Ring-Opening Polymerization of L-Lactide Catalyzed by Potassium-Based Complexes: Mechanistic Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Syntesis of Homometallic Compounds
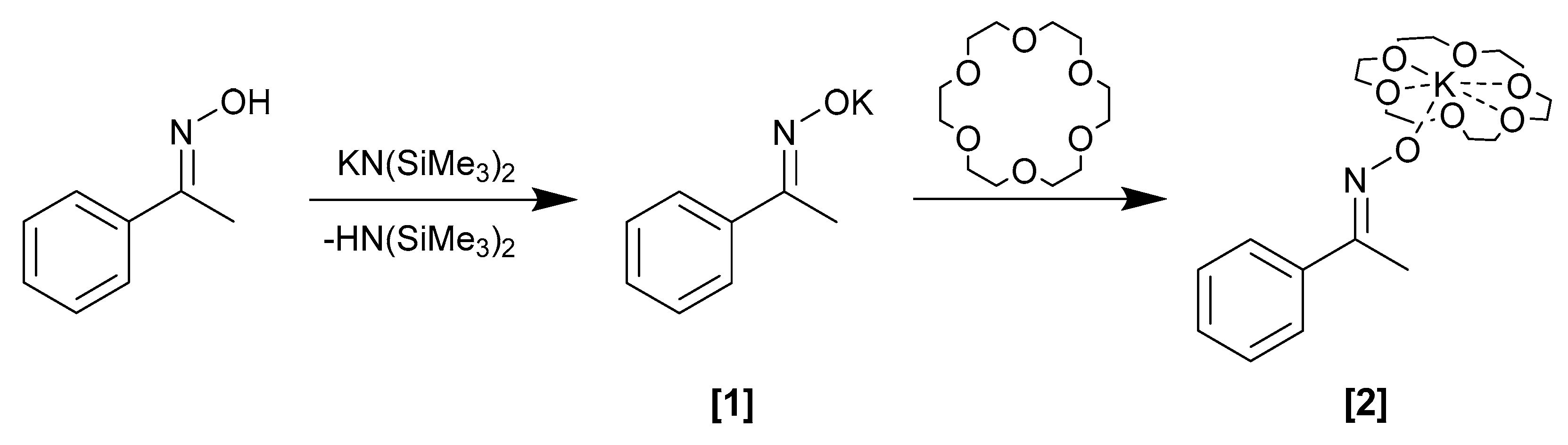
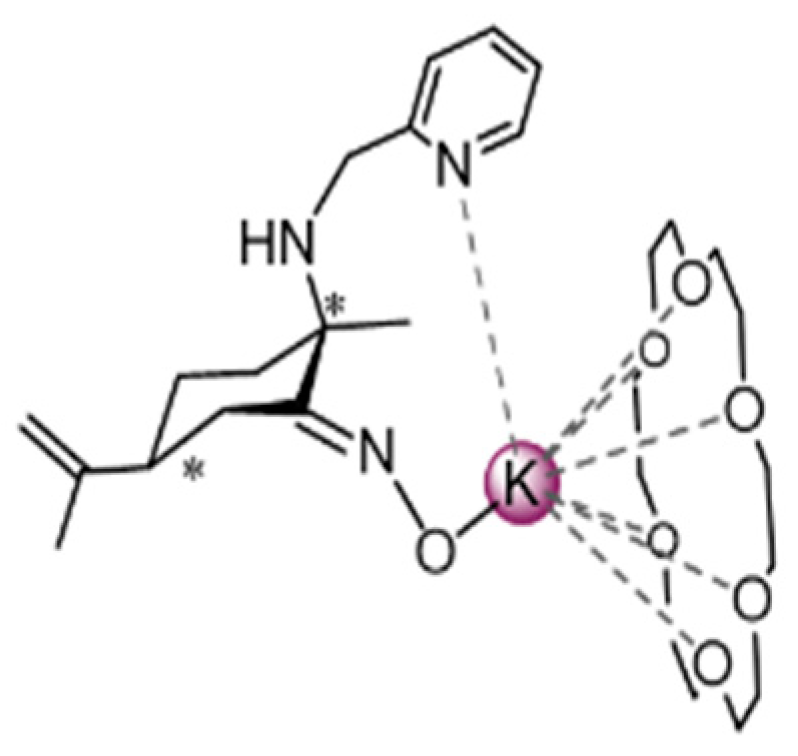
2.2.1. Potassium (E)-Acetophenone Oximate, (1)
2.2.2. Potassium (18-Crown-6 Ether) (E)-Acetophenone Oximate, (2)
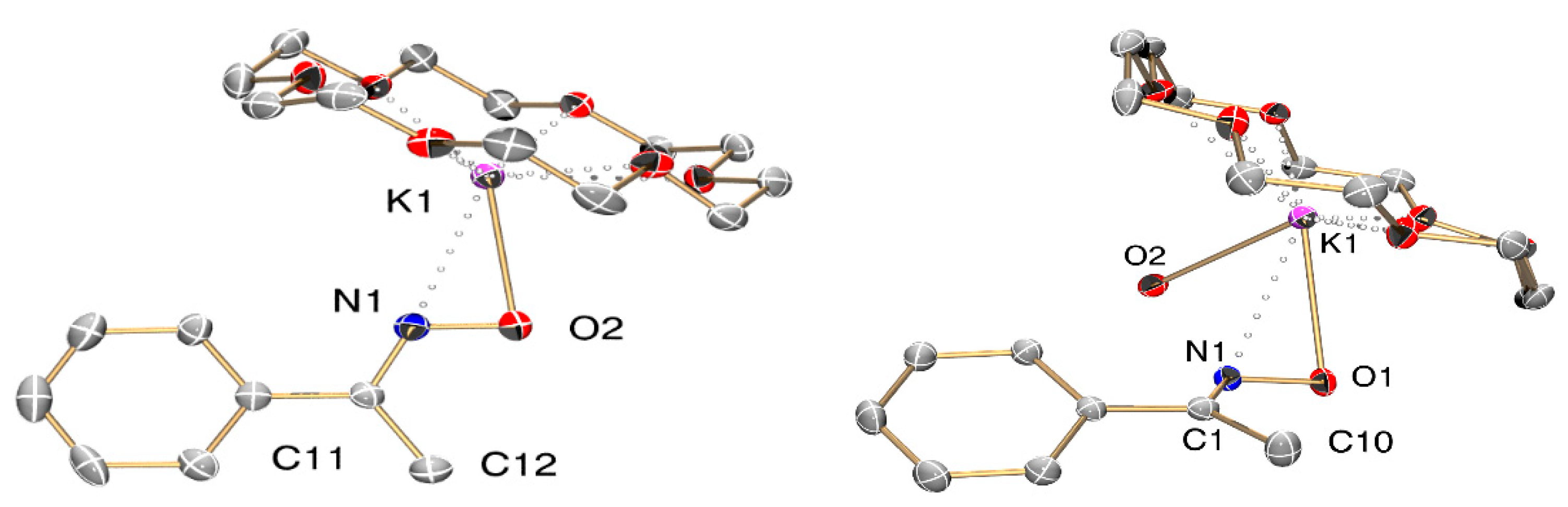
2.3. Single-Crystal X-ray Structure Determination
2.4. Polymerization Procedure
2.5. Gel Permeation Chromatography (GPC)
2.6. Mass Spectroscopy (MS)
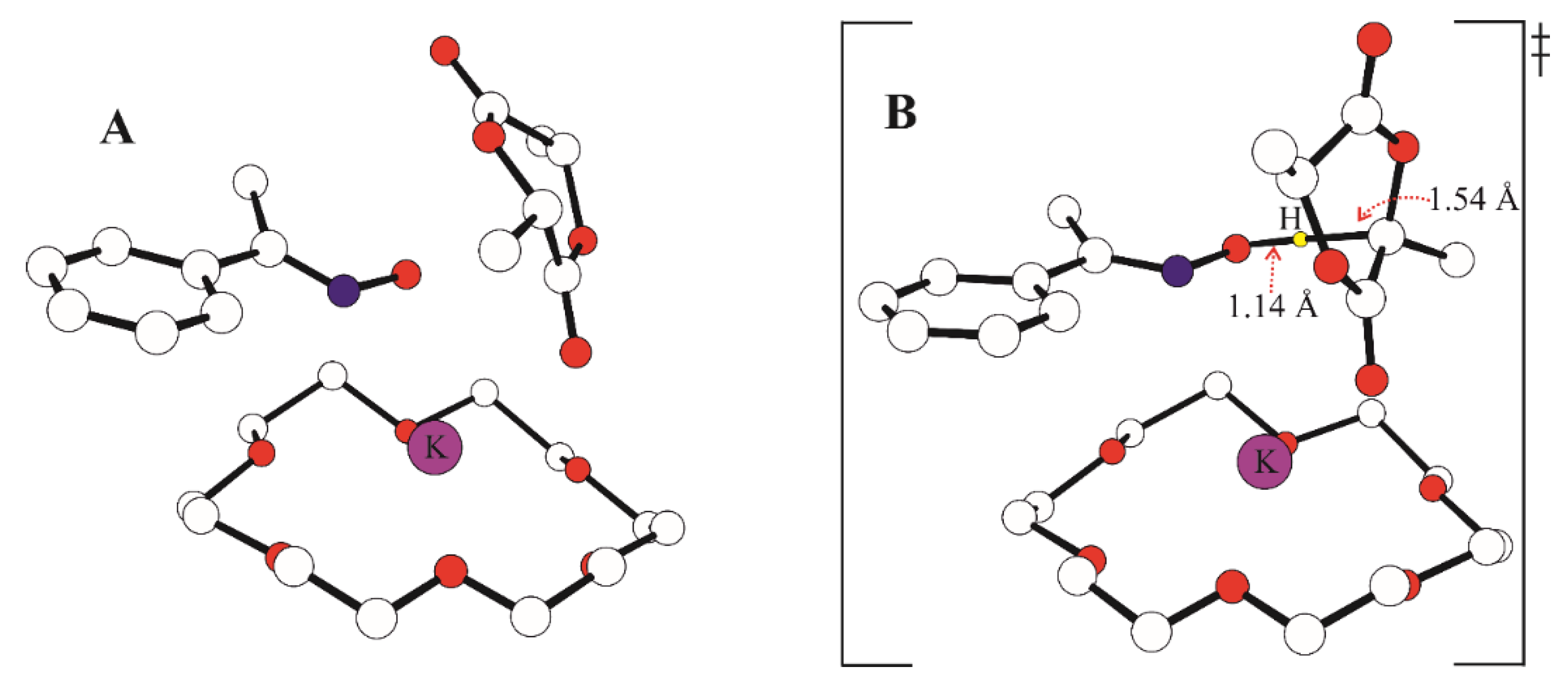
2.7. Density Functional Theory (DFT) Calculations
3. Results and Discussion
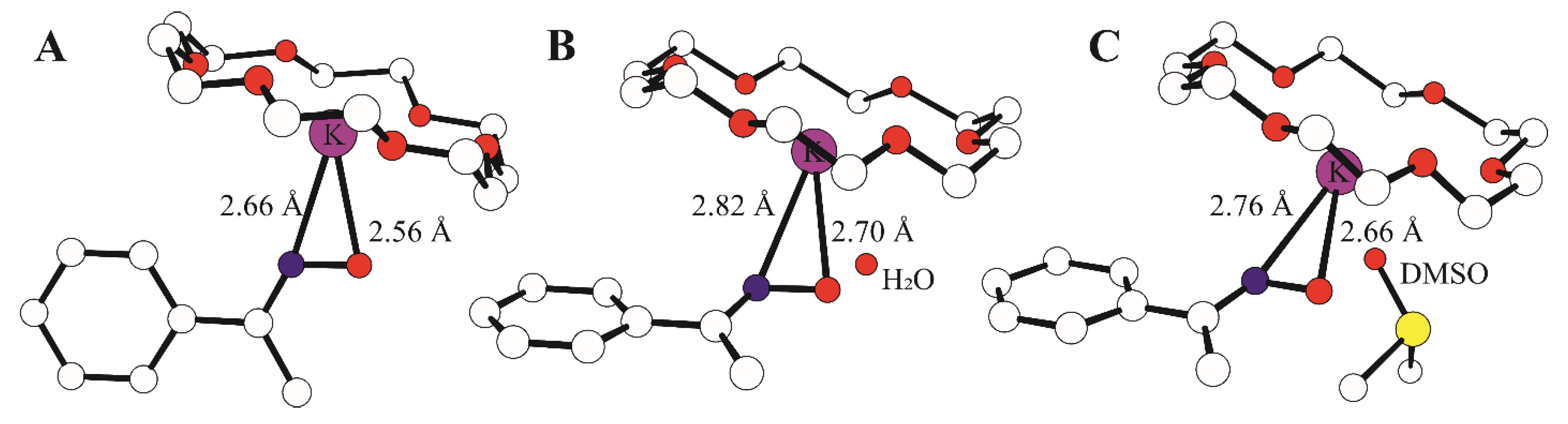
3.1. Synthesis and Behavior of the Metallic Complexes
3.2. L-Lactide Polymerization
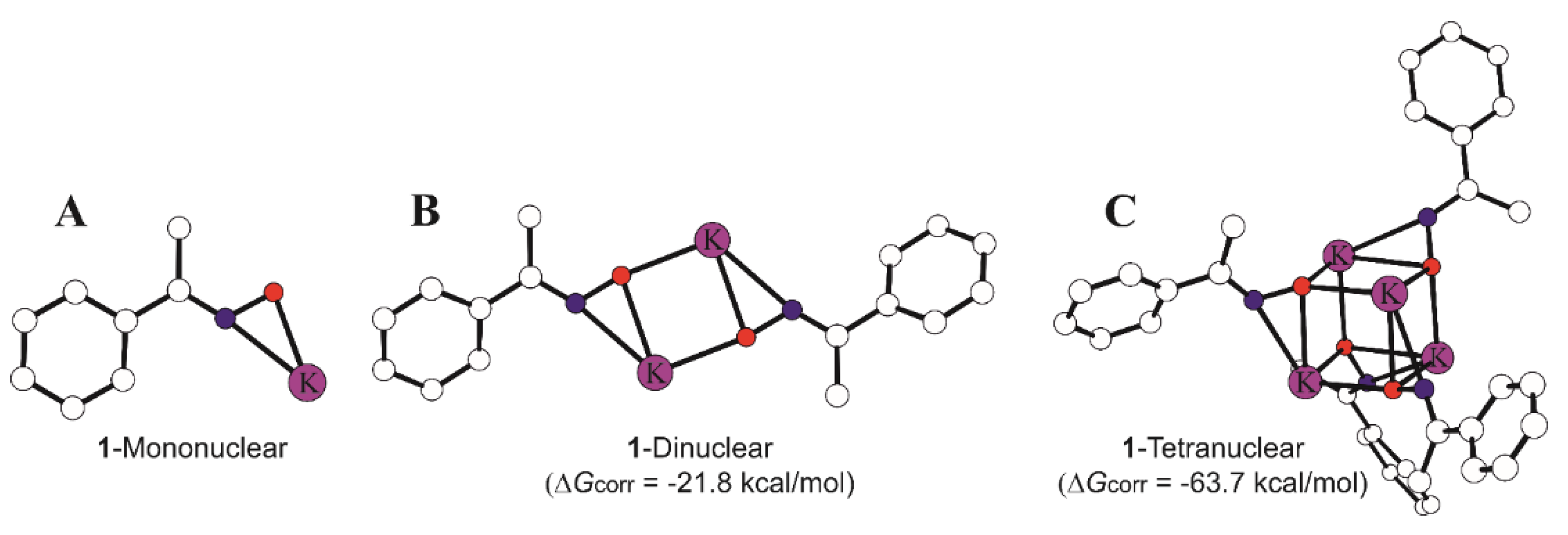
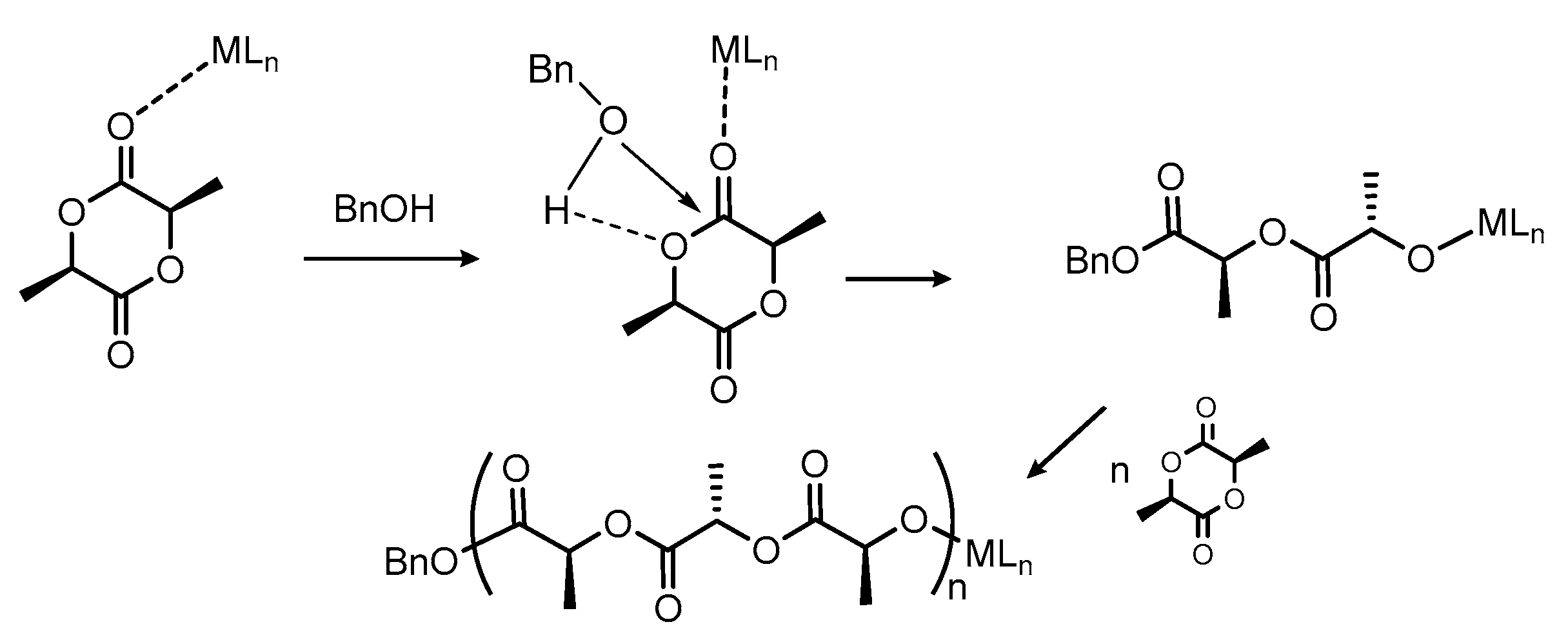
3.3. Mechanistic Studies
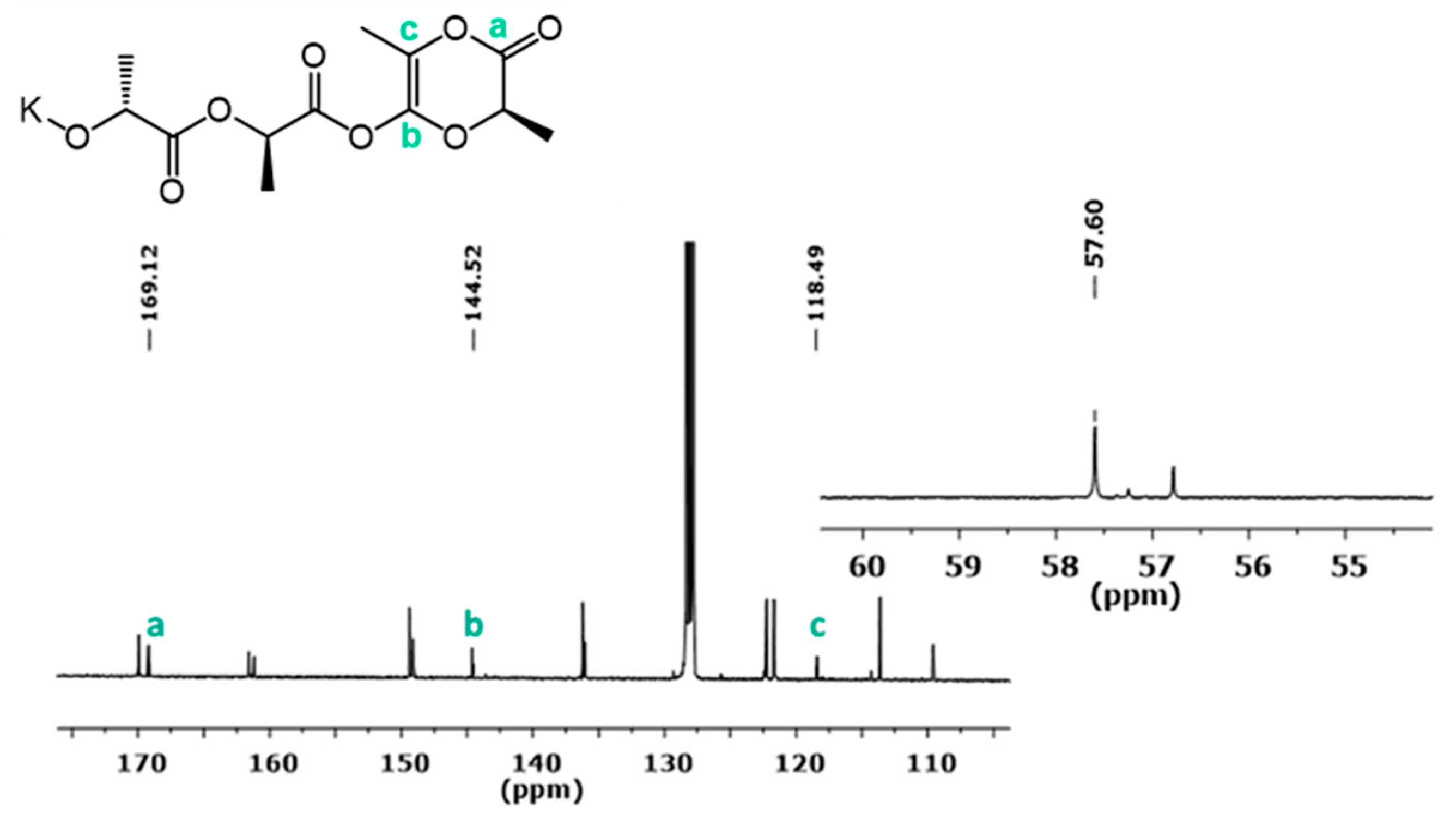
3.3.1. Initiator:monomer:BnOH Ratio 1:1:0
3.3.2. Initiator:Monomer:BnOH Ratio 1:1:1
3.3.3. Initiator:Monomer:BnOH Ratio 1:1:5 and 1:1:2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cywar, R.M.; Rorrer, N.A.; Hoyt, C.B.; Beckham, G.T.; Chen, E.Y.X. Bio-based polymers with performance-advantaged properties. Nat. Rev. Mat. 2022, 7, 83–103. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B 2009, 364, 2127–2139. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qiu, J.; Sakai, E. Microstructures and mechanical properties of polylactic acid prepared by a cold rolling process. J. Mater. Process. Technol. 2016, 232, 184–194. [Google Scholar] [CrossRef]
- Fukada, E. Piezoelectricity of biopolymers. Biorheology 1995, 32, 593–609. [Google Scholar] [CrossRef]
- Fernández-Millán, M.; Ortega, P.; Cuenca, T.; Cano, J.; Mosquera, M.E.G. Alkali-Metal Compounds with Bio-Based Ligands as Catalysts for Isoselective Lactide Polymerization: Influence of the Catalyst Aggregation on the Polymerization Control. Organometallics 2020, 39, 2278–2286. [Google Scholar] [CrossRef]
