, A.; Green, J.J. Biodegradable Polymeric Nanoparticles Show High Efficacy and Specificity at DNA Delivery to Human Glioblastoma In Vitro and In Vivo. ACS Nano 2014, 8, 5141–5153. [Google Scholar] [CrossRef] [PubMed]
- Negron, K.; Zhu, C.; Chen, S.-W.; Shahab, S.; Rao, D.; Raabe, E.H.; Eberhart, C.G.; Hanes, J.; Suk, J.S. Non-adhesive and highly stable biodegradable nanoparticles that provide widespread and safe transgene expression in orthotopic brain tumors. Drug Deliv. Transl. Res. 2020, 10, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef] [Green Version]
- Caprifico, A.E.; Foot, P.J.S.; Polycarpou, E.; Calabrese, G. Overcoming the Blood-Brain Barrier: Functionalised Chitosan Nanocarriers. Pharmaceutics 2020, 12, 1013. [Google Scholar] [CrossRef]
- Shevtsov, M.; Nikolaev, B.; Marchenko, Y.; Yakovleva, L.; Skvortsov, N.; Mazur, A.; Tolstoy, P.; Ryzhov, V.; Multhoff, G. Targeting experimental orthotopic glioblastoma with chitosan-based superparamagnetic iron oxide nanoparticles (CS-DX-SPIONs). Int. J. Nanomed. 2018, 13, 1471–1482. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-L.; Chen, J.-P.; Wei, K.-C.; Chen, J.-Y.; Huang, C.-W.; Liao, Z.-X. Release of Doxorubicin by a Folate-Grafted, Chitosan-Coated Magnetic Nanoparticle. Nanomaterials 2017, 7, 85. [Google Scholar] [CrossRef] [Green Version]
- Fana, M.; Gallien, J.; Srinageshwar, B.; Dunbar, G.L.; Rossignol, J. PAMAM Dendrimer Nanomolecules Utilized as Drug Delivery Systems for Potential Treatment of Glioblastoma: A Systematic Review. Int. J. Nanomed. 2020, 15, 2789–2808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, R.V.D.; Santos, S.D.S.; Igne Ferreira, E.; Giarolla, J. New Advances in General Biomedical Applications of PAMAM Dendrimers. Molecules 2018, 23, 2849. [Google Scholar] [CrossRef] [Green Version]
- Florendo, M.; Figacz, A.; Srinageshwar, B.; Sharma, A.; Swanson, D.; Dunbar, G.L.; Rossignol, J. Use of Polyamidoamine Dendrimers in Brain Diseases. Molecules 2018, 23, 2238. [Google Scholar] [CrossRef] [Green Version]
- Sarin, H.; Kanevsky, A.S.; Wu, H.; Brimacombe, K.R.; Fung, S.H.; Sousa, A.A.; Auh, S.; Wilson, C.M.; Sharma, K.; Aronova, A.M.; et al. Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. J. Transl. Med. 2008, 6, 80. [Google Scholar] [CrossRef] [Green Version]
- Moscariello, P.; Ng, D.; Jansen, M.; Weil, T.; Luhmann, H.J.; Hedrich, J. Brain Delivery of Multifunctional Dendrimer Protein Bioconjugates. Adv. Sci. 2018, 5, 1700897. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.; Jagani, S.; Johnson, K.; Jose, M.V.; Dean, D.R.; Vohra, Y.K.; Nyairo, E. Electrospun Bioactive Nanocomposite Scaffolds of Polycaprolactone and Nanohydroxyapatite for Bone Tissue Engineering. J. Nanosci. Nanotechnol. 2006, 6, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Sha, X.; Jiang, X.; Zhang, W.; Chen, L.; Fang, X. Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials 2012, 33, 8167–8176. [Google Scholar] [CrossRef]
- Belykh, E.; Shaffer, K.V.; Lin, C.; Byvaltsev, V.A.; Preul, M.C.; Chen, L. Blood-Brain Barrier, Blood-Brain Tumor Barrier, and Fluorescence-Guided Neurosurgical Oncology: Delivering Optical Labels to Brain Tumors. Front. Oncol. 2020, 10, 739. [Google Scholar] [CrossRef]
- Gou, M.; Wei, X.; Men, K.; Wang, B.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. PCL/PEG Copolymeric Nanoparticles: Potential Nanoplatforms for Anticancer Agent Delivery. Curr. Drug Targets 2011, 12, 1131–1150. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, S.; Diban, N.; Urtiaga, A. Hydrolytic Degradation and Mechanical Stability of Poly(epsilon-Caprolactone)/Reduced Graphene Oxide Membranes as Scaffolds for In Vitro Neural Tissue Regeneration. Membranes 2018, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zeng, Y.; Shi, S.; Xu, L.; Zhang, H.; Pathak, J.L.; Pan, Y. Design of polyaspartic acid peptide-poly (ethylene glycol)-poly (epsilon-caprolactone) nanoparticles as a carrier of hydrophobic drugs targeting cancer metastasized to bone. Int. J. Nanomed. 2017, 12, 3561–3575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulheim, E.; Baghirov, H.; Von Haartman, E.; Bøe, A.; Åslund, A.K.O.; Mørch, Y.; Davies, C.D.L. Cellular uptake and intracellular degradation of poly(alkyl cyanoacrylate) nanoparticles. J. Nanobiotechnol. 2016, 14, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, R.H.; Lherm, C.; Herbert, J.; Couvreur, P. In vitro model for the degradation of alkylcyanoacrylate nanoparticles. Biomaterials 1990, 11, 590–595. [Google Scholar] [CrossRef]
- Kante, B.; Couvreur, P.; Dubois-Krack, G.; De Meester, C.; Guiot, P.; Roland, M.; Mercier, M.; Speiseru, P. Toxicity of Polyalkylcyanoacrylate Nanoparticles I: Free Nanoparticles. J. Pharm. Sci. 1982, 71, 786–790. [Google Scholar] [CrossRef]
- Vauthier, C.; Dubernet, C.; Fattal, E.; Pinto-Alphandary, H.; Couvreur, P. Poly(alkylcyanoacrylates) as biodegradable materials for biomedical applications. Adv. Drug Deliv. Rev. 2003, 55, 519–548. [Google Scholar] [CrossRef]
