Innovative Coating–Etching Method of Biocarrier Fabrication for Treating Wastewater with a Low C/N Ratio
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
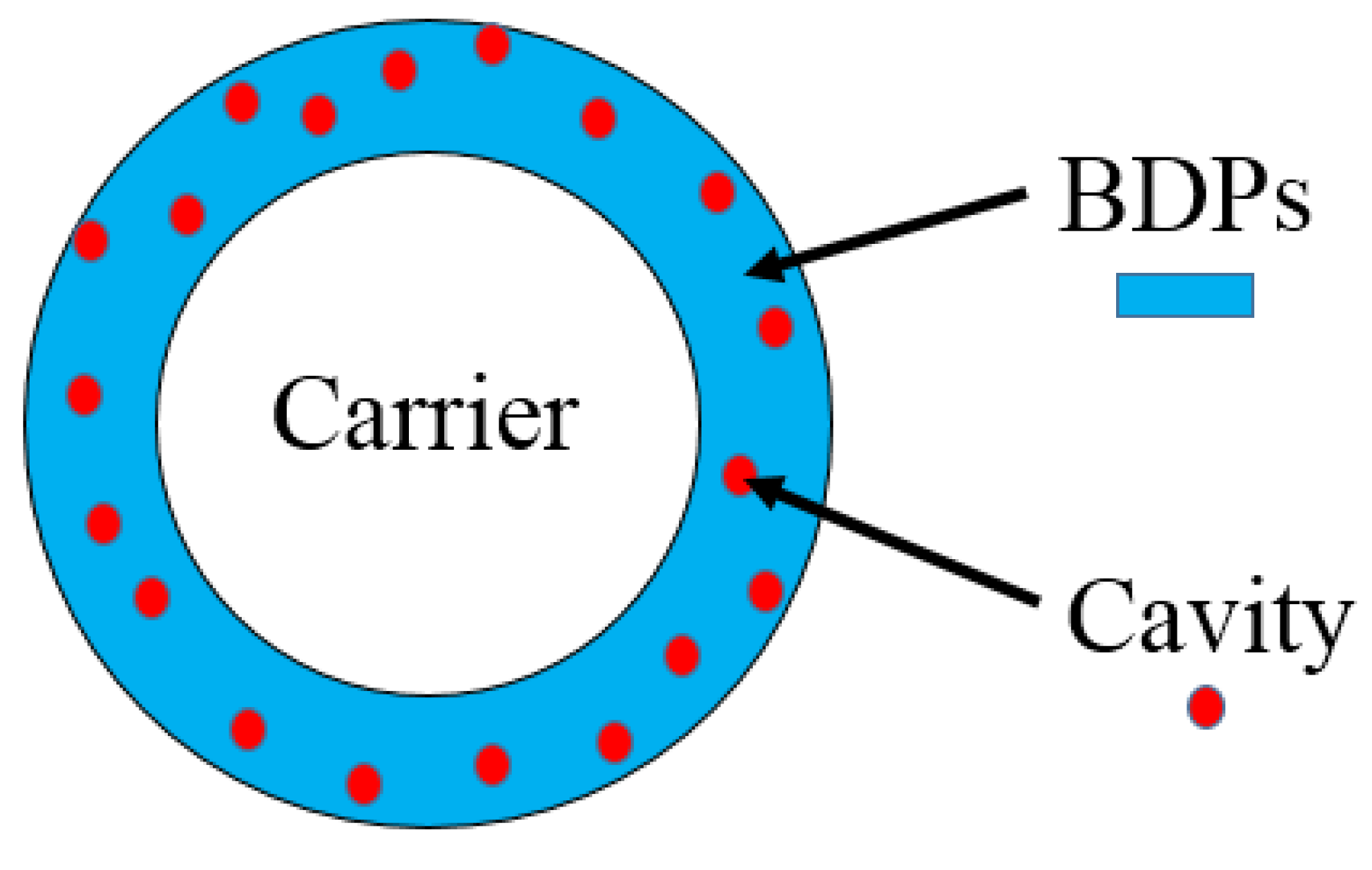
2.2. Two-Step Coating–Etching Method
2.3. Synthetic Wastewater
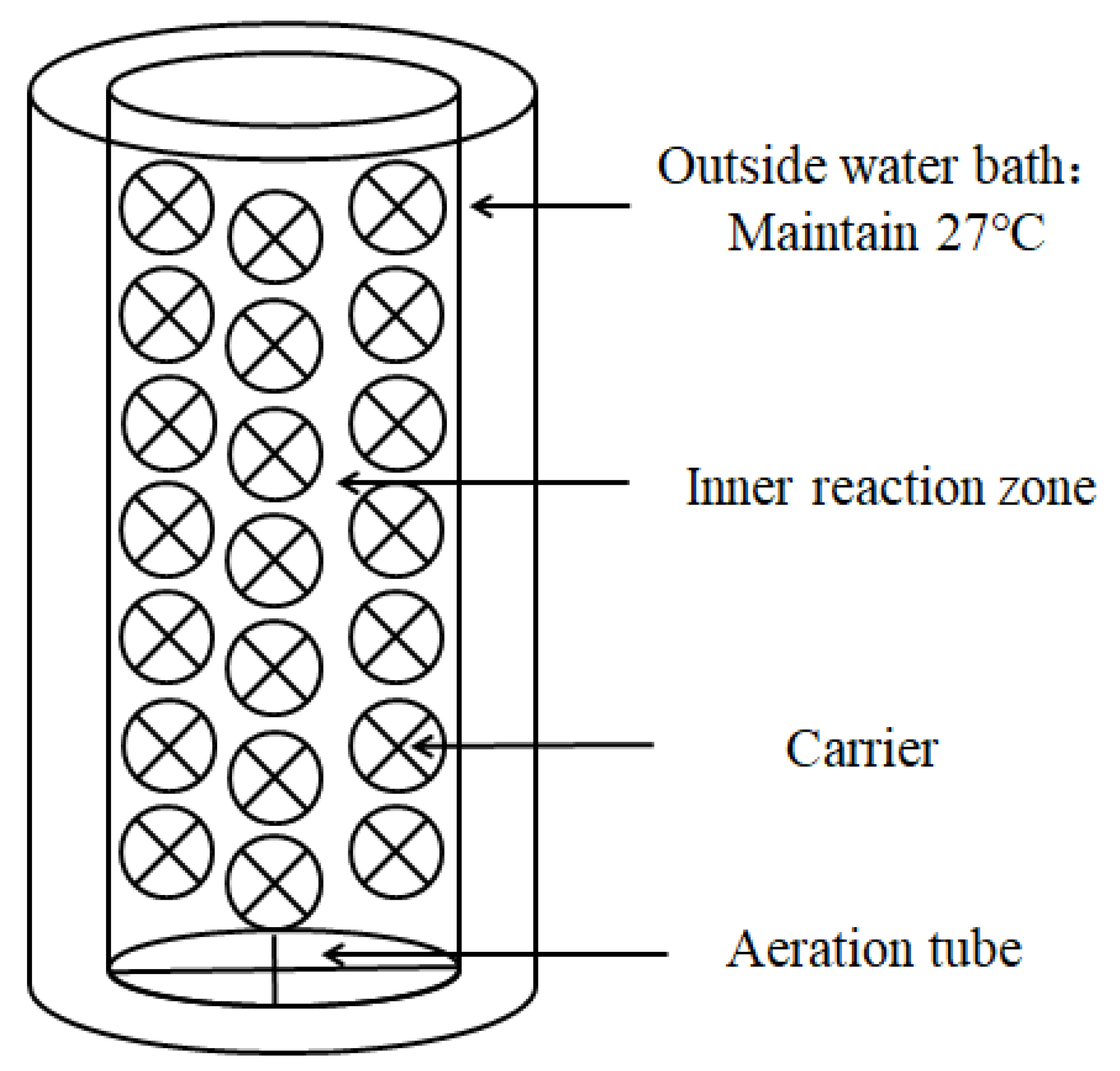
2.4. Reactor Set-Ups and Operation
2.5. Characterization of the Carrier and Biofilm
2.6. DNA Extraction and Microbial Community Analysis
2.7. Analytical Method for Wastewater
3. Results and Discussion
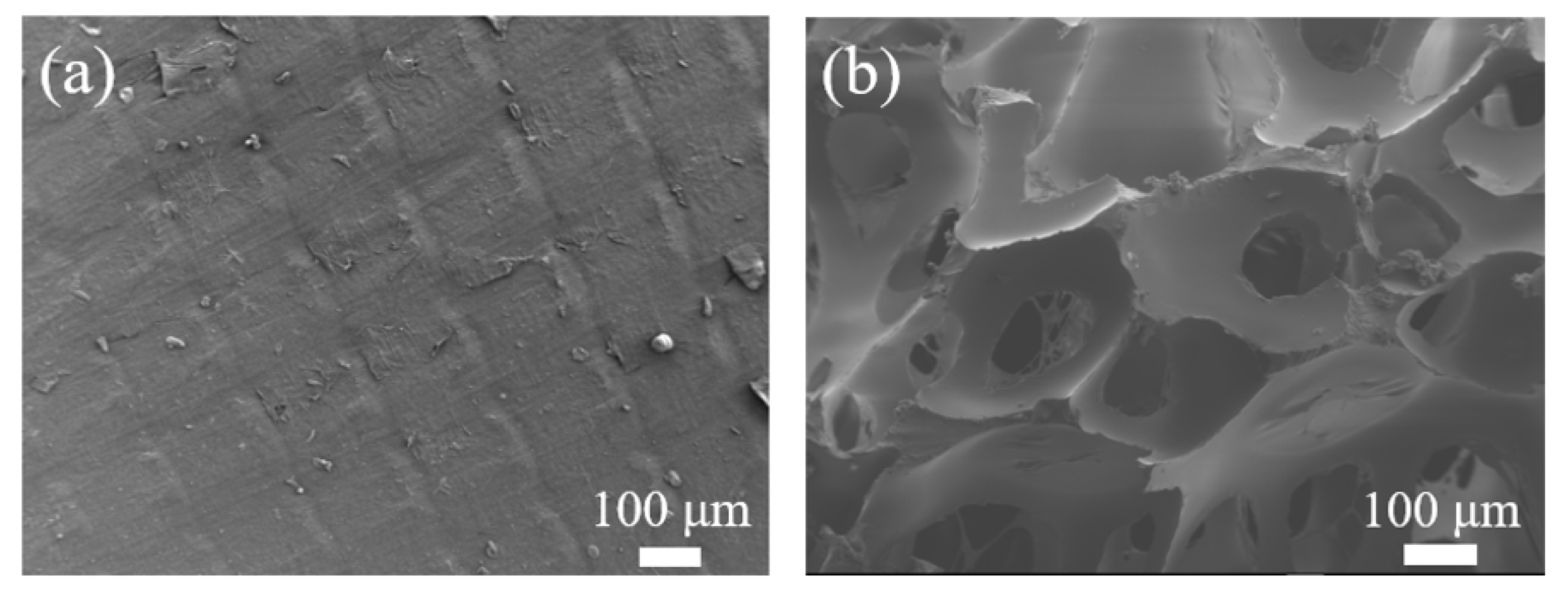
3.1. Structure of PLA Films by Light Microscopy
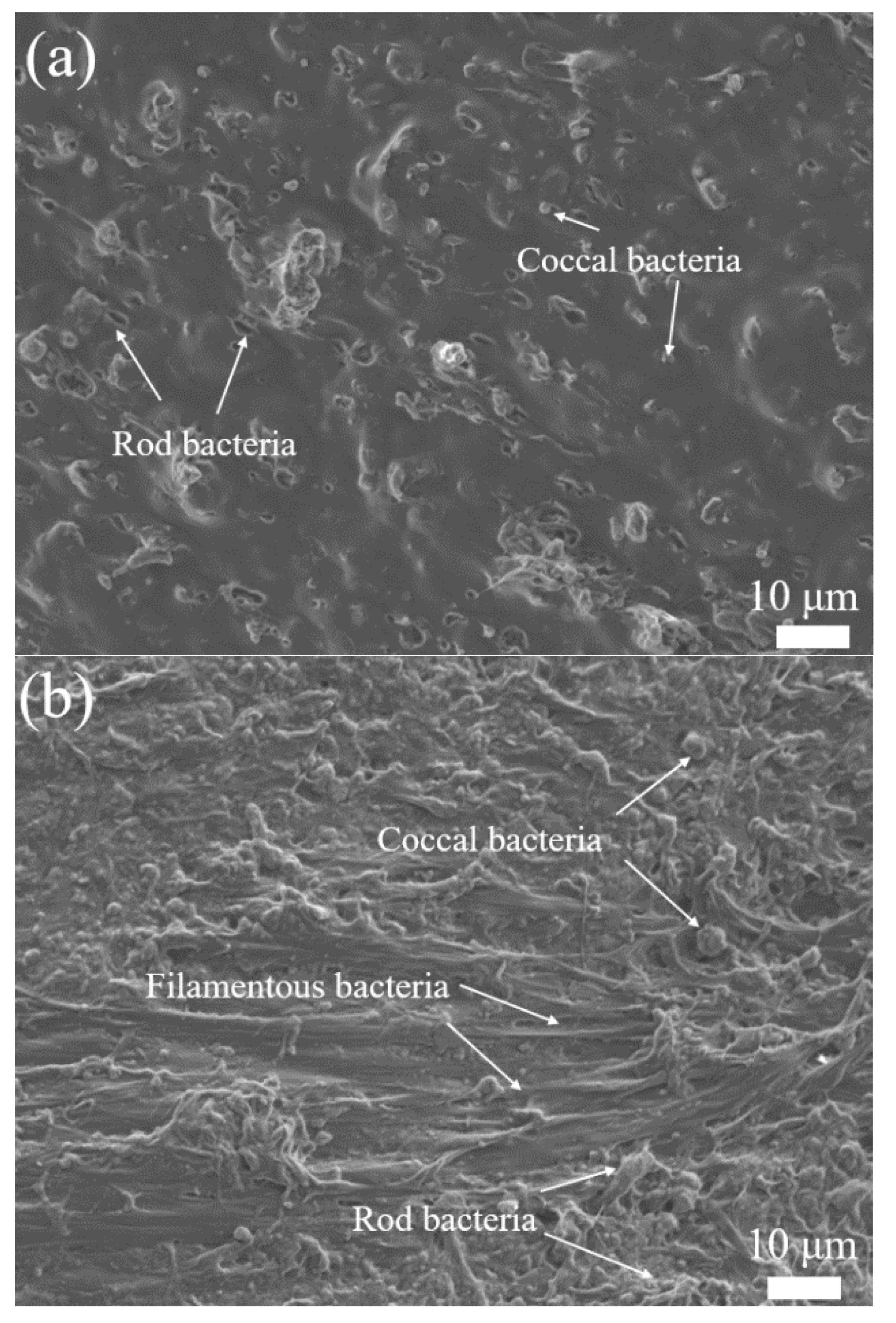
3.2. Biofilm on the Carriers
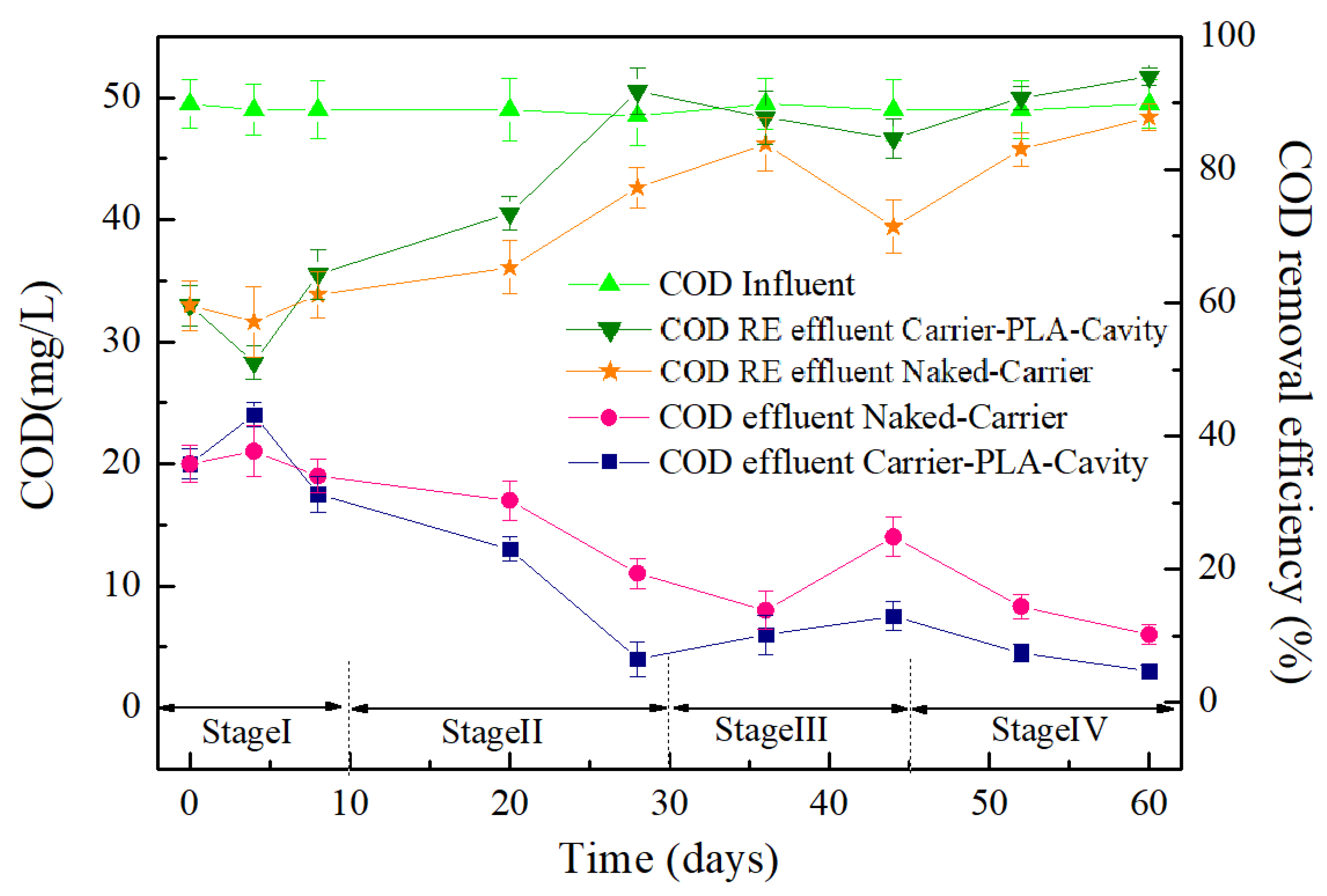
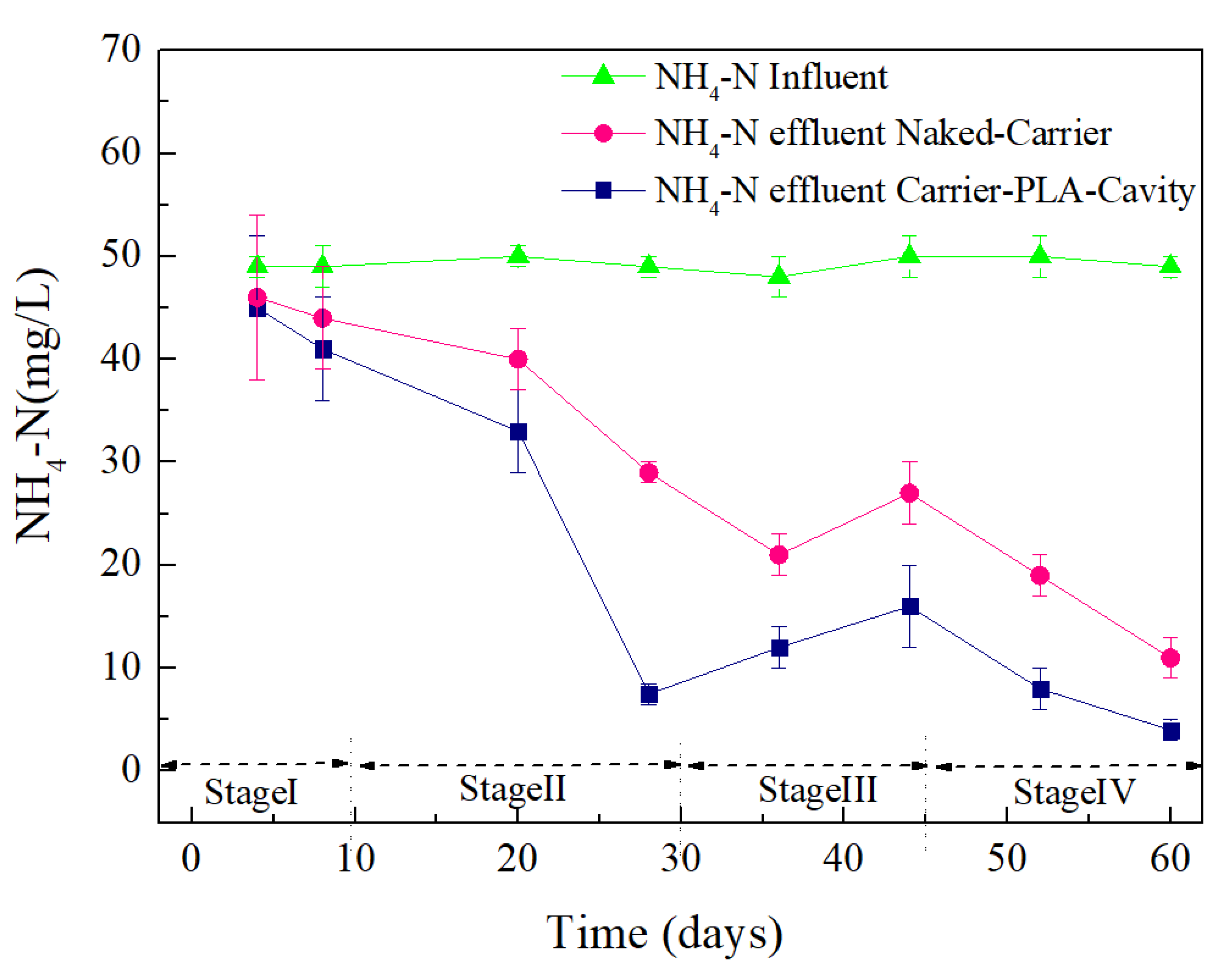
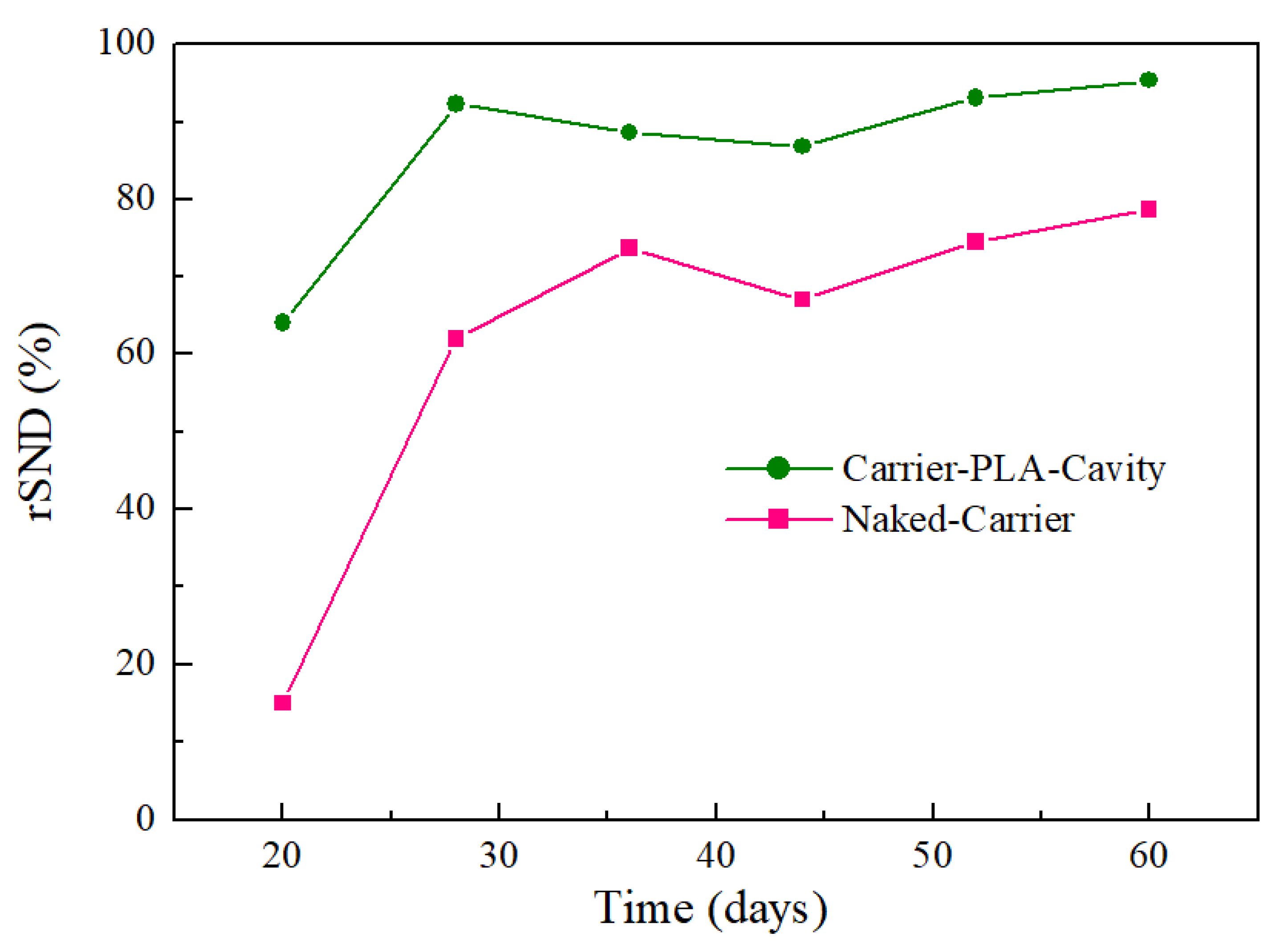
3.3. Wastewater Treatment Performance of Bio-Carriers
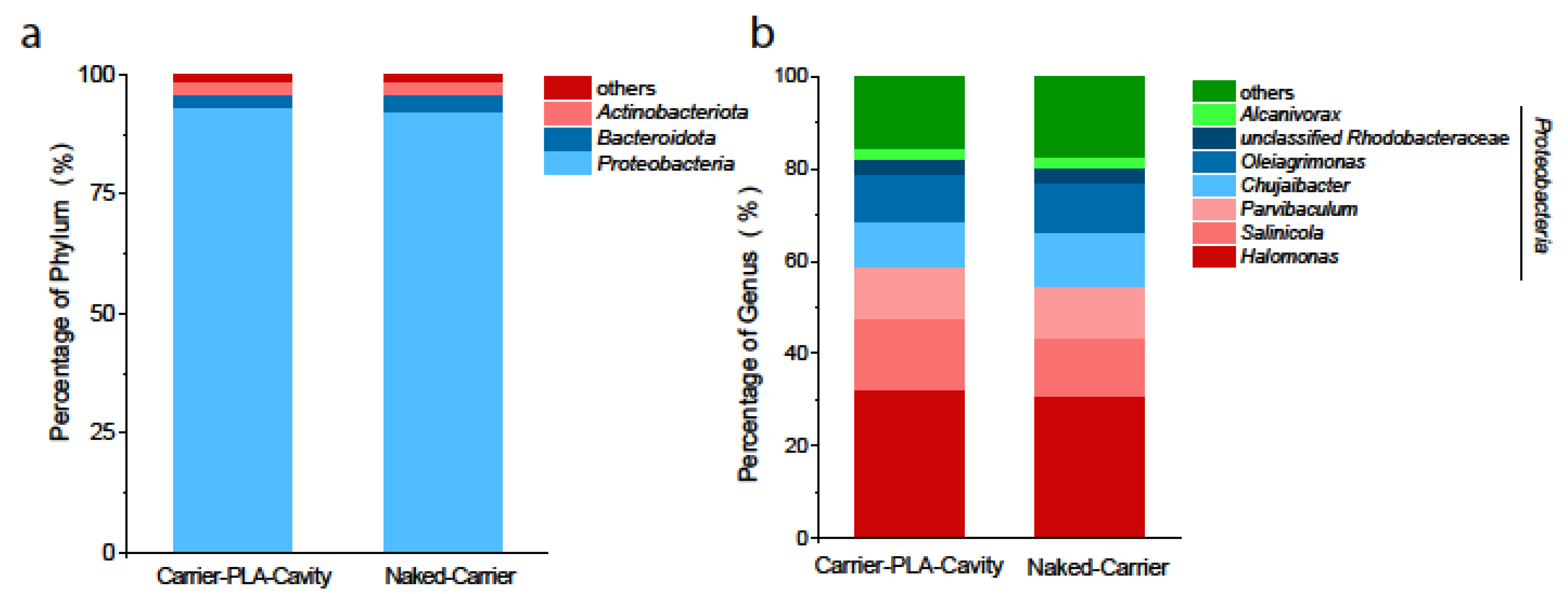
3.4. Microbial Community Analysis
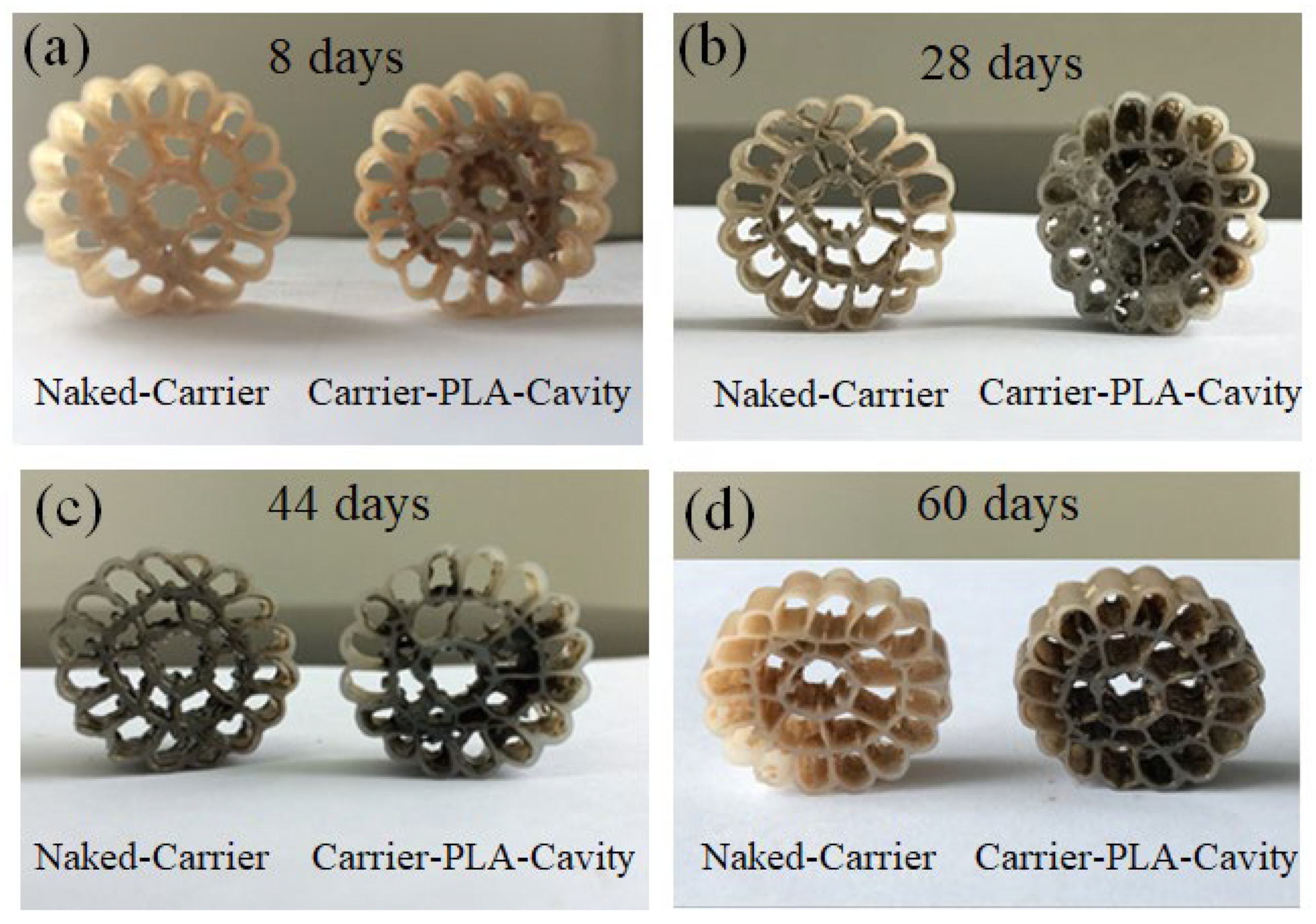
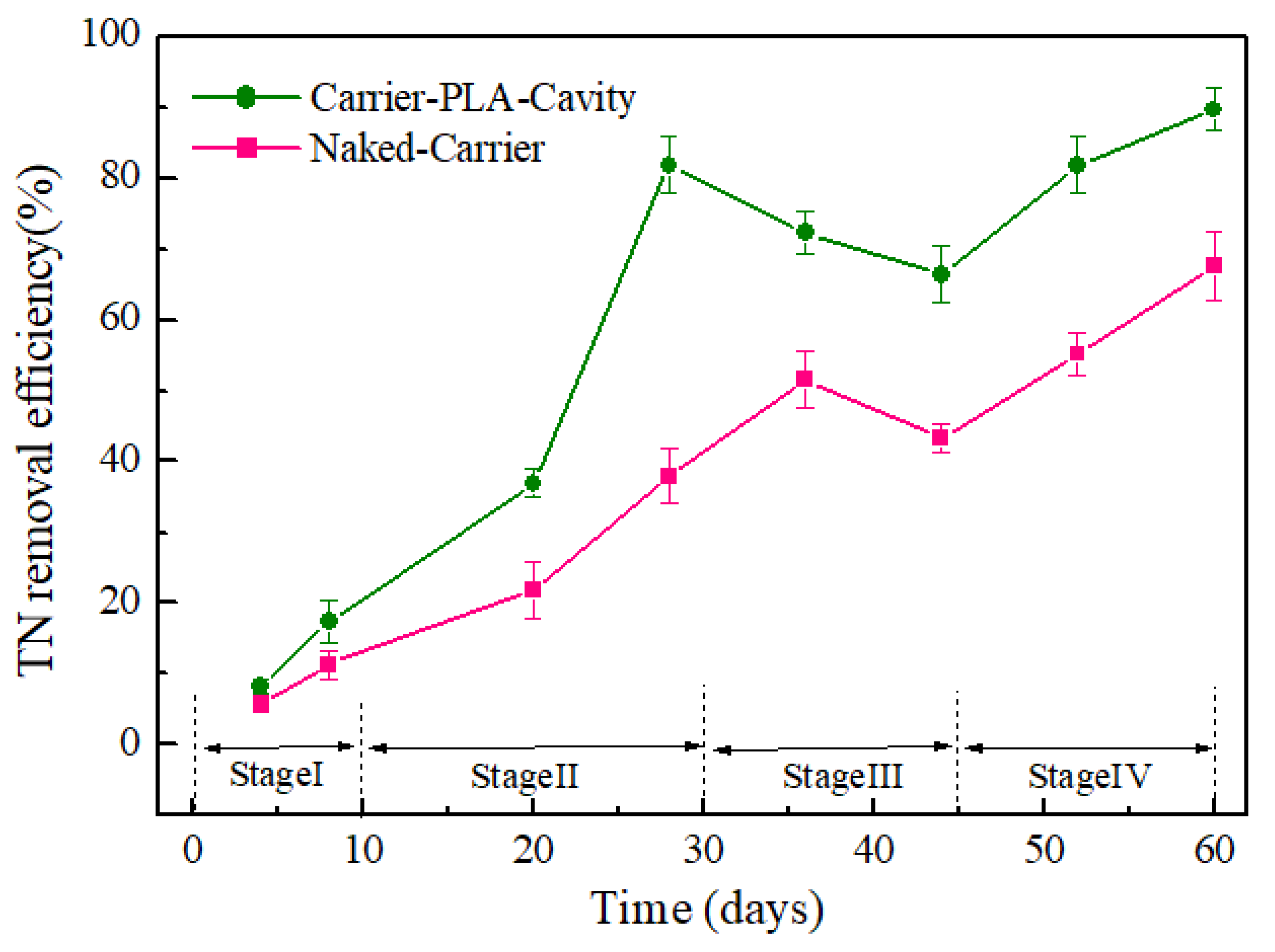
3.5. Degradation of Carrier-PLA-Cavity and Denitrification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, L.; Wang, J. Denitrification Performance and Biofilm Characteristics Using Biodegradable Polymers PCL as Carriers and Carbon Source. Chemosphere 2013, 91, 1310–1316. [Google Scholar] [CrossRef]
- Tarjányi-Szikora, S.; Oláh, J.; Makó, M.; Palkó, G.; Barkács, K.; Záray, G. Comparison of Different Granular Solids as Biofilm Carriers. Microchem. J. 2013, 107, 101–107. [Google Scholar] [CrossRef]
