Characteristics of Water Transport of Membrane Electrolyte over Selected Temperature for Proton Exchange Membrane Fuel Cell
Abstract
:1. Introduction
2. Experimental Approach
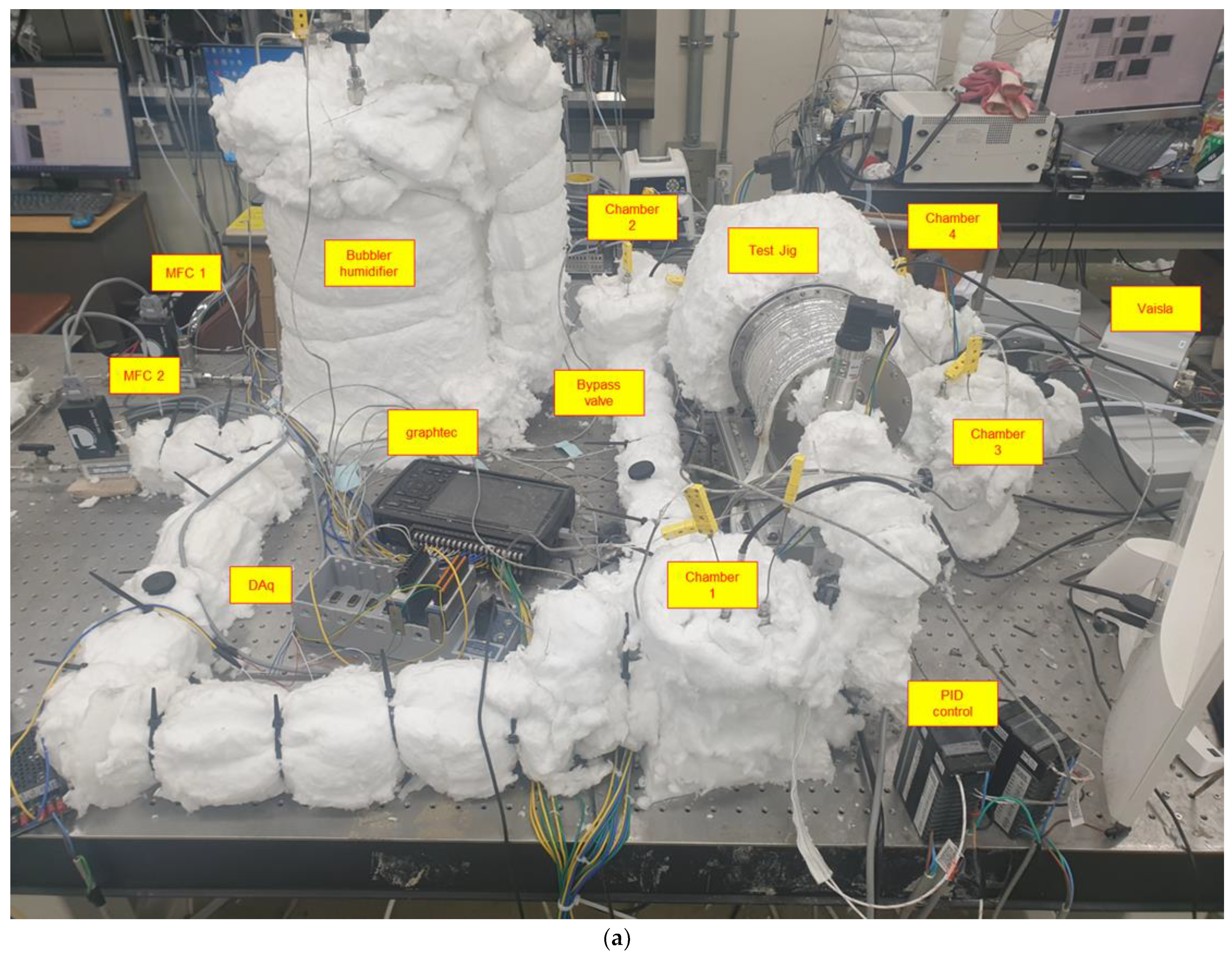
2.1. Experimental Configuration
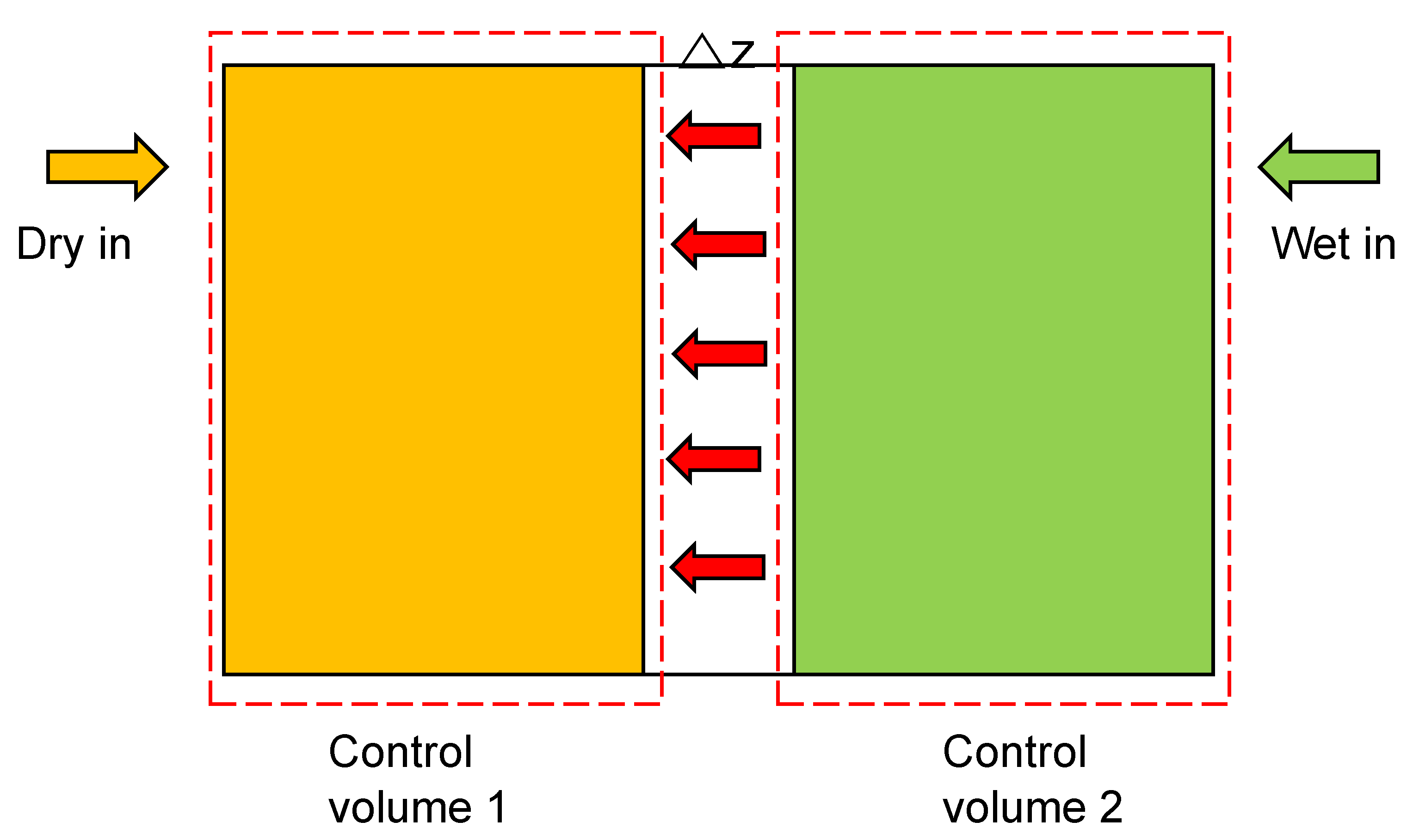
2.2. Mathematical Background
- The experiments are conducted under isothermal steady-state conditions for both air streams.
- There are no chemical reactions either side of the test jig.
- No condensation occurs inside or outside the test jig. The wet air is considered an ideal gas.
- The membrane is a stationary, nonreacting medium of uniform total molar concentration.
3. Results and Discussion
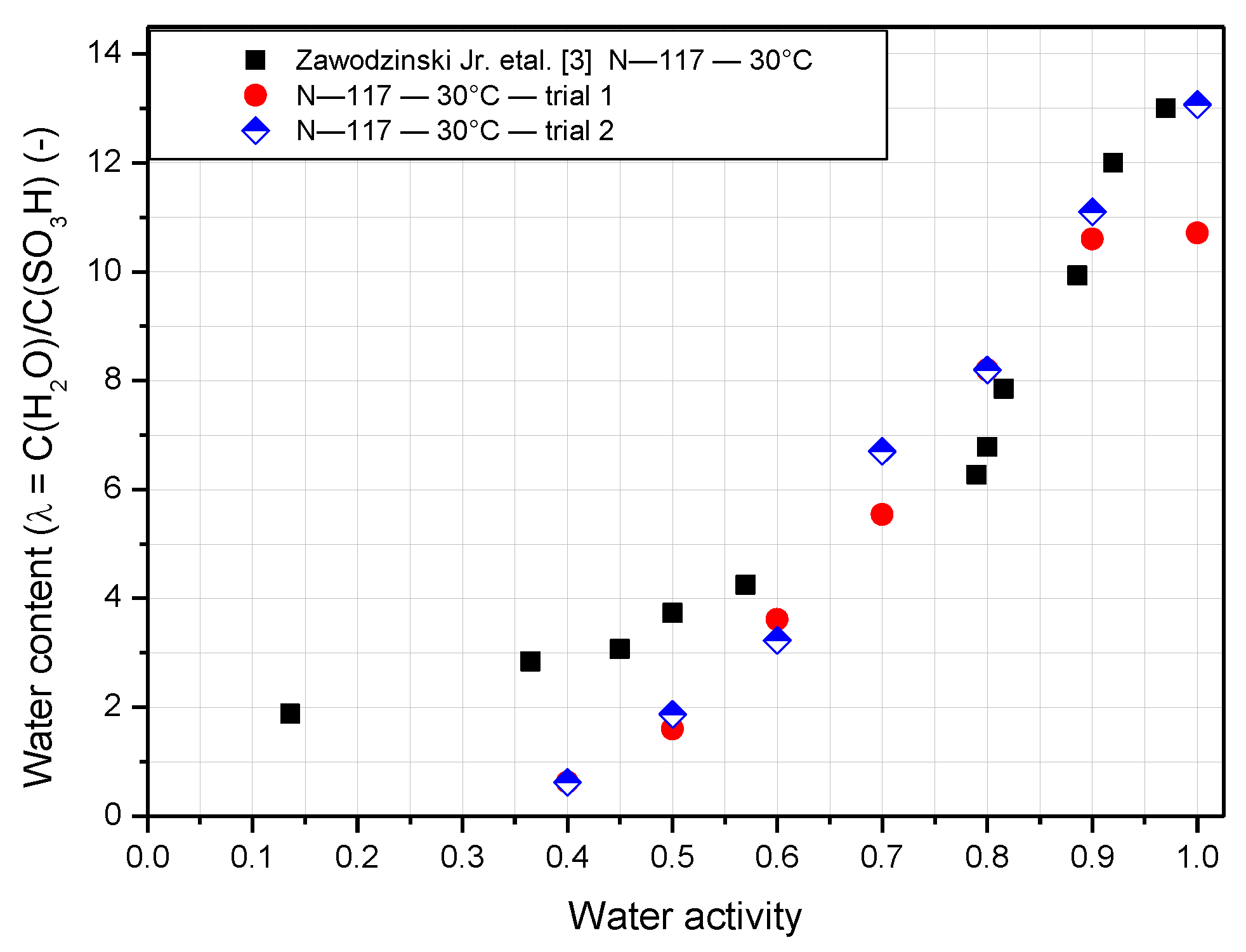
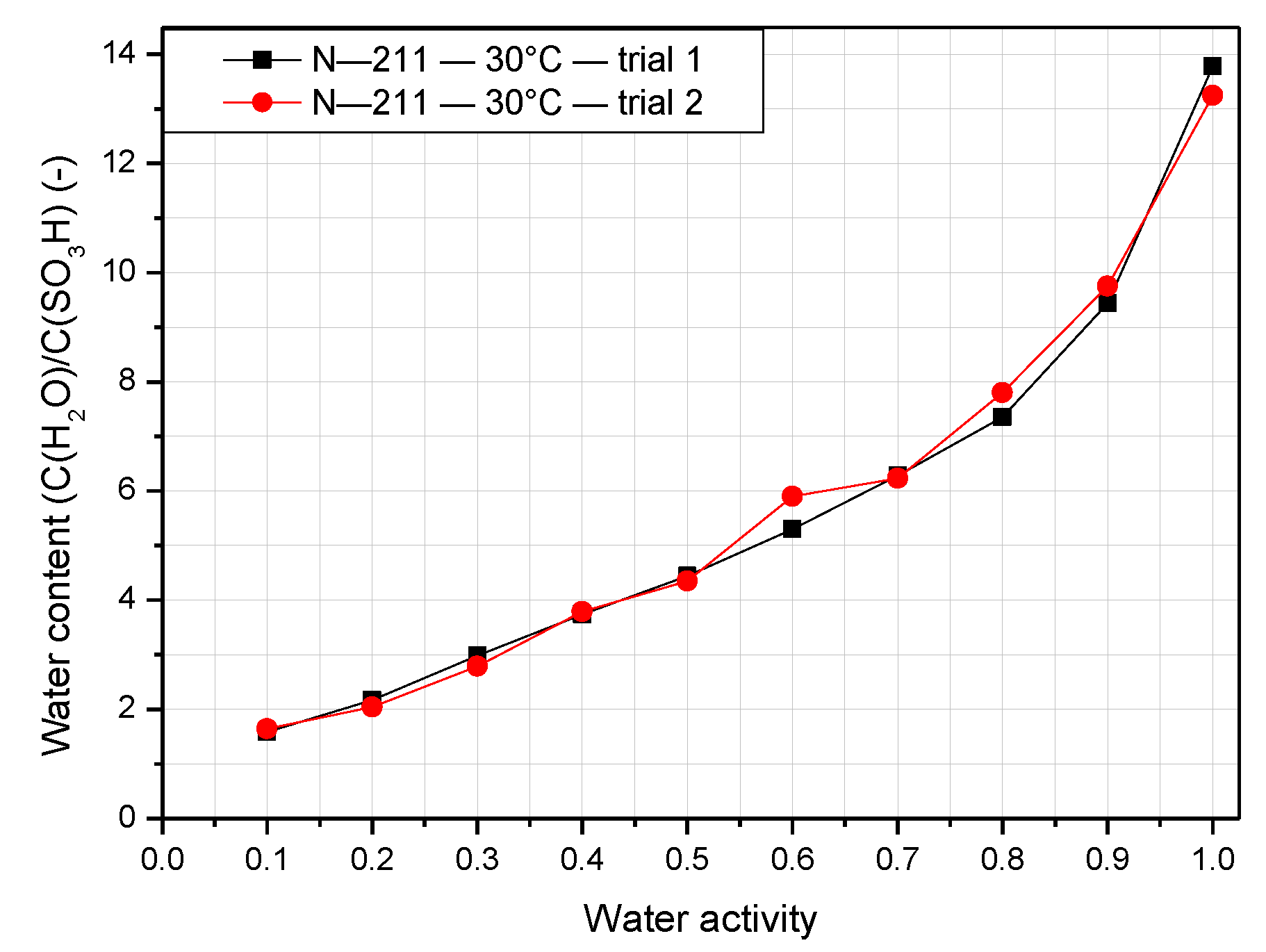
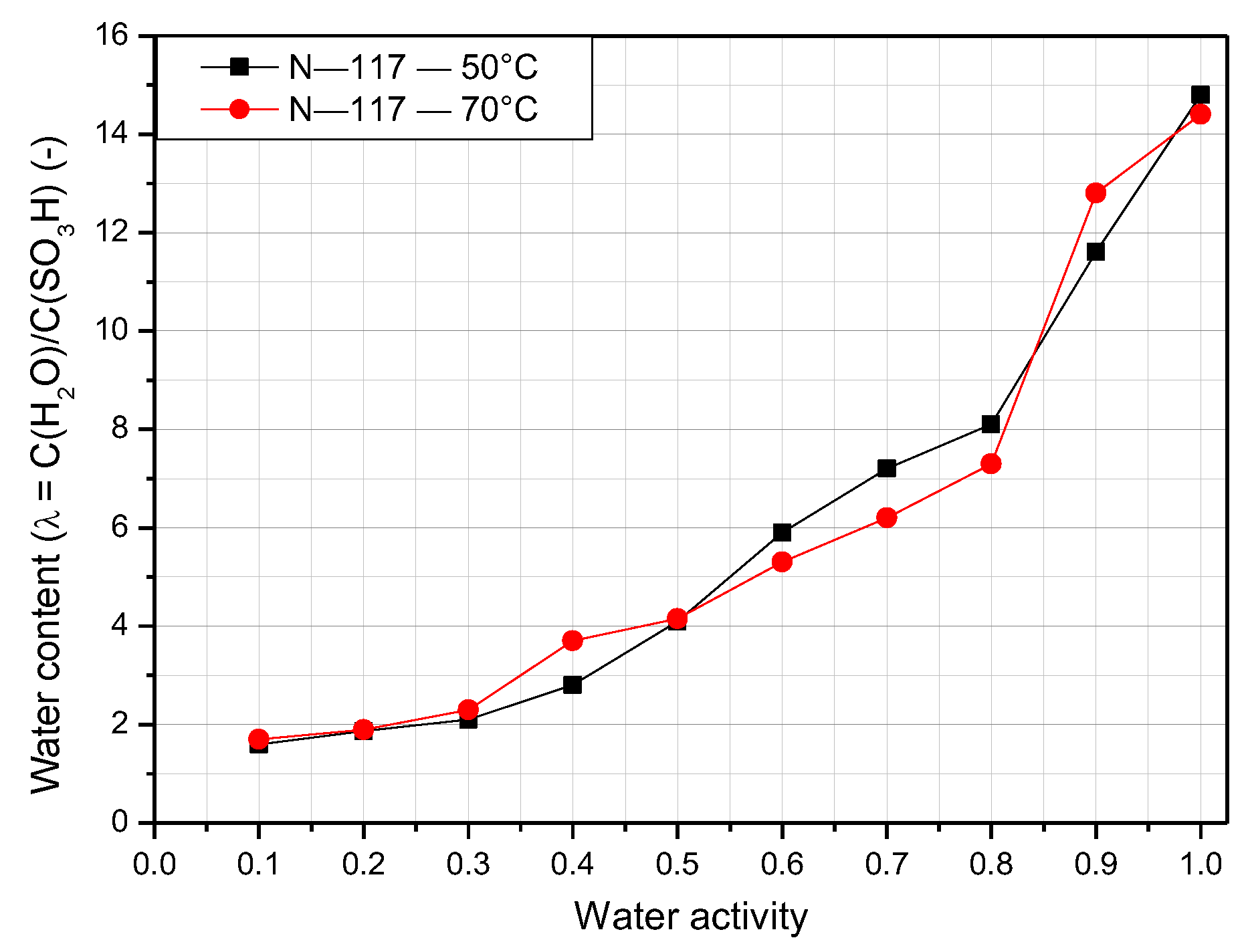
3.1. Water Content of Nafion Membranes with Varying Temperature and Humidity
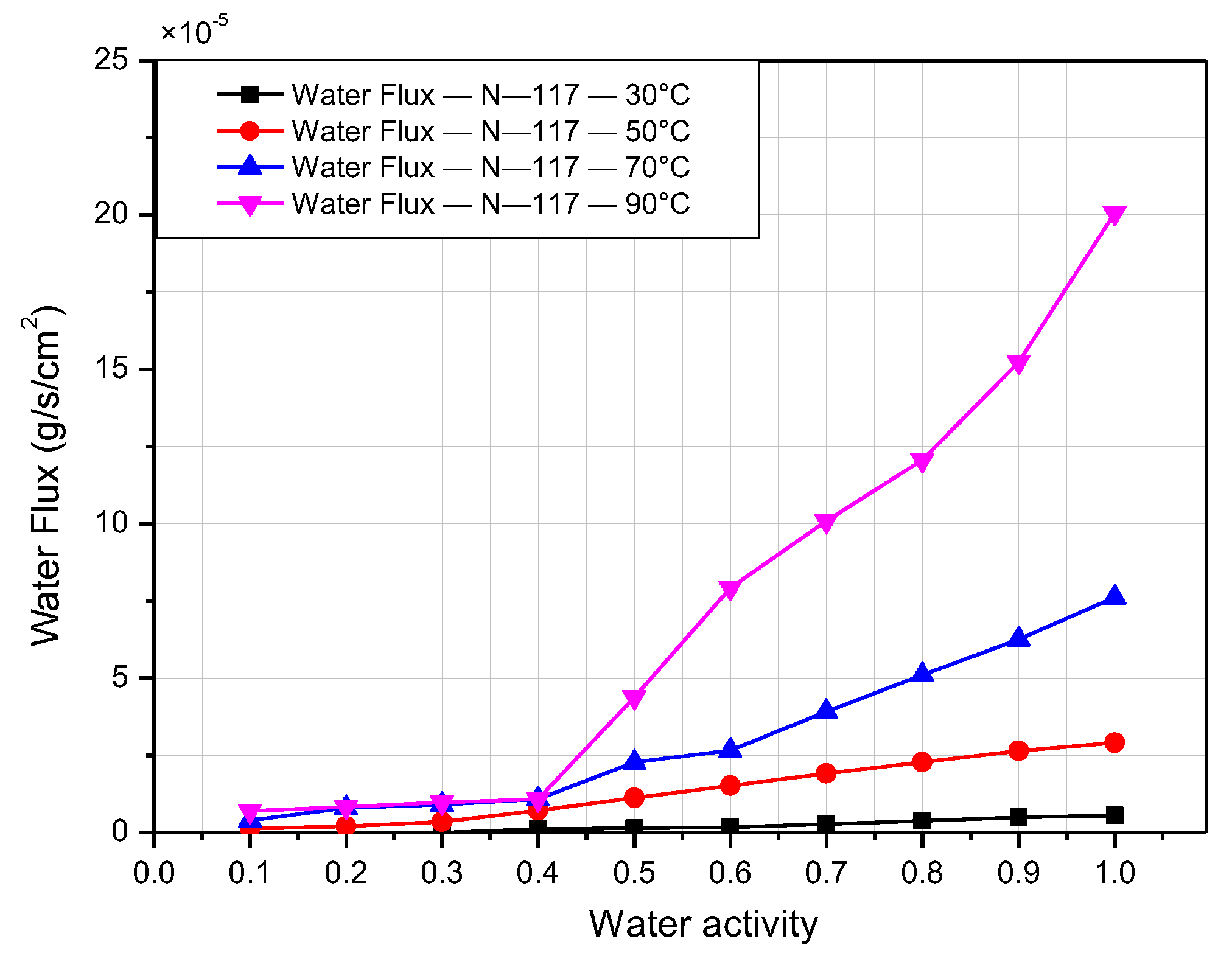
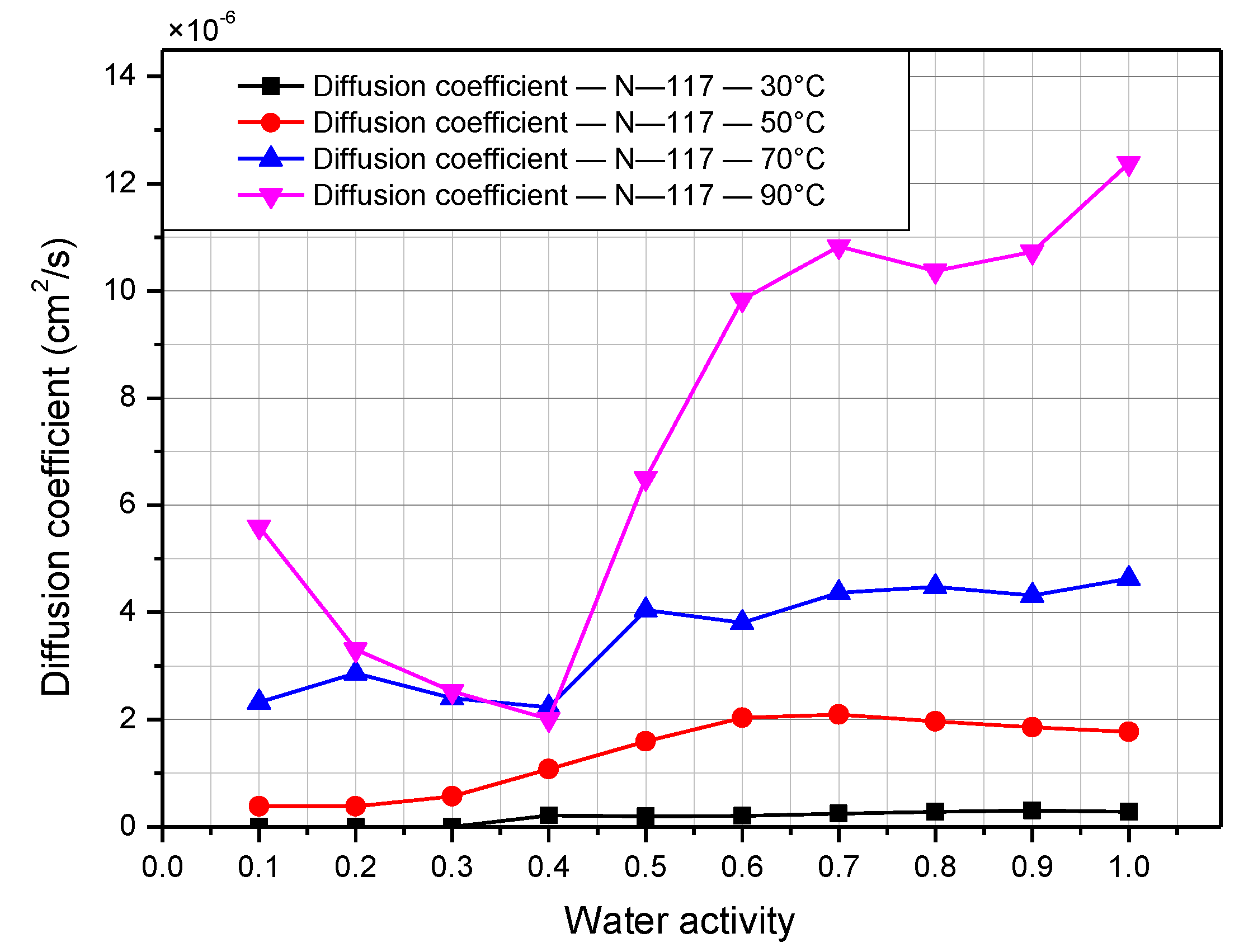
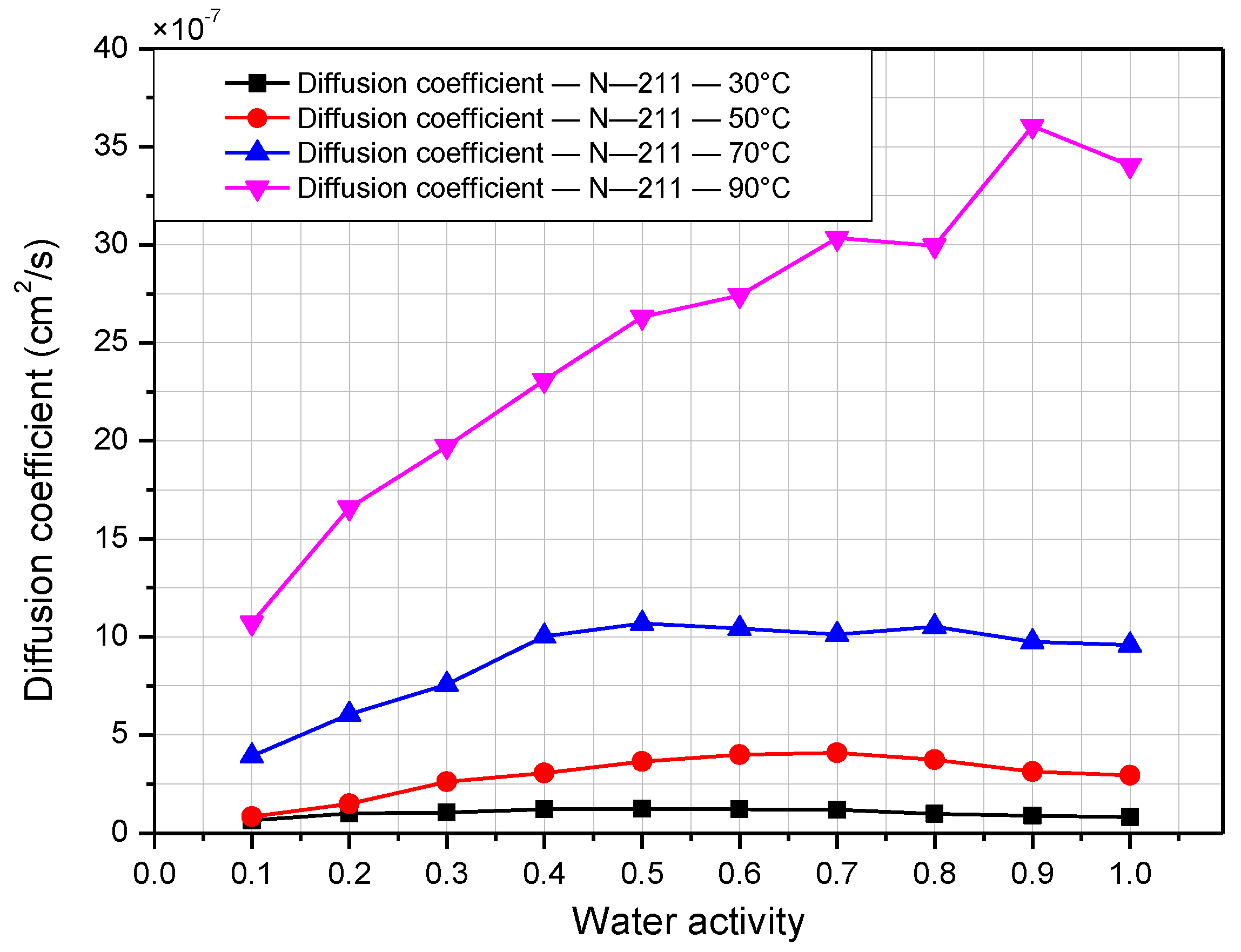