- García-Valle, F.M.; Estivill, R.; Gallegos, C.; Cuenca, T.; Mosquera, M.E.G.; Tabernero, V.; Cano, J. Metal and Ligand-Substituent Effects in the Immortal Polymerization of rac-Lactide with Li, Na, and K Phenoxo-imine Complexes. Organometallics 2015, 34, 477–487. [Google Scholar] [CrossRef]
- Bhattacharjee, J.; Sarkar, A.; Panda, T.K. Recent development of alkali metal complex promoted iso-selective ring-opening polymerization of rac-Lactide. Curr. Opin. Green Sustain. Chem. 2021, 31, 100545. [Google Scholar] [CrossRef]
- Gentner, T.X.; Mulvey, R.E. Alkali-Metal Mediation: Diversity of Applications in Main-Group Organometallic Chemistry. Angew. Chem. Int. Edit. 2021, 60, 9247–9262. [Google Scholar] [CrossRef]
- Dai, Z.; Sun, Y.; Xiong, J.; Pan, X.; Tang, N.; Wu, J. Simple sodium and potassium phenolates as catalysts for highly isoselective polymerization of rac-lactide. Catal. Sci. Technol. 2016, 6, 515–520. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, C.; Sun, Y.; Wu, J.; Pan, X. Isoselective mechanism of the ring-opening polymerization of rac-lactide catalyzed by chiral potassium binolates. Inorg. Chem. Front. 2017, 4, 261–269. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, J.; Sun, Y.; Tang, N.; Wu, J. Highly Iso-Selective and Active Catalysts of Sodium and Potassium Monophenoxides Capped by a Crown Ether for the Ring-Opening Polymerization of rac-Lactide. Macromolecules 2014, 47, 7789–7796. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, J.; Sun, Y.; Dai, Z.; Pan, X.; Wu, J. Iso-Selective Ring-Opening Polymerization of rac-Lactide Catalyzed by Crown Ether Complexes of Sodium and Potassium Naphthalenolates. Inorg. Chem. 2015, 54, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Sun, Y.; Xiong, J.; Pan, X.; Wu, J. Alkali-Metal Monophenolates with a Sandwich-Type Catalytic Center as Catalysts for Highly Isoselective Polymerization of rac-Lactide. ACS Macro Lett. 2015, 4, 556–560. [Google Scholar] [CrossRef]