- Masłoń, A.; Tomaszek, J.A. A Study on the Use of the BioBall® as a Biofilm Carrier in a Sequencing Batch Reactor. Bioresour. Technol. 2015, 196, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.C.; Li, X.; Raskin, L.; Morgenroth, E. Chemisorption of Oxygen onto Activated Carbon Can Enhance the Stability of Biological Perchlorate Reduction in Fixed Bed Biofilm Reactors. Water Res. 2008, 42, 3425–3434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felföldi, T.; Jurecska, L.; Vajna, B.; Barkács, K.; Makk, J.; Cebe, G.; Szabó, A.; Záray, G.; Márialigeti, K. Texture and Type of Polymer Fiber Carrier Determine Bacterial Colonization and Biofilm Properties in Wastewater Treatment. Chem. Eng. J. 2015, 264, 824–834. [Google Scholar] [CrossRef]
- Gapes, D.J.; Keller, J. Impact of Oxygen Mass Transfer on Nitrification Reactions in Suspended Carrier Reactor Biofilms. Process Biochem. 2009, 44, 43–53. [Google Scholar] [CrossRef]
- Dong, Y.; Fan, S.-Q.; Shen, Y.; Yang, J.-X.; Yan, P.; Chen, Y.-P.; Li, J.; Guo, J.-S.; Duan, X.-M.; Fang, F.; et al. A Novel Bio-Carrier Fabricated Using 3D Printing Technique for Wastewater Treatment. Sci. Rep. 2015, 5, 12400. [Google Scholar] [CrossRef]
- Drugă, B.; Ukrainczyk, N.; Weise, K.; Koenders, E.; Lackner, S. Interaction between Wastewater Microorganisms and Geopolymer or Cementitious Materials: Biofilm Characterization and Deterioration Characteristics of Mortars. Int. Biodeterior. Biodegrad. 2018, 134, 58–67. [Google Scholar] [CrossRef]