3.2. Water-Transport and Water-Diffusion Coefficient through Nafion Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| λ | Water content |
| C.V. | Control volume of test Jig |
| H2O molar mass, kg/kmol | |
| SO3—molar mass, kg/kmol | |
| Vapor mass flow, kg/s | |
| Air mass flow, kg/s | |
| Relative humidity | |
| Saturation pressure, kPa | |
| Air pressure, kPa | |
| C.V. 1 inlet dry air, kg/s | |
| Water permeation through the membrane, kg/s | |
| C.V. 1 outlet dry air, kg/s | |
| C.V. 1 outlet vapor, kg/s | |
| C.V. 2 inlet wet air, kg/s | |
| C.V. 2 outlet dry air, kg/s | |
| C.V. 2 outlet vapor, kg/s | |
| Molar flux, mol/cm2 s | |
| Membrane dry density, g/cm3 | |
| Equivalent weight of the membrane, g/mol | |
| Diffusion coefficient, cm2/s | |
| Membrane thickness, cm | |
| Membrane area, cm2 | |
| Water flux through the membrane, kg/cm2 s |
References
- Sun, C.; Zhang, H. Review of the Development of First-Generation Redox Flow Batteries: Iron-Chromium System. ChemSusChem 2022, 15, e202101798. [Google Scholar] [CrossRef] [PubMed]