- Sun, Y.; Xiong, J.; Dai, Z.; Pan, X.; Tang, N.; Wu, J. Stereoselective Alkali-Metal Catalysts for Highly Isotactic Poly(rac-lactide) Synthesis. Inorg. Chem. 2016, 55, 136–143. [Google Scholar] [CrossRef]
- Xiong, J.; Sun, Y.; Jiang, J.; Chen, C.; Pan, X.; Wang, C.; Wu, J. Metal-size influence of alkali metal complexes for polymerization of rac-lactide. Polyhedron 2018, 141, 118–124. [Google Scholar] [CrossRef]
- Wu, B.-B.; Tian, L.-L.; Wang, Z.-X. Ring-opening polymerization of rac-lactide catalyzed by crown ether complexes of sodium and potassium iminophenoxides. RSC Adv. 2017, 7, 24055–24063. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.-L.; Wu, B.-B.; Wang, Z.-X. Crown ether complexes of sodium and potassium 2-(benzotriazol-2-yl)phenolates: Synthesis and catalysis towards the ring-opening polymerization of rac-lactide. J. Macromol. Sci. Part A 2017, 54, 944–950. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Lin, C.-C.; Blake, M.P.; Clark, L.; Schwarz, A.D.; Mountford, P. Potassium, zinc, and magnesium complexes of a bulky OOO-tridentate bis(phenolate) ligand: Synthesis, structures, and studies of cyclic ester polymerisation. Dalton Trans. 2013, 42, 9313–9324. [Google Scholar] [CrossRef]
- Roşca, S.-C.; Roşca, D.-A.; Dorcet, V.; Kozak, C.M.; Kerton, F.M.; Carpentier, J.-F.; Sarazin, Y. Alkali aminoether-phenolate complexes: Synthesis, structural characterization and evidence for an activated monomer ROP mechanism. Dalton Trans. 2013, 42, 9361–9375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, C.; Yang, Y.; Xu, S.; Ma, H. Potassium complexes supported by monoanionic tetradentate amino-phenolate ligands: Synthesis, structure and catalysis in the ring-opening polymerization of rac-lactide. Dalton Trans. 2017, 46, 6087–6097. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jiang, J.; Mao, X.; Cong, Y.; Cui, Y.; Pan, X.; Wu, J. Isoselective Polymerization of rac-Lactide Catalyzed by Ion-Paired Potassium Amidinate Complexes. Inorg. Chem. 2018, 57, 3158–3168. [Google Scholar] [CrossRef]