- Vauthier, C. A journey through the emergence of nanomedicines with poly(alkylcyanoacrylate) based nanoparticles. J. Drug Target. 2019, 27, 502–524. [Google Scholar] [CrossRef] [PubMed]
- Baghirov, H.; Snipstad, S.; Sulheim, E.; Berg, S.; Hansen, R.; Thorsen, F.; Mørch, Y.; Davies, C.D.L.; Åslund, A.K.O. Ultrasound-mediated delivery and distribution of polymeric nanoparticles in the normal brain parenchyma of a metastatic brain tumour model. PLoS ONE 2018, 13, e0191102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrieux, K.; Couvreur, P. Polyalkylcyanoacrylate nanoparticles for delivery of drugs across the blood-brain barrier. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 463–474. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Zhang, N.; Tang, C.; Jiang, P.; Liu, X.; Yan, F.; Zheng, H. Mechanisms of enhanced antiglioma efficacy of polysorbate 80-modified paclitaxel-loaded PLGA nanoparticles by focused ultrasound. J. Cell. Mol. Med. 2018, 22, 4171–4182. [Google Scholar] [CrossRef]
- Vauthier, C.; Dubernet, C.; Chauvierre, C.; Brigger, I.; Couvreur, P. Drug delivery to resistant tumors: The potential of poly(alkyl cyanoacrylate) nanoparticles. J. Control Release 2003, 93, 151–160. [Google Scholar] [CrossRef]
- McCrorie, P.; Vasey, C.E.; Smith, S.J.; Marlow, M.; Alexander, C.; Rahman, R. Biomedical engineering approaches to enhance therapeutic delivery for malignant glioma. J. Control Release 2020, 328, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhang, Q.; Zhang, M.; Lv, X.; Li, Z.; Mohammadniaei, M.; Zhou, N.; Sun, Y. A novel biodegradable injectable chitosan hydrogel for overcoming postoperative trauma and combating multiple tumors. Carbohydr. Polym. 2021, 265, 118065. [Google Scholar] [CrossRef] [PubMed]
- Helmlinger, G.; Yuan, F.; Dellian, M.; Jain, R.K. Interstitial pH and pO2 gradients in solid tumors in vivo: High-resolution measurements reveal a lack of correlation. Nat. Med. 1997, 3, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, H.; Tie, C.; Yan, C.; Deng, Z.; Wan, Q.; Liu, X.; Yan, F.; Zheng, H. MR imaging tracking of inflammation-activatable engineered neutrophils for targeted therapy of surgically treated glioma. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef]
- Zhou, M.; Jiang, N.; Fan, J.; Fu, S.; Luo, H.; Su, P.; Zhang, M.; Shi, H.; Huang, Y.; Li, Y.; et al. H7K(R2)2-modified pH-sensitive self-assembled nanoparticles delivering small interfering RNA targeting hepatoma-derived growth factor for malignant glioma treatment. J. Control Release 2019, 310, 24–35. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Hickey, J.W.; Santos, J.L.; Williford, J.-M.; Mao, H.-Q. Control of polymeric nanoparticle size to improve therapeutic delivery. J. Control Release 2015, 219, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Cruz, L.J.; Stammes, M.A.; Que, I.; van Beek, E.R.; Knol-Blankevoort, V.T.; Snoeks, T.J.; Chan, A.; Kaijzel, E.L.; Löwik, C.W. Effect of PLGA NP size on efficiency to target traumatic brain injury. J. Control Release 2016, 223, 31–41. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Feng, S.-S. Effects of Particle Size and Surface Modification on Cellular Uptake and Biodistribution of Polymeric Nanoparticles for Drug Delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Brown, T.D.; Graham, A.; Helgeson, M.E.; Mitragotri, S. Size, shape, and flexibility influence nanoparticle transport across brain endothelium under flow. Bioeng. Transl. Med. 2020, 5, e10153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voigt, N.; Henrich-Noack, P.; Kockentiedt, S.; Hintz, W.; Tomas, J.; Sabel, B.A. Surfactants, not size or zeta-potential influence blood-brain barrier passage of polymeric nanoparticles. Eur. J. Pharm. Biopharm. 2014, 87, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Thorne, R.G.; Nicholson, C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc. Natl. Acad. Sci. USA 2006, 103, 5567–5572. [Google Scholar] [CrossRef] [Green Version]
- Nance, E.A.; Woodworth, G.F.; Sailor, K.A.; Shih, T.Y.; Xu, Q.; Swaminathan, G.; Xiang, D.; Eberhart, C.; Hanes, J. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci. Transl. Med. 2012, 4, 149ra119. [Google Scholar] [CrossRef] [Green Version]
- Truong, N.; Whittaker, M.; Mak, C.W.; Davis, T.P. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142. [Google Scholar] [CrossRef]
- Christian, D.A.; Cai, S.; Garbuzenko, O.B.; Harada, T.; Zajac, A.L.; Minko, T.; Discher, D.E. Flexible Filaments for in Vivo Imaging and Delivery: Persistent Circulation of Filomicelles Opens the Dosage Window for Sustained Tumor Shrinkage. Mol. Pharm. 2009, 6, 1343–1352. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Li, L.; Liu, T.; Hao, N.; Liu, H.; Chen, D.; Tang, F. The Shape Effect of Mesoporous Silica Nanoparticles on Biodistribution, Clearance, and Biocompatibility In Vivo. ACS Nano 2011, 5, 5390–5399. [Google Scholar] [CrossRef]
- Doshi, N.; Prabhakarpandian, B.; Rea-Ramsey, A.; Pant, K.; Sundaram, S.; Mitragotri, S. Flow and adhesion of drug carriers in blood vessels depend on their shape: A study using model synthetic microvascular networks. J. Control Release 2010, 146, 196–200. [Google Scholar] [CrossRef] [Green Version]