- Hong, P.; Wu, X.; Shu, Y.; Wang, C.; Tian, C.; Wu, H.; Xiao, B. Bioaugmentation Treatment of Nitrogen-Rich Wastewater with a Denitrifier with Biofilm-Formation and Nitrogen-Removal Capacities in a Sequencing Batch Biofilm Reactor. Bioresour. Technol. 2020, 303, 122905. [Google Scholar] [CrossRef]
- Tan, L.C.; Papirio, S.; Luongo, V.; Nancharaiah, Y.V.; Cennamo, P.; Esposito, G.; van Hullebusch, E.D.; Lens, P.N.L. Comparative Performance of Anaerobic Attached Biofilm and Granular Sludge Reactors for the Treatment of Model Mine Drainage Wastewater Containing Selenate, Sulfate and Nickel. Chem. Eng. J. 2018, 345, 545–555. [Google Scholar] [CrossRef]
- Wu, X.; Cen, Q.; Addy, M.; Zheng, H.; Luo, S.; Liu, Y.; Cheng, Y.; Zhou, W.; Chen, P.; Ruan, R. A Novel Algal Biofilm Photobioreactor for Efficient Hog Manure Wastewater Utilization and Treatment. Bioresour. Technol. 2019, 292, 121925. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Fan, X.; Peng, P.; Peng, C.; Gao, Y.; Zhang, X.; Ren, H. Two Birds with One Stone: Simultaneous Improvement of Biofilm Formation and Nitrogen Transformation in MBBR Treating High Ammonia Nitrogen Wastewater via Exogenous N-Acyl Homoserine Lactones. Chem. Eng. J. 2020, 386, 124001. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Hu, H.; Wu, B.; Zhang, X.; Ren, H. Insight into Mature Biofilm Quorum Sensing in Full-Scale Wastewater Treatment Plants. Chemosphere 2019, 234, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.I.; Cheng, L.; Cord-Ruwisch, R. Energy Efficient COD and N-Removal from High-Strength Wastewater by a Passively Aerated GAO Dominated Biofilm. Bioresour. Technol. 