- Wu, B.-B.; Wang, Z.-X. Crown ether complexes of potassium quinolin-8-olates: Synthesis, characterization and catalysis toward the ring-opening polymerization of rac-lactide. RSC Adv. 2017, 7, 11657–11664. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Cui, Y.; Mao, X.; Pan, X.; Wu, J. Suppressing Cyclic Polymerization for Isoselective Synthesis of High-Molecular-Weight Linear Polylactide Catalyzed by Sodium/Potassium Sulfonamidate Complexes. Macromolecules 2017, 50, 83–96. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.; Mao, X.; Pan, X.; Wu, J. Structures of potassium calix[4]arene crown ether inclusion complexes and application in polymerization of rac-lactide. Dalton Trans. 2016, 45, 9636–9645. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, A. Structural chemistry of transition metal complexes of oximes. Coordin. Chem. Rev. 1974, 13, 1–46. [Google Scholar] [CrossRef]
- Milios, C.J.; Stamatatos, T.C.; Perlepes, S.P. The coordination chemistry of pyridyl oximes. Polyhedron 2006, 25, 134–194. [Google Scholar] [CrossRef]
- Singh, N.; Karpichev, Y.; Tiwari, A.K.; Kuca, K.; Ghosh, K.K. Oxime functionality in surfactant self-assembly: An overview on combating toxicity of organophosphates. J. Mol. Liq. 2015, 208, 237–252. [Google Scholar] [CrossRef]
- Mitzel, N.W.; Lustig, C.; Woski, M. Organoaluminium and -Gallium Compounds with O-Oximato Substituents. Z. Nat. B 2003, 58, 363–368. [Google Scholar] [CrossRef]
- Ullrich, M.; Mitzel, N.W.; Bergander, K.; Fröhlich, R. Organoaluminium complexes with sterically demanding oximato ligands: Does a bulky and rigid ligand backbone change the aggregation motif? Dalton Trans. 2006, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Millán, M.; Temprado, M.; Cano, J.; Cuenca, T.; Mosquera, M.E.G. Synthesis of novel chiral heterometallic terpene oximates: Unusual generation of an aluminium enolate by a cooperative effect. Dalton Trans. 2016, 45, 10514–10518. [Google Scholar] [CrossRef] [PubMed]