- Kolhar, P.; Anselmo, A.C.; Gupta, V.; Pant, K.; Prabhakarpandian, B.; Ruoslahti, E.; Mitragotri, S. Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proc. Natl. Acad. Sci. USA 2013, 110, 10753–10758. [Google Scholar] [CrossRef] [Green Version]
- Grabrucker, A.M.; Ruozi, B.; Belletti, D.; Pederzoli, F.; Forni, F.; Vandelli, M.A.; Tosi, G. Nanoparticle transport across the blood brain barrier. Tissue Barriers 2016, 4, e1153568. [Google Scholar] [CrossRef] [Green Version]
- Lu, W. Adsorptive-Mediated Brain Delivery Systems. Curr. Pharm. Biotechnol. 2012, 13, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, C.; Duschmalé, M.; Gavrilov, A.; Brandenberg, N.; Hoehnel, S.; Ceroni, C.; Lassalle, E.; Kassianidou, E.; Knoetgen, H.; Niewoehner, J.; et al. Investigating receptor-mediated antibody transcytosis using blood–brain barrier organoid arrays. Fluids Barriers CNS 2021, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for delivering therapeutics across the blood–brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef] [PubMed]
- Niewoehner, J.; Bohrmann, B.; Collin, L.; Urich, E.; Sade, H.; Maier, P.; Rueger, P.; Stracke, J.O.; Lau, W.; Tissot, A.C.; et al. Increased Brain Penetration and Potency of a Therapeutic Antibody Using a Monovalent Molecular Shuttle. Neuron 2014, 81, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Boado, R.J.; Zhang, Y.; Zhang, Y.; Pardridge, W.M. Humanization of anti-human insulin receptor antibody for drug targeting across the human blood–brain barrier. Biotechnol. Bioeng. 2007, 96, 381–391. [Google Scholar] [CrossRef]
- Zuchero, Y.J.Y.; Chen, X.; Bien-Ly, N.; Bumbaca, D.; Tong, R.K.; Gao, X.; Zhang, S.; Hoyte, K.; Luk, W.; Huntley, M.A.; et al. Discovery of Novel Blood-Brain Barrier Targets to Enhance Brain Uptake of Therapeutic Antibodies. Neuron 2016, 89, 70–82. [Google Scholar] [CrossRef] [Green Version]
- Dehouck, B.; Fenart, L.; Dehouck, M.-P.; Pierce, A.; Torpier, G.; Cecchelli, R. A New Function for the LDL Receptor: Transcytosis of LDL across the Blood–Brain Barrier. J. Cell Biol. 1997, 138, 877–889. [Google Scholar] [CrossRef]
- Ramalho, M.; Sevin, E.; Gosselet, F.; Lima, J.; Coelho, M.; Loureiro, J.; Pereira, M. Receptor-mediated PLGA nanoparticles for glioblastoma multiforme treatment. Int. J. Pharm. 2018, 545, 84–92. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Moos, T. Revisiting nanoparticle technology for blood–brain barrier transport: Unfolding at the endothelial gate improves the fate of transferrin receptor-targeted liposomes. J. Control Release 2016, 222, 32–46. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Loureiro, J.A.; Coelho, M.A.N.; Pereira, M.C. Transferrin Receptor-Targeted Nanocarriers: Overcoming Barriers to Treat Glioblastoma. Pharmaceutics 2022, 14, 279. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Jiang, X.; Zhang, Y.; Lu, Y.; Ma, H.; Guo, Y.; Zhang, Y.; An, S.; Li, J.; Liu, L.; et al. Dual Functional Peptide-Driven Nanoparticles for Highly Efficient Glioma-Targeting and Drug Codelivery. Mol. Pharm. 2016, 13, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Shilo, M.; Motiei, M.; Hana, P.; Popovtzer, R. Transport of nanoparticles through the blood–brain barrier for imaging and therapeutic applications. Nanoscale 2014, 6, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Betzer, O.; Shilo, M.; Opochinsky, R.; Barnoy, E.; Motiei, M.; Okun, E.; Yadid, G.; Popovtzer, R. The effect of nanoparticle size on the ability to cross the blood–brain barrier: An in vivo study. Nanomedicine 2017, 12, 1533–1546. [Google Scholar] [CrossRef]
- Ulbrich, K.; Knobloch, T.; Kreuter, J. Targeting the insulin receptor: Nanoparticles for drug delivery across the blood–brain barrier (BBB). J. Drug Target. 2011, 19, 125–132. [Google Scholar] [CrossRef]
- Tamaru, M.; Akita, H.; Kajimoto, K.; Sato, Y.; Hatakeyama, H.; Harashima, H. An apolipoprotein E modified liposomal nanoparticle: Ligand dependent efficiency as a siRNA delivery carrier for mouse-derived brain endothelial cells. Int. J. Pharm. 2014, 465, 77–82. [Google Scholar] [CrossRef]
- Neves, A.R.; Queiroz, J.F.; Lima, S.A.C.; Reis, S. Apo E-Functionalization of Solid Lipid Nanoparticles Enhances Brain Drug Delivery: Uptake Mechanism and Transport Pathways. Bioconj. Chem. 2017, 28, 995–1004. [Google Scholar] [CrossRef]
- Spencer, B.; Verma, I.; Desplats, P.; Morvinski, D.; Rockenstein, E.; Adame, A.; Masliah, E. A Neuroprotective Brain-penetrating Endopeptidase Fusion Protein Ameliorates Alzheimer Disease Pathology and Restores Neurogenesis. J. Biol. Chem. 2014, 289, 17917–17931. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Sun, X.; Mei, H.; Wang, Y.; Liao, Z.; Chen, J.; Zhang, Q.; Hu, Y.; Pang, Z.; Jiang, X. LDLR-mediated peptide-22-conjugated nanoparticles for dual-targeting therapy of brain glioma. Biomaterials 2013, 34, 9171–9182. [Google Scholar] [CrossRef]