2019, 283, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Han, Y.; Xu, C.; Han, H. Enhanced Biodegradation of Coal Gasification Wastewater with Anaerobic Biofilm on Polyurethane (PU), Powdered Activated Carbon (PAC), and Biochar. Bioresour. Technol. 2019, 289, 121487. [Google Scholar] [CrossRef]
- Fuseini, M.; Zaghloul, M.M.Y.; Elkady, M.F.; El-Shazly, A.H. Evaluation of Synthesized Polyaniline Nanofibres as Corrosion Protection Film Coating on Copper Substrate by Electrophoretic Deposition. J. Mater. Sci. 2022, 57, 6085–6101. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Zaghloul, M.Y.M.; Zaghloul, M.M.Y. Experimental and Modeling Analysis of Mechanical-Electrical Behaviors of Polypropylene Composites Filled with Graphite and MWCNT Fillers. Polym. Test. 2017, 63, 467–474. [Google Scholar] [CrossRef]
- Yu, N.; Sun, X.; Wang, Z.; Zhang, D.; Li, J. Effects of Auxiliary Heat on Warpage and Mechanical Properties in Carbon Fiber/ABS Composite Manufactured by Fused Deposition Modeling. Mater. Des. 2020, 195, 108978. [Google Scholar] [CrossRef]
- Fuseini, M.; Zaghloul, M.M.Y. Statistical and Qualitative Analyses of the Kinetic Models Using Electrophoretic Deposition of Polyaniline. J. Ind. Eng. Chem. 2022. [Google Scholar] [CrossRef]
- Mahmoud Zaghloul, M.Y.; Yousry Zaghloul, M.M.; Yousry Zaghloul, M.M. Developments in Polyester Composite Materials—An in-Depth Review on Natural Fibres and Nano Fillers. Compos. Struct. 2021, 278, 114698. [Google Scholar] [CrossRef]