- Tsehaye, M.T.; Alloin, F.; Iojoiu, C.; Tufa, R.A.; Aili, D.; Fischer, P.; Velizarov, S. Membranes for zinc-air batteries: Recent progress, challenges and perspectives. J. Power Sources 2020, 475, 228689. [Google Scholar] [CrossRef]
- Zawodzinski, T.A.; Neeman, M.; Sillerud, L.O.; Gottesfeld, S. Determination of water diffusion-coefficients in perfluorosulfonate ionomeric membranes. J. Phys. Chem. 1991, 95, 6040–6044. [Google Scholar] [CrossRef]
- Zawodzinski, T.A.; Derouinm, C.; Radzinski, S.; Sherman, R.J.; Smith, V.T.; Springer, T.E.; Gottesfeld, S. Water uptake by and transport through Nafion® 117 membranes. J. Electrochem. Soc. 1991, 137, 2334–2337. [Google Scholar] [CrossRef]
- Takamatsu, T.; Hashiyama, M.; Eisenberg, A. Sorption phenomena in Nafion membranes. J. Appl. Polym. Sci. 1979, 24, 2199–2220. [Google Scholar] [CrossRef]
- Monroe, C.W.; Romero, T.; Merida, W.; Eikerling, M. A vaporization-exchange model for water sorption and flux in Nafion. J. Membr. Sci. 2008, 324, 1–6. [Google Scholar] [CrossRef]