- Lugo-González, J.C.; Gómez-Tagle, P.; Flores-Alamo, M.; Yatsimirsky, A.K. Mechanistic study of carboxylic acid and phosphate ester cleavage by oximate metal complexes surpassing the limiting reactivity of highly basic free oximate anions. Dalton Trans. 2020, 49, 2452–2467. [Google Scholar] [CrossRef] [PubMed]
- Matveevskaya, V.V.; Pavlov, D.I.; Samsonenko, D.G.; Bonfili, L.; Cuccioloni, M.; Benassi, E.; Pettinari, R.; Potapov, A.S. Arene-ruthenium(II) complexes with tetracyclic oxime derivatives: Synthesis, structure and antiproliferative activity against human breast cancer cells. Inorg. Chim Acta. 2022, 535, 120879. [Google Scholar] [CrossRef]
- Mancin, F.; Tecilla, P.; Tonellato, U. Metallomicelles Made of Ni(II) and Zn(II) Complexes of 2-Pyridinealdoxime-Based Ligands as Catalyst of the Cleavage of Carboxylic Acid Esters. Langmuir 2000, 16, 227–233. [Google Scholar] [CrossRef]
- Sharutin, V.V.; Sharutina, O.K.; Efremov, A.N. Syntheses and structures of tris(para-tolyl)-, tris(3-fluorophenyl)-, and tris(4-fluorophenyl)antimony dioximates. Russ. J. Coord. Chem. 2017, 43, 526–534. [Google Scholar] [CrossRef]
- Alaimo, A.A.; Worrell, A.; Gupta, S.D.; Abboud, K.A.; Lampropoulos, C.; Christou, G.; Stamatatos, T.C. Structural and Magnetic Variations in a Family of Isoskeletal, Oximate-Bridged {MnIV2MIII} Complexes (MIII=Mn, Gd, Dy). Chem. Eur. J. 2018, 24, 2588–2592. [Google Scholar] [CrossRef]
- Alaimo, A.A.; Alexandropoulos, D.I.; Lampropoulos, C.; Stamatatos, T.C. New insights in Mn–Ca chemistry from the use of oximate-based ligands: {MnII/III22Ca2} and {MnIV2Ca2} complexes with relevance to both low- and high-valent states of the oxygen-evolving complex. Polyhedron 2018, 149, 39–44. [Google Scholar] [CrossRef]
- Francos, J.; Borge, J.; Conejero, S.; Cadierno, V. Platinum Complexes with a Phosphino-Oxime/Oximate Ligand. Eur. J. Inorg. Chem. 2018, 2018, 3176–3186. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Fang, W.-H.; Zhang, L.; Zhang, J. Synthesis, Structures, and Photocurrent Responses of Polyoxo-Titanium Clusters with Oxime Ligands: From Ti4 to Ti18. Inorg. Chem. 2018, 57, 8850–8856. [Google Scholar] [CrossRef]