- Karkan, D.; Pfeifer, C.; Vitalis, T.Z.; Arthur, G.; Ujiie, M.; Chen, Q.; Tsai, S.; Koliatis, G.; Gabathuler, R.; Jefferies, W.A. A unique carrier for delivery of therapeutic compounds beyond the blood-brain barrier. PLoS ONE 2008, 3, e2469. [Google Scholar] [CrossRef]
- Nounou, M.I.; Adkins, C.E.; Rubinchik, E.; Terrell-Hall, T.B.; Afroz, M.; Vitalis, T.; Gabathuler, R.; Tian, M.M.; Lockman, P.R. Anti-cancer Antibody Trastuzumab-Melanotransferrin Conjugate (BT2111) for the Treatment of Metastatic HER2+ Breast Cancer Tumors in the Brain: An In-Vivo Study. Pharm. Res. 2016, 33, 2930–2942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Canup, B.S.B.; Ngo, V.L.; Denning, T.L.; Garg, P.; Laroui, H. Internalization of Garlic-Derived Nanovesicles on Liver Cells is Triggered by Interaction with CD98. ACS Omega 2020, 5, 23118–23128. [Google Scholar] [CrossRef] [PubMed]
- Feral, C.C.; Nishiya, N.; Fenczik, C.A.; Stuhlmann, H.; Slepak, M.; Ginsberg, M.H. CD98hc (SLC3A2) mediates integrin signaling. Proc. Natl. Acad. Sci. USA 2005, 102, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Xiao, B.; Laroui, H.; Viennois, E.; Ayyadurai, S.; Charania, M.A.; Zhang, Y.; Zhang, Z.; Baker, M.T.; Zhang, B.; Gewirtz, A.T.; et al. Nanoparticles with Surface Antibody Against CD98 and Carrying CD98 Small Interfering RNA Reduce Colitis in Mice. Gastroenterology 2014, 146, 1289–1300.e19. [Google Scholar] [CrossRef] [Green Version]
- Nawashiro, H.; Otani, N.; Shinomiya, N.; Fukui, S.; Nomura, N.; Yano, A.; Shima, K.; Matsuo, H.; Kanai, Y. The Role of CD98 in Astrocytic Neoplasms. Hum. Cell 2002, 15, 25–31. [Google Scholar] [CrossRef]
- Kim, K.J.; Li, B.; Winer, J.; Armanini, M.; Gillett, N.; Phillips, H.S.; Ferrara, N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993, 362, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Abakumov, M.A.; Nukolova, N.V.; Sokolsky-Papkov, M.; Shein, S.A.; Sandalova, T.O.; Vishwasrao, H.M.; Grinenko, N.F.; Gubsky, I.L.; Abakumov, A.M.; Kabanov, A.V.; et al. VEGF-targeted magnetic nanoparticles for MRI visualization of brain tumor. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 825–833. [Google Scholar] [CrossRef]
- Alves, A.D.C.S.; Lavayen, V.; Dias, A.D.F.; Bruinsmann, F.A.; Scholl, J.N.; Cé, R.; Visioli, F.; Battastini, A.M.O.; Guterres, S.S.; Figueiró, F.; et al. EGFRvIII peptide nanocapsules and bevacizumab nanocapsules: A nose-to-brain multitarget approach against glioblastoma. Nanomedicine 2021, 16, 1775–1790. [Google Scholar] [CrossRef]
- Shein, S.A.; Nukolova, N.V.; Korchagina, A.A.; Abakumova, T.; Kiuznetsov, I.I.; Abakumov, M.; Baklaushev, V.P.; Gurina, O.I.; Chekhonin, V.P. Site-Directed Delivery of VEGF-Targeted Liposomes into Intracranial C6 Glioma. Bull. Exp. Biol. Med. 2015, 158, 371–376. [Google Scholar] [CrossRef]
- Shein, S.A.; Kuznetsov, I.I.; Abakumova, T.; Chelushkin, P.S.; Melnikov, P.A.; Korchagina, A.A.; Bychkov, D.A.; Seregina, I.F.; Bolshov, M.A.; Kabanov, A.V.; et al. VEGF- and VEGFR2-Targeted Liposomes for Cisplatin Delivery to Glioma Cells. Mol. Pharm. 2016, 13, 3712–3723. [Google Scholar] [CrossRef]
- Whittle, J.R.; Lickliter, J.D.; Gan, H.K.; Scott, A.M.; Simes, J.; Solomon, B.J.; MacDiarmid, J.A.; Brahmbhatt, H.; Rosenthal, M.A. First in human nanotechnology doxorubicin delivery system to target epidermal growth factor receptors in recurrent glioblastoma. J. Clin. Neurosci. 2015, 22, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.-W.; Weiss, W.A. Epidermal growth factor receptor and EGFRvIII in glioblastoma: Signaling pathways and targeted therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Nabors, L.; Mason, W.P.; Perry, J.R.; Shapiro, W.; Kavan, P.; Mathieu, D.; Phuphanich, S.; Cseh, A.; Fu, Y.; et al. Phase I/randomized phase II study of afatinib, an irreversible ErbB family blocker, with or without protracted temozolomide in adults with recurrent glioblastoma. Neuro-Oncology 2015, 17, 430–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westphal, M.; Maire, C.L.; Lamszus, K. EGFR as a Target for Glioblastoma Treatment: An Unfulfilled Promise. CNS Drugs 2017, 31, 723–735. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, J.H.; Jeppesen, M.; Pilgaard, L.; Agger, R.; Duroux, M.; Zachar, V.; Moos, T. Targeted Antiepidermal Growth Factor Receptor (Cetuximab) Immunoliposomes Enhance Cellular Uptake In Vitro and Exhibit Increased Accumulation in an Intracranial Model of Glioblastoma Multiforme. J. Drug Deliv. 2013, 2013, 209205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erel-Akbaba, G.; Carvalho, L.A.; Tian, T.; Zinter, M.; Akbaba, H.; Obeid, P.J.; Chiocca, E.A.; Weissleder, R.; Kantarci, A.G.; Tannous, B.A. Radiation-Induced Targeted Nanoparticle-Based Gene Delivery for Brain Tumor Therapy. ACS Nano 2019, 13, 4028–4040. [Google Scholar] [CrossRef]
- A Study to Evaluate the Safety, Tolerability and Immunogenicity of EGFR(V)-EDV-Dox in Subjects with Recurrent Glioblastoma Multiforme (GBM). Available online: https://www.clinicaltrials.gov/ct2/show/NCT02766699 (accessed on 23 May 2022).