- Kientiz, B.; Yamada, H.; Nonoyama, N.; Weber, A.Z. Interfacial water transport effects in proton-exchange membranes. J. Fuel Cell Sci. Technol. 2011, 8, 011013. [Google Scholar] [CrossRef]
- Majsztrik, P.W.; Satterfield, M.B.; Bocarsly, A.B.; Benziger, J.B. Water sorption, desorption and transport in Nafion membranes. J. Membr. Sci. 2007, 301, 93–106. [Google Scholar] [CrossRef]
- Hirata, Y.; Miura, Y.; Nakagawa, T. Oxygen permeability and the state of water in Nation® membranes with alkali metal and amino sugar counterions. J. Membr. Sci. 1999, 163, 357–366. [Google Scholar] [CrossRef]
- Pineri, M.; Gebel, G.; Davies, R.J.; Diat, O. Water sorption–desorption in Nafion (R) membranes at low temperature, probed by micro X-ray diffraction. J. Power Sources 2007, 172, 587–596. [Google Scholar] [CrossRef]
- Wu, X.; Wang, X.; He, G.; Benziger, J. Differences in water sorption and proton conductivity between Nafion and SPEEK. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1437–1445. [Google Scholar] [CrossRef]
- Zhao, Q.; Majsztrik, P.; Benziger, J. Diffusion and interfacial transport of water in Nafion. J. Phys. Chem. B 2011, 115, 2717–2727. [Google Scholar] [CrossRef] [PubMed]
- Rubatat, L.; Diat, O. Stretching effect on Nafion fibrillar nanostructure. Macromolecules 2007, 40, 9455–9462. [Google Scholar] [CrossRef]
- Satterfield, M.B.; Benziger, J.B. Viscoelastic properties of nafion at elevated temperature and humidity. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Gebel, G.; Lyonnard, S.; Mendil-Jakani, H.; Morin, A. The kinetics of water sorption in Nafion membranes: A small-angle neutron scattering study. J. Phys. Condens. Matter 2011, 23, 234107. [Google Scholar] [CrossRef]
- Maldonado, L.; Perrin, J.C.; Dillet, J.; Lottin, O. Characterization of polymer electrolyte Nafion membranes: Influence of temperature, heat treatment and drying protocol on sorption and transport properties. J. Membr. Sci. 2012, 389, 43–56. [Google Scholar] [CrossRef]
