Chitosan Effect on Hardening Dynamics of Calcium Phosphate Cement: Low-Field NMR Relaxometry Investigations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. NMR Relaxometry Technique
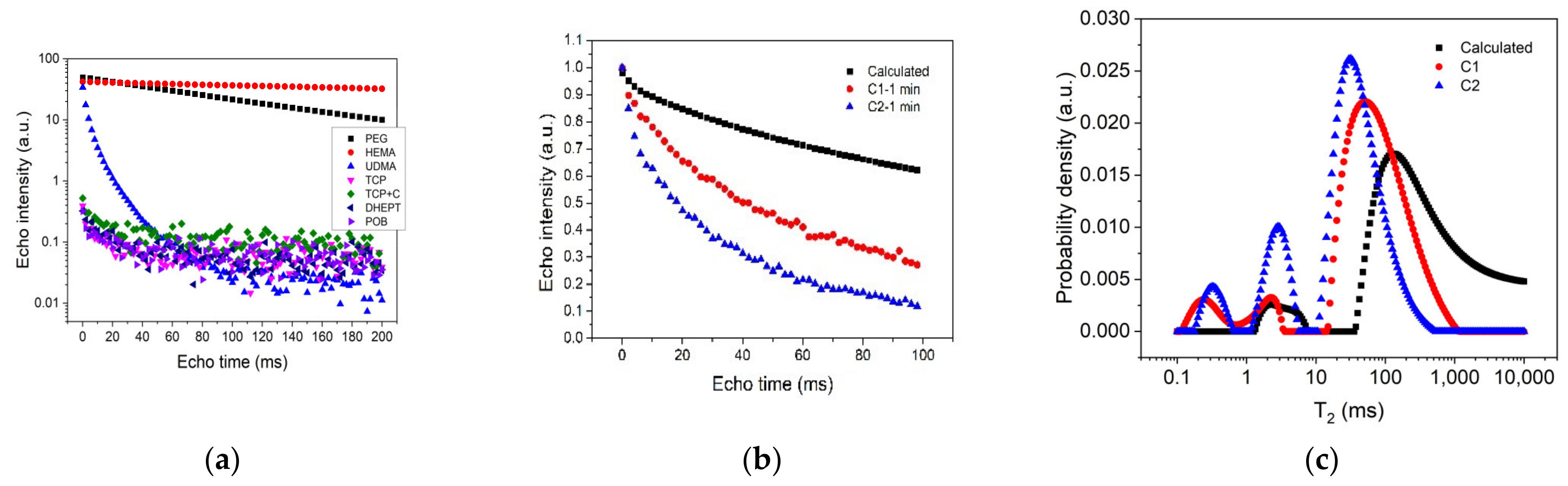