- Beirakhov, A.G.; Rotov, A.V. Structural Features of Uranyl Hydroxylaminate and Oximate Complexes. Russ. J. Inorg. Chem. 2018, 63, 1689–1703. [Google Scholar] [CrossRef]
- Feng, Q.; Li, B.; Du, R.; Jiang, F.; Liu, T. Syntheses, crystal structures, spectroscopic and magnetic properties of two trinuclear iron complexes with carboxylate and oximate mixed-bridge ligands. Transit. Met. Chem. 2019, 44, 49–55. [Google Scholar] [CrossRef]
- Pool, J.A.; Scott, B.L.; Kiplinger, J.L. Carbon–nitrogen bond cleavage in pyridine ring systems mediated by organometallic thorium(iv) complexes. Chem. Commun. 2005, 2591–2593. [Google Scholar] [CrossRef]
- Kim, M.; Gabbaï, F.P. Stoichiometric reactions of methylparathion with a palladium aryl oxime metallacycle. Dalton Trans. 2004, 3403–3407. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kashiwame, Y.; Kuwata, S.; Ikariya, T. Synthesis, Structures, and Transfer Hydrogenation Catalysis of Bifunctional Iridium Complexes Bearing a C–N Chelate Oxime Ligand. Eur. J. Inorg. Chem. 2012, 2012, 504–511. [Google Scholar] [CrossRef]
- Buncel, E.; Kumar, A.; Xie, H.-Q.; Moir, R.Y.; Purdon, J.G. Solvent and crown ether/cryptand effects on the oximate-promoted 1,2-elimination from β-phenylmercaptoethyl p-nitrophenolate. Formation and reactivity of a crown ether-complexed potassium oximate ion pair. Can. J. Chem. 1994, 72, 437–447. [Google Scholar] [CrossRef]
- Bannard, R.A.; Casselman, A.A.; Purdon, J.G.; Bovenkamp, J.W. Broad spectrum chemical decontaminant system. U.S. Patent 5,075,297, 1991. [Google Scholar]
- Blessing, R. An empirical correction for absorption anisotropy. Acta Crystallogr. Sect. A 1995, 51, 33–38. [Google Scholar] [CrossRef]