- Burnett, D.J.; Garcia, A.R.; Thielmann, F. Measuring moisture sorption and diffusion kinetics on proton exchange membranes using a gravimetric vapor sorption apparatus. J. Power Sources 2006, 160, 426–430. [Google Scholar] [CrossRef]
- Duan, Q.; Wang, H.; Benziger, J. Transport of liquid water through Nafion membranes. J. Membr. Sci. 2012, 392–393, 88–94. [Google Scholar] [CrossRef]
- Chadha, K.; Martemianov, S.; Thomas, A. Estimation of the effective water diffusion coefficient in Nafion® membrane by water balance measurements. Fuel Cells 2021, 21, 139–148. [Google Scholar] [CrossRef]
- Rodgers, R.C.; Hill, G.E. Equations for vapour pressure versus temperature: Derivation and use of the antoine equation on a hand-held programmable calculator. Br. J. Anaesth. 1978, 50, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Springer, T.E.; Zawodzinski, T.A.; Gottesfeld, S. Polymer electrolyte fuel cell model. J. Electrochem. Soc. 1993, 140, 1041–1047. [Google Scholar] [CrossRef]
- Suresh, G.; Scindia, Y.M.; Pandey, A.K.; Goswami, A. Self-diffusion coefficient of water in Nafion-117 membrane with different monovalent counterions: A radiotracer study. J. Membr. Sci. 2005, 250, 39–45. [Google Scholar] [CrossRef]




| Parameter | Nafion 211 | Nafion 117 | Unit |
|---|---|---|---|
| Surface area | 4900 | 4900 | mm2 |
| Thickness | 25.4 | 183 | µm |
| Width | 7 | 7 | cm |
| Length | 7 | 7 | cm |
| Equivalent weight | 1100 | 1100 | g/mol |
| Density | 1.97 | 0.2 | g/cm3 |
| Instrument | Model | Range | Accuracy |
|---|---|---|---|
| Mass flow controller | Line tech, Korea | 100 L/min | ±1% |
| Pressure sensor | IS-3, Wika | 10 bar | ±0.5% |
| Temperature controller | UT-32A, Yokogawa | −270–1370 °C | |
| Thermo-couple | K-type | −100–650 °C | ±0.1 °C |
| Electronic scale | GX-200 | 0.001–210 g | ±0.001 g |
| Vacuum dryer | OV-11, Jeiotech | 25–250 °C |
| Operating Variables | Value | Units |
|---|---|---|
| Flow rate | 8 | L/min |
| Temperature | 30, 50, 70, 90 | °C |
| Pressure | 1 | bar |
| Relative humidity | 10–100 | % |