- Chen, Z.; Gan, L.; Chang, P.R.; Liu, C.; Huang, J.; Gao, S. Cost Reduction and Mechanical Enhancement of Biopolyesters Using an Agricultural Byproduct from Konjac Glucomannan Processing. ACS Sustain. Chem. Eng. 2017, 5, 6498–6506. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, J. Biological Denitrification Using Cross-Linked Starch/PCL Blends as Solid Carbon Source and Biofilm Carrier. Bioresour. Technol. 2011, 102, 8835–8838. [Google Scholar] [CrossRef]
- Zhu, S.-M.; Deng, Y.-L.; Ruan, Y.-J.; Guo, X.-S.; Shi, M.-M.; Shen, J.-Z. Biological Denitrification Using Poly(Butylene Succinate) as Carbon Source and Biofilm Carrier for Recirculating Aquaculture System Effluent Treatment. Bioresour. Technol. 2015, 192, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, V.; Socol, G.; Grumezescu, A.M.; Holban, A.M.; Ficai, A.; Truşcǎ, R.; Bleotu, C.; Balaure, P.C.; Cristescu, R.; Chifiriuc, M.C. Functionalized Antibiofilm Thin Coatings Based on PLA–PVA Microspheres Loaded with Usnic Acid Natural Compounds Fabricated by MAPLE. Appl. Surf. Sci. 2014, 302, 262–267. [Google Scholar] [CrossRef]
- Chu, L.; Wang, J. Nitrogen Removal Using Biodegradable Polymers as Carbon Source and Biofilm Carriers in a Moving Bed Biofilm Reactor. Chem. Eng. J. 2011, 170, 220–225. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Mohamed, Y.S.; El-Gamal, H. Fatigue and Tensile Behaviors of Fiber-Reinforced Thermosetting Composites Embedded with Nanoparticles. J. Compos. Mater. 2019, 53, 709–718. [Google Scholar] [CrossRef]