- Arif, W.M.; Elsinga, P.H.; Gasca-Salas, C.; Versluis, M.; Martínez-Fernández, R.; Dierckx, R.A.; Borra, R.J.; Luurtsema, G. Focused ultrasound for opening blood-brain barrier and drug delivery monitored with positron emission tomography. J. Control Release 2020, 324, 303–316. [Google Scholar] [CrossRef]
- McDannold, N.; Vykhodtseva, N.; Hynynen, K. Use of Ultrasound Pulses Combined with Definity for Targeted Blood-Brain Barrier Disruption: A Feasibility Study. Ultrasound Med. Biol. 2007, 33, 584–590. [Google Scholar] [CrossRef] [Green Version]
- Sirsi, S.; Borden, M. Microbubble Compositions, Properties and Biomedical Applications. Bubble Sci. Eng. Technol. 2009, 1, 3–17. [Google Scholar] [CrossRef]
- Burgess, A.; Dubey, S.; Yeung, S.; Hough, O.; Eterman, N.; Aubert, I.; Hynynen, K. Alzheimer Disease in a Mouse Model: MR Imaging–guided Focused Ultrasound Targeted to the Hippocampus Opens the Blood-Brain Barrier and Improves Pathologic Abnormalities and Behavior. Radiology 2014, 273, 736–745. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Zhou, Y.; Chen, J.; Huang, N.; Wang, Z.; Cheng, Y. Gene Therapy for Drug-Resistant Glioblastoma via Lipid-Polymer Hybrid Nanoparticles Combined with Focused Ultrasound. Int. J. Nanomed. 2021, 16, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Nance, E.; Timbie, K.; Miller, G.W.; Song, J.; Louttit, C.; Klibanov, A.L.; Shih, T.-Y.; Swaminathan, G.; Tamargo, R.J.; Woodworth, G.F.; et al. Non-invasive delivery of stealth, brain-penetrating nanoparticles across the blood-brain barrier using MRI-guided focused ultrasound. J. Control Release 2014, 189, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timbie, K.F.; Afzal, U.; Date, A.; Zhang, C.; Song, J.; Miller, G.W.; Suk, J.S.; Hanes, J.; Price, R.J. MR image-guided delivery of cisplatin-loaded brain-penetrating nanoparticles to invasive glioma with focused ultrasound. J. Control Release 2017, 263, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.P.; Mastorakos, P.; Suk, J.S.; Klibanov, A.L.; Hanes, J.; Price, R.J. Targeted gene transfer to the brain via the delivery of brain-penetrating DNA nanoparticles with focused ultrasound. J. Control Release 2016, 223, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.B.; Di Ianni, T.; Vyas, D.B.; Huang, Z.; Park, S.; Hosseini-Nassab, N.; Aryal, M.; Airan, R.D. Focused Ultrasound for Noninvasive, Focal Pharmacologic Neurointervention. Front. Neurosci. 2020, 14, 675. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Sheikov, N.A.; Jolesz, F.A.; Vykhodtseva, N. Local and reversible blood–brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. NeuroImage 2005, 24, 12–20. [Google Scholar] [CrossRef]
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-Enhanced Delivery. Neurotherapeutics 2017, 14, 358–371. [Google Scholar] [CrossRef] [Green Version]
- Saucier-Sawyer, J.K.; Seo, Y.-E.; Gaudin, A.; Quijano, E.; Song, E.; Sawyer, A.J.; Deng, Y.; Huttner, A.; Saltzman, W.M. Distribution of polymer nanoparticles by convection-enhanced delivery to brain tumors. J. Control Release 2016, 232, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef] [Green Version]
- Kunwar, S.; Chang, S.; Westphal, M.; Vogelbaum, M.; Sampson, J.; Barnett, G.; Shaffrey, M.; Ram, Z.; Piepmeier, J.; Prados, M.; et al. Phase III randomized trial of CED of IL13-PE38QQR vs. Gliadel wafers for recurrent glioblastoma. Neuro-Oncology 2010, 12, 871–881. [Google Scholar] [CrossRef] [Green Version]
- Vogelbaum, M.; Healy, A. Convection-enhanced drug delivery for gliomas. Surg. Neurol. Int. 2015, 6 (Suppl. S1), S59–S67. [Google Scholar] [CrossRef] [PubMed]
- Mastorakos, P.; Zhang, C.; Song, E.; Kim, Y.E.; Park, H.W.; Berry, S.; Choi, W.K.; Hanes, J.; Suk, J.S. Biodegradable brain-penetrating DNA nanocomplexes and their use to treat malignant brain tumors. J. Control Release 2017, 262, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negron, K.; Khalasawi, N.; Lu, B.; Ho, C.-Y.; Lee, J.; Shenoy, S.; Mao, H.-Q.; Wang, T.-H.; Hanes, J.; Suk, J.S. Widespread gene transfer to malignant gliomas with In vitro-to-In vivo correlation. J. Control Release 2019, 303, 1–11. [Google Scholar] [CrossRef]
- Ansari, M.A.; Chung, I.-M.; Rajakumar, G.; Alzohairy, M.A.; Alomary, M.; Thiruvengadam, M.; Pottoo, F.H.; Ahmad, N. Current Nanoparticle Approaches in Nose to Brain Drug Delivery and Anticancer Therapy—A Review. Curr. Pharm. Des. 2020, 26, 1128–1137. [Google Scholar] [CrossRef]
- Dufes, C.; Olivier, J.-C.; Gaillard, F.; Gaillard, A.; Couet, W.; Muller, J.-M. Brain delivery of vasoactive intestinal peptide (VIP) following nasal administration to rats. Int. J. Pharm. 2003, 255, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Su, Y.; Sun, B.; Gao, X.; Dong, X.; Fu, L.; Zhang, Y.; Li, Z.; Wang, Y.; Jiang, H.; Han, B. Intranasal Delivery of Targeted Nanoparticles Loaded With miR-132 to Brain for the Treatment of Neurodegenerative Diseases. Front. Pharmacol. 2020, 11, 1165. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Taki, H.; Okada, H. Nose-to-brain drug delivery system with ligand/cell-penetrating peptide-modified polymeric nano-micelles for intracerebral gliomas. Eur. J. Pharm. Biopharm. 2020, 152, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, P.G.; Pulakkat, S.; Patravale, V.B. Nose-to-brain delivery: Exploring newer domains for glioblastoma multiforme management. Drug Deliv. Transl. Res. 2020, 10, 1044–1056. [Google Scholar] [CrossRef]