- Farrugia, L. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.; Trucks, G.; Schlegel, H. GAUSSIAN09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Falivene, L.; Barone, V.; Talarico, G. Unraveling the role of entropy in tuning unimolecular vs. bimolecular reaction rates: The case of olefin polymerization catalyzed by transition metals. Mol. Catal. 2018, 452, 138–144. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [Green Version]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- D’Alterio, M.C.; De Rosa, C.; Talarico, G. Syndiotactic PLA from meso-LA polymerization at the Al-chiral complex: A probe of DFT mechanistic insights. Chem. Commun. 2021, 57, 1611–1614. [Google Scholar] [CrossRef]
- D’Alterio, M.C.; De Rosa, C.; Talarico, G. Stereoselective Lactide Polymerization: The Challenge of Chiral Catalyst Recognition. ACS Catal. 2020, 10, 2221–2225. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Kricheldorf, H.R.; Kreiser-Saunders, I. Polylactones, 19. Anionic polymerization of L-lactide in solution. Makromol. Chem. 1990, 191, 1057–1066. [Google Scholar] [CrossRef]
- Bhaw-Luximon, A.; Jhurry, D.; Spassky, N.; Pensec, S.; Belleney, J. Anionic polymerization of D, L-lactide initiated by lithium diisopropylamide. Polymer 2001, 42, 9651–9656. [Google Scholar] [CrossRef]
- Sipos, L.; Zsuga, M. Anionic polymerization of L-lactide effect of lithium and potassium as counterions. J. Macromol. Sci. Part A 1997, 34, 1269–1284. [Google Scholar] [CrossRef]
- Sauer, A.; Kapelski, A.; Fliedel, C.; Dagorne, S.; Kol, M.; Okuda, J. Structurally well-defined group 4 metal complexes as initiators for the ring-opening polymerization of lactide monomers. Dalton Trans. 2013, 42, 9007–9023. [Google Scholar] [CrossRef]
- Tsuji, H. Poly (lactide) stereocomplexes: Formation, structure, properties, degradation, and applications. Macromol. Biosci. 2005, 5, 569–597. [Google Scholar] [CrossRef]



| Ent. | Cat. | [Cat]:[LLA]: [BnOH] | [LLA] (M) | Conv. (%) 2 | t (min) 2 | Mn theo. (kDa) 5 | Mn exp. (kDa) 6 | Ð 6 |
|---|---|---|---|---|---|---|---|---|
| 1 | [1] | 1:100:5 | 0.2 | >99 | 1 | 2.99 | 3.83 | 1.95 |
| 2 | [2] | 1:100:5 | 0.2 | >99 | 1 | 2.99 | 1.60 | 1.94 |
| 3 | [1] | 1:100:5 | 0.8 | >99 | 1 | 2.99 | 3.28 | 1.89 |