- Zaghloul, M.Y.; Zaghloul, M.M.Y.; Zaghloul, M.M.Y. Influence of Stress Level and Fibre Volume Fraction on Fatigue Performance of Glass Fibre-Reinforced Polyester Composites. Polymers 2022, 14, 2662. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, F.; Yang, L. Biological Denitrification with a Novel Biodegradable Polymer as Carbon Source and Biofilm Carrier. Bioresour. Technol. 2012, 118, 136–140. [Google Scholar] [CrossRef]
- Pawlowski, L. Standard Methods for the Examination of Water and Wastewater, 18th Edition. Sci. Total Environ. 1994, 142, 227–228. [Google Scholar] [CrossRef]
- Song, L.; Wang, Y.; Tang, W.; Lei, Y. Bacterial Community Diversity in Municipal Waste Landfill Sites. Appl. Microbiol. Biotechnol. 2015, 99, 7745–7756. [Google Scholar] [CrossRef]
- Guillaume, O.; Garric, X.; Lavigne, J.-P.; Van Den Berghe, H.; Coudane, J. Multilayer, Degradable Coating as a Carrier for the Sustained Release of Antibiotics: Preparation and Antimicrobial Efficacy in Vitro. J. Control. Release 2012, 162, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Tombola, R.; Buttiglieri, G.; Auset, M.; Gonzalez-Olmos, R. Recycled Corrugated Wire Hose Cover as Biological Carriers for Greywater Treatment in a Sequential Batch Biofilm Reactor. J. Environ. Manag. 2019, 240, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Yurtsever, A.; Basaran, E.; Ucar, D. Process Optimization and Filtration Performance of an Anaerobic Dynamic Membrane Bioreactor Treating Textile Wastewaters. J. Environ. Manag. 2020, 273, 111114. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues de Assis, L.; Calijuri, M.L.; Assemany, P.P.; Silva, T.A.; Teixeira, J.S. Innovative Hybrid System for Wastewater Treatment: High-Rate Algal Ponds for Effluent Treatment and Biofilm Reactor for Biomass Production and Harvesting. J. Environ. Manag. 2020, 274, 111183. [Google Scholar] [CrossRef]
- Sheng, G.-P.; Yu, H.-Q.; Li, X.-Y. Extracellular Polymeric Substances (EPS) of Microbial Aggregates in Biological Wastewater Treatment Systems: A Review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef]
- Liu, X.-M.; Sheng, G.-P.; Luo, H.-W.; Zhang, F.; Yuan, S.-J.; Xu, J.; Zeng, R.J.; Wu, J.-G.; Yu, H.-Q. Contribution of Extracellular Polymeric Substances (EPS) to the Sludge Aggregation. Environ. Sci. Technol. 2010, 44, 4355–4360. [Google Scholar] [CrossRef]
- Ming, H.; Ji, W.; Li, M.; Zhao, Z.; Cheng, L.; Niu, M.; Zhang, L.; Wang, Y.; Nie, G. Halomonas Lactosivorans sp. Nov., Isolated from Salt-Lake Sediment. Int. J. Syst. Evol. Microbiol. 2020, 70, 3504–3512. [Google Scholar] [CrossRef]




| Materials | Melting Point (°C) | Boiling Point (°C) | Density (g/cm3) | Tensile Strength (MPa) | Elongation (%) | Form |
|---|---|---|---|---|---|---|
| K3 | 105 | - | 0.96 | 15 | 90 | Particle |
| PLA | 170 | - | 1.25 | 55 | 5 | Powder |
| NaOH | 318 | 1388 | 2.13 | - | - | Powder |
| CH2Cl2 | −97 | 39 | 1.32 | - | - | Liquid |
| Component | Dosage (g/L) |
|---|---|
| EDTA | 15.000 |
| ZnSO4·7H2O | 0.430 |
| CoCl2·6H2O | 0.240 |
| MnCl2·4H2O | 0.990 |
| CuSO4·5H2O | 0.250 |
| NaMoO4·2H2O | 0.220 |
| NiCl2·2H2O | 0.190 |