- Craparo, E.F.; Musumeci, T.; Bonaccorso, A.; Pellitteri, R.; Romeo, A.; Naletova, I.; Cucci, L.M.; Cavallaro, G.; Satriano, C. mPEG-PLGA Nanoparticles Labelled with Loaded or Conjugated Rhodamine-B for Potential Nose-to-Brain Delivery. Pharmaceutics 2021, 13, 1508. [Google Scholar] [CrossRef]
- Chu, L.; Wang, A.; Ni, L.; Yan, X.; Song, Y.; Zhao, M.; Sun, K.; Mu, H.; Liu, S.; Wu, Z.; et al. Nose-to-brain delivery of temozolomide-loaded PLGA nanoparticles functionalized with anti-EPHA3 for glioblastoma targeting. Drug Deliv. 2018, 25, 1634–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanazawa, T.; Morisaki, K.; Suzuki, S.; Takashima, Y. Prolongation of Life in Rats with Malignant Glioma by Intranasal siRNA/Drug Codelivery to the Brain with Cell-Penetrating Peptide-Modified Micelles. Mol. Pharm. 2014, 11, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Cornelio, D.; Roesler, R.; Schwartsmann, G. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Ann. Oncol. 2007, 18, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-Hydrogel: A Hybrid Biomaterial System for Localized Drug Delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef] [Green Version]
- Bastiancich, C.; Vanvarenberg, K.; Ucakar, B.; Pitorre, M.; Bastiat, G.; Lagarce, F.; Préat, V.; Danhier, F. Lauroyl-gemcitabine-loaded lipid nanocapsule hydrogel for the treatment of glioblastoma. J. Control Release 2016, 225, 283–293. [Google Scholar] [CrossRef]
- Pillai, J.J.; Thulasidasan, A.K.T.; Anto, R.J.; Chithralekha, D.N.; Narayanan, A.; Kumar, G.S.V. Folic acid conjugated cross-linked acrylic polymer (FA-CLAP) hydrogel for site specific delivery of hydrophobic drugs to cancer cells. J. Nanobiotechnol. 2014, 12, 25. [Google Scholar] [CrossRef] [Green Version]
- Basso, J.; Miranda, A.; Nunes, S.; Cova, T.; Sousa, J.; Vitorino, C.; Pais, A. Hydrogel-Based Drug Delivery Nanosystems for the Treatment of Brain Tumors. Gels 2018, 4, 62. [Google Scholar] [CrossRef] [Green Version]
- Eckmann, D.M.; Composto, R.J.; Tsourkas, A.; Muzykantov, V.R. Nanogel carrier design for targeted drug delivery. J. Mater. Chem. B 2014, 2, 8085–8097. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Guo, X.; Temenoff, J.S.; Tabata, Y.; Caplan, A.I.; Kasper, F.K.; Mikos, A.G. Effect of Swelling Ratio of Injectable Hydrogel Composites on Chondrogenic Differentiation of Encapsulated Rabbit Marrow Mesenchymal Stem Cells In Vitro. Biomacromolecules 2009, 10, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Žuržul, N.; Ilseng, A.; Prot, V.E.; Sveinsson, H.M.; Skallerud, B.H.; Stokke, B.T. Donnan Contribution and Specific Ion Effects in Swelling of Cationic Hydrogels are Additive: Combined High-Resolution Experiments and Finite Element Modeling. Gels 2020, 6, 31. [Google Scholar] [CrossRef]
- Shatsberg, Z.; Zhang, X.; Ofek, P.; Malhotra, S.; Krivitsky, A.; Scomparin, A.; Tiram, G.; Calderon, M.; Haag, R.; Satchi-Fainaro, R. Functionalized nanogels carrying an anticancer microRNA for glioblastoma therapy. J. Control Release 2016, 239, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, F.; Chen, Y.; Liu, J.; Wang, X.; Chen, A.T.; Deng, G.; Zhang, H.; Liu, J.; Hong, Z.; et al. Targeted Delivery of CRISPR/Cas9-Mediated Cancer Gene Therapy via Liposome-Templated Hydrogel Nanoparticles. Adv. Funct. Mater. 2017, 27, 1703036. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Zong, H.; Kopelman, R. Click Conjugation of Peptide to Hydrogel Nanoparticles for Tumor-Targeted Drug Delivery. Biomacromolecules 2014, 15, 3728–3734. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.J.; Baek, S.-K.; Makkouk, A.R.; Krasieva, T.; Hirschberg, H. Macrophages as Cell-Based Delivery Systems for Nanoshells in Photothermal Therapy. Ann. Biomed. Eng. 2012, 40, 507–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibarra, L.E.; Beaugé, L.; Arias-Ramos, N.; Rivarola, V.A.; Chesta, C.A.; López-Larrubia, P.; Palacios, R.E. Trojan horse monocyte-mediated delivery of conjugated polymer nanoparticles for improved photodynamic therapy of glioblastoma. Nanomedicine 2020, 15, 1687–1707. [Google Scholar] [CrossRef]
- Xue, J.; Zhao, Z.; Zhang, L.; Xue, L.; Shen, S.; Wen, Y.; Wei, Z.; Wang, L.; Kong, L.; Sun, H.; et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 2017, 12, 692–700. [Google Scholar] [CrossRef]
- Wang, X.; Gao, J.-Q.; Ouyang, X.; Wang, J.; Sun, X.; Lv, Y. Mesenchymal stem cells loaded with paclitaxel–poly(lactic-co-glycolic acid) nanoparticles for glioma-targeting therapy. Int. J. Nanomed. 2018, 13, 5231–5248. [Google Scholar] [CrossRef] [Green Version]
- Roger, M.; Clavreul, A.; Venier-Julienne, M.-C.; Passirani, C.; Sindji, L.; Schiller, P.; Montero-Menei, C.; Menei, P. Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials 2010, 31, 8393–8401. [Google Scholar] [CrossRef]