| 4 | [2] | 1:100:5 | 0.8 | >99 | 1 | 2.99 | 2.40 | 4.06 |
| 5 | [1] | 1:200:5 | 0.2 | >99 | 1 | 5.87 | 6.32 | 1.77 |
| 6 | [2] | 1:200:5 | 0.2 | >99 | 1 | 5.87 | 4.79 | 1.63 |
| 7 | [1] | 1:200:5 | 0.8 | >99 | 1 | 5.87 | 9.48 | 3.10 |
| 8 | [2] | 1:200:5 | 0.8 | >99 | 1 | 5.87 | 2.82 | 2.64 |
| 9 | [1] | 1:100:2 | 0.8 | >99 | 1 | 7.32 | 5.94 | 2.16 |
| 10 | [2] | 1:100:2 | 0.8 | >99 | 1 | 7.32 | 5.63 | 1.99 |
| 11 | [1] | 1:200:2 | 0.8 | >99 | 1 | 14.52 | 7.24 | 2.18 |
| 12 | [2] | 1:200:2 | 0.8 | >99 | 1 | 14.52 | 7.72 | 2.69 |
| 13 | [1] | 1:400:2 | 0.8 | >99 | 1 | 28.93 | 9.68 | 3.29 |
| 14 | [2] | 1:400:2 | 0.8 | >99 | 1 | 28.93 | 13.24 | 3.08 |
| 15 | [1] | 1:1000:2 | 0.8 | 35 3 | 1 3 | 29.66 | 29.25 | 1.35 |
| 16 | [2] | 1:1000:2 | 0.8 | 33 4 | 1 4 | 25.33 | 21.54 | 1.78 |
| 17 | [1] | 1:100:0 | 0.4 | >99 | 1 | 14.41 | 55.83 | 2.98 |
| 18 | [2] | 1:100:0 | 0.4 | >99 | 1 | 14.41 | 20.35 | 1.76 |
| 19 | [1] | 1:200:0 | 0.4 | >99 | 8 | 28.83 | 113.66 | 2.67 |
| 20 | [2] | 1:200:0 | 0.4 | >99 | 2 | 28.83 | 43.53 | 2.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rentero, C.; Damián, J.; Medel, A.; Fernández-Millán, M.; Rusconi, Y.; Talarico, G.; Cuenca, T.; Sessini, V.; Mosquera, M.E.G. Ring-Opening Polymerization of L-Lactide Catalyzed by Potassium-Based Complexes: Mechanistic Studies. Polymers 2022, 14, 2982. https://doi.org/10.3390/polym14152982
Rentero C, Damián J, Medel A, Fernández-Millán M, Rusconi Y, Talarico G, Cuenca T, Sessini V, Mosquera MEG. Ring-Opening Polymerization of L-Lactide Catalyzed by Potassium-Based Complexes: Mechanistic Studies. Polymers. 2022; 14(15):2982. https://doi.org/10.3390/polym14152982
Chicago/Turabian StyleRentero, Christian, Jesús Damián, Asier Medel, María Fernández-Millán, Yolanda Rusconi, Giovanni Talarico, Tomás Cuenca, Valentina Sessini, and Marta E. G. Mosquera. 2022. "Ring-Opening Polymerization of L-Lactide Catalyzed by Potassium-Based Complexes: Mechanistic Studies" Polymers 14, no. 15: 2982. https://doi.org/10.3390/polym14152982