| Na2SeO4·10H2O | 0.210 |
| H3BO4 | 0.014 |
| Na2WO4·2H2O | 0.050 |
| Component | Dosage (g/L) |
|---|---|
| NaH2PO4·2H2O | 0.029 |
| CaCl2·2H2O | 0.300 |
| MgSO4·7H2O | 0.200 |
| FeSO4·7H2O | 0.00625 |
| EDTA | 0.00625 |
| Component | Dosage |
|---|---|
| COD in influent | 50 mg/L |
| NH4+-N in influent | 50 mg/L |
| NO3−-N in influent | 10 mg/L |
| NaCl in influent | 30 g/L |
| pH | 7.0~8.2 |
| Trace elements | 1.25 mL/L |
| Mineral medium | As Table 2 showed |
| Sample | Sequence Number | OUT | Alpha Diversity Index | ||||
|---|---|---|---|---|---|---|---|
| Ace | Chao | Shannon | Simpson | Coverage | |||
| Carrier-PLA-Cavity | 53,617 | 195 | 212 | 206 | 2.427 | 0.163 | 0.999 |
| Naked-Carrier | 58,259 | 192 | 223 | 222 | 2.496 | 0.151 | 0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, N.; Zhang, D.; Lei, Y.; Wang, J.; Dong, Y.; Chen, Y. Innovative Coating–Etching Method of Biocarrier Fabrication for Treating Wastewater with a Low C/N Ratio. Polymers 2022, 14, 3010. https://doi.org/10.3390/polym14153010
Yu N, Zhang D, Lei Y, Wang J, Dong Y, Chen Y. Innovative Coating–Etching Method of Biocarrier Fabrication for Treating Wastewater with a Low C/N Ratio. Polymers. 2022; 14(15):3010. https://doi.org/10.3390/polym14153010
Chicago/Turabian StyleYu, Ning, Daijun Zhang, Yu Lei, Jianhui Wang, Yang Dong, and Youpeng Chen. 2022. "Innovative Coating–Etching Method of Biocarrier Fabrication for Treating Wastewater with a Low C/N Ratio" Polymers 14, no. 15: 3010. https://doi.org/10.3390/polym14153010