- Clavreul, A.; Lautram, N.; Franconi, F.; Passirani, C.; Montero-Menei, C.; Menei, P.; Tetaud, C.; Montagu, A.; Laine, A.-L.; Vessieres, A. Targeting and treatment of glioblastomas with human mesenchymal stem cells carrying ferrociphenol lipid nanocapsules. Int. J. Nanomed. 2015, 10, 1259–1271. [Google Scholar] [CrossRef] [Green Version]
- Arshad, A.; Yang, B.; Bienemann, A.S.; Barua, N.U.; Wyatt, M.J.; Woolley, M.; Johnson, D.E.; Edler, K.; Gill, S.S. Convection-Enhanced Delivery of Carboplatin PLGA Nanoparticles for the Treatment of Glioblastoma. PLoS ONE 2015, 10, e0132266. [Google Scholar] [CrossRef]
- Danhier, F.; Messaoudi, K.; Lemaire, L.; Benoit, J.P.; Lagarce, F. Combined anti-Galectin-1 and anti-EGFR siRNA-loaded chitosan-lipid nanocapsules decrease temozolomide resistance in glioblastoma: In vivo evaluation. Int. J. Pharm. 2015, 481, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, W.G.; Oskolkov, N.; Song, X.; Lal, B.; Yang, X.; Pomper, M.; Laterra, J.; Nimmagadda, S.; McMahon, M.T. Salicylic Acid Conjugated Dendrimers Are a Tunable, High Performance CEST MRI NanoPlatform. Nano Lett. 2016, 16, 2248–2253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, J.S.; Bernal, G.; Polster, S.; Nunez, L.; Larsen, G.F.; Mansour, N.; Podell, M.; Yamini, B. Convection-Enhanced Delivery of Polymeric Nanoparticles Encapsulating Chemotherapy in Canines with Spontaneous Supratentorial Tumors. World Neurosurg. 2018, 117, e698–e704. [Google Scholar] [CrossRef]
- Panja, S.; Dey, G.; Bharti, R.; Mandal, P.; Mandal, M.; Chattopadhyay, S. Metal Ion Ornamented Ultrafast Light-Sensitive Nanogel for Potential in Vivo Cancer Therapy. Chem. Mater. 2016, 28, 8598–8610. [Google Scholar] [CrossRef]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Shen, M.; Li, Y.; Sun, Y.; Teng, Y.; Wang, Y.; Duan, Y. The synergic antitumor effects of paclitaxel and temozolomide co-loaded in mPEG-PLGA nanoparticles on glioblastoma cells. Oncotarget 2016, 7, 20890–20901. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Polymer Type | Common Synthesis Techniques | Advantages | Disadvantages | Specific Uses Cited |
|---|---|---|---|---|
| Polyanhydride | Most often via polycondensations from diacids or diacyl anhydrides; can also be prepared via solvent evaporation from emulsion, or thiol-ene ‘click’ polymerization, or melt condensation; NP synthesis via nanoprecipitation | Well-characterized; biocompatible; biodegradable; modifiable (depending on copolymer and surface ligand composition); hydrophobic; predictable rate of release/erosion | Rapid erosion can lead to inadequate ligand retention times; difficult to synthesize; limited stability of loaded peptides and proteins due to nucleophile acylation | Gliadel® (BCNU) wafer for local, sustained-release chemotherapy [14]; drug delivery across the BBB [21]; delivery of non-proteinaceous cargo [16] |
| Poly (lactic-co-glycolic acid) | Co-polymerization of cyclic dimers of glycolic acid and lactic acid; NP synthesis via emulsification-evaporation, nanoprecipitation, phase-inversion, and solvent diffusion; emulsification-evaporation and nanoprecipitation are most commonly used when loading hydrophobic moieties | Widely used; biocompatible; simple biodegradability; easily synthesized; modifiable charge, hydrophobicity, and degradation rate; sustained drug-release; good BBB/tumor penetration | Poor drug loading efficiency; poor drug target delivery efficiency due to high burst release; destabilization of acid-sensitive drugs/peptides | Encapsulation of chemotherapeutics with toxicity profiles indicating sustained, low dosing [7]; microsphere and microparticle drug delivery systems [23] |
| Poly (β-amino ester) | Conjugate addition of amines to bis(acrylamides) and copolymerization; NP synthesis via solvent/anti-solvent formulation | Established safety profile; biocompatible; biodegradable; easily synthesized; high efficacy; pH buffering capacity; able to escape endosomes and allow intracellular expression of nucleic acids | Instability in blood (rapid hydrolysis) without surface modifications; limited ability to achieve widespread gene transfer due to adhesive interactions with ECM | Delivery of polynucleotides and other acid-labile compounds [36]; delivery of nucleic acids to cells [44] |
| Chitosan | Enzymatic or chemical deacetylation of chitin, usually through hydrolysis, produces chitosan; NP synthesis via emulsification and crosslinking, microemulsion, precipitation, or ionic gelation | Biodegradable; capable of mucous membrane adherence and transcytosis; sustained drug release; putative preferential release in tumor acidic environment | Low solubility at physiological pH; tendency to aggregate | Nose-to-brain delivery (via mucous membrane adherence) [46]; in situ gelation [73]; tumor targeting via differential pH [47] |