| Flow arrangement | Co-flow |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, N.V.; Nguyen, X.L.; Kim, Y.; Yu, S. Characteristics of Water Transport of Membrane Electrolyte over Selected Temperature for Proton Exchange Membrane Fuel Cell. Polymers 2022, 14, 2972. https://doi.org/10.3390/polym14152972
Trinh NV, Nguyen XL, Kim Y, Yu S. Characteristics of Water Transport of Membrane Electrolyte over Selected Temperature for Proton Exchange Membrane Fuel Cell. Polymers. 2022; 14(15):2972. https://doi.org/10.3390/polym14152972
Chicago/Turabian StyleTrinh, Ngoc Van, Xuan Linh Nguyen, Younghyeon Kim, and Sangseok Yu. 2022. "Characteristics of Water Transport of Membrane Electrolyte over Selected Temperature for Proton Exchange Membrane Fuel Cell" Polymers 14, no. 15: 2972. https://doi.org/10.3390/polym14152972
APA StyleTrinh, N. V., Nguyen, X. L., Kim, Y., & Yu, S. (2022). Characteristics of Water Transport of Membrane Electrolyte over Selected Temperature for Proton Exchange Membrane Fuel Cell. Polymers, 14(15), 2972. https://doi.org/10.3390/polym14152972