| Poly(amidoamine) dendrimers | Convergent (beginning with exterior and adding end groups while working towards the core) or divergent synthesis (beginning with core and adding end groups towards the exterior); end group additions via conjugate addition | Biocompatible; flexible, non-toxic; stable; highly soluble; small; modifiable; large hydrophilic surface area; presence of cavities; resistance to denaturation after freezing/thawing | Associated with (modifiable) cytotoxicity; synthesis can lead to heterogeneous mixture of dendrimers unless additional purification steps are completed | Precision-targeting [52]; delivery across the BBB [54]; encapsulating particularly insoluble contents [53] |
| Poly(caprolactone) | Polycondensation of 6-hydroxyhexanoic acid, or ring-opening polymerization of ε-caprolactone; NP synthesis via nanoemulsification, supercritical fluid extraction of emulsion, or solvent evaporation | Biodegradable; non-toxic; modifiable; stable | High hydrophobicity (slow degradation rate of months/years) | Combination with other copolymers to tailor NP suitability to cargo [56] |
| Poly(alkyl cyanoacrylate) | Free radical, anionic, and zwitterionic polymerization; NP synthesis via polymerization in aqueous acidic phase or through interfacial emulsion polymerization | Biodegradable; modifiable; enhanced intracellular penetration; capable of overcoming multidrug resistance | BBB translocation ability remains controversial | Hydrogel-incorporated drug delivery [66]; delivery of nucleic acids and peptides [66]; continuous drug delivery (vs. bursts) [66]; instances of multidrug resistance [70] |
| Polymer Modification | Advantages | Disadvantages | General Uses | |
| Polyethylene glycol | Widely used; classified as GRAS; increases systemic circulation time of NPs; reduces recognition of NPs by immune cells; decreases NP aggregation, opsonization, and phagocytosis | Reduced cellular uptake of PEGylated NPs | Modify NP to reduce immunogenicity | |
| pH | Can improve selective tumor targeting via triggered drug release | Limits the types of cargo able to be carried within the NP | Modify NP to selectively target tumor tissue and spare surrounding parenchyma | |
| Size | Can increase NP stability; can potentially increase BBB/BBTB penetration and brain parenchymal spread | Conflicting in vitro/in vivo results on ideal size of NPs for BBB/BBTB penetrance, brain tissue spread, and cellular uptake | Modify NP to increase intra-tumoral spread | |
| Shape | Can modulate NP circulation time, cellular uptake, and BBB penetration | Certain shapes promote accumulation in non-target organs; ideal shape, depending on delivery mechanism, requires further investigation | Modify NP to maximize efficacy based on delivery mechanism (e.g., nose-to-brain vs. across BBB) |
| Mechanism of Delivery | Type(s) of Polymeric NPs Used | Advantages | Limitations |
|---|---|---|---|
| Focused Ultrasound | PLGA [133] | Can reversibly open BBB; targeted delivery; safety supported via clinical trials; minimal systemic effects | Acute complications such as microhemorrhages reported; invasive |
| Convection-Enhanced Delivery | PLGA [171], PBAE [143], Chitosan [172], PAMAM [173], PCL [174] | High volume of distribution reported; targeted delivery; multiple ongoing clinical trials; potential for use post-resection; minimal systemic effects | No definitive increase in glioma patient survival time reported; infection; limited therapeutic administration windows; invasive |
| Nose-to-Brain Delivery | PLGA [151], Chitosan [46], PCL [153] | Minimally invasive; easier to study in vivo; bypasses BBB; minimal systemic effects | Exact delivery mechanism and clearance pathways unclear; non-targeted delivery; bioavailability can be low compared to other delivery mechanisms; limited NP clinical studies |
| Intracranial Hydrogel Delivery | PLGA [155], Chitosan [73], PCL [175] | Potential for use post-resection; targeted delivery; passively controlled drug release; variety of potential approaches; minimal systemic effects | Difficult to use with hydrophobic NPs; invasive; non-targeted delivery |
| Cell-Based Delivery | PLGA [168] | Minimally invasive; limited clearance via reticuloendothelial system compared to other systemic delivery approaches | Limited NP clinical studies; non-targeted delivery |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caraway, C.A.; Gaitsch, H.; Wicks, E.E.; Kalluri, A.; Kunadi, N.; Tyler, B.M. Polymeric Nanoparticles in Brain Cancer Therapy: A Review of Current Approaches. Polymers 2022, 14, 2963. https://doi.org/10.3390/polym14142963
Caraway CA, Gaitsch H, Wicks EE, Kalluri A, Kunadi N, Tyler BM. Polymeric Nanoparticles in Brain Cancer Therapy: A Review of Current Approaches. Polymers. 2022; 14(14):2963. https://doi.org/10.3390/polym14142963
Chicago/Turabian StyleCaraway, Chad A., Hallie Gaitsch, Elizabeth E. Wicks, Anita Kalluri, Navya Kunadi, and Betty M. Tyler. 2022. "Polymeric Nanoparticles in Brain Cancer Therapy: A Review of Current Approaches" Polymers 14, no. 14: 2963. https://doi.org/10.3390/polym14142963
APA StyleCaraway, C. A., Gaitsch, H., Wicks, E. E., Kalluri, A., Kunadi, N., & Tyler, B. M. (2022). Polymeric Nanoparticles in Brain Cancer Therapy: A Review of Current Approaches. Polymers, 14(14), 2963. https://doi.org/10.3390/polym14142963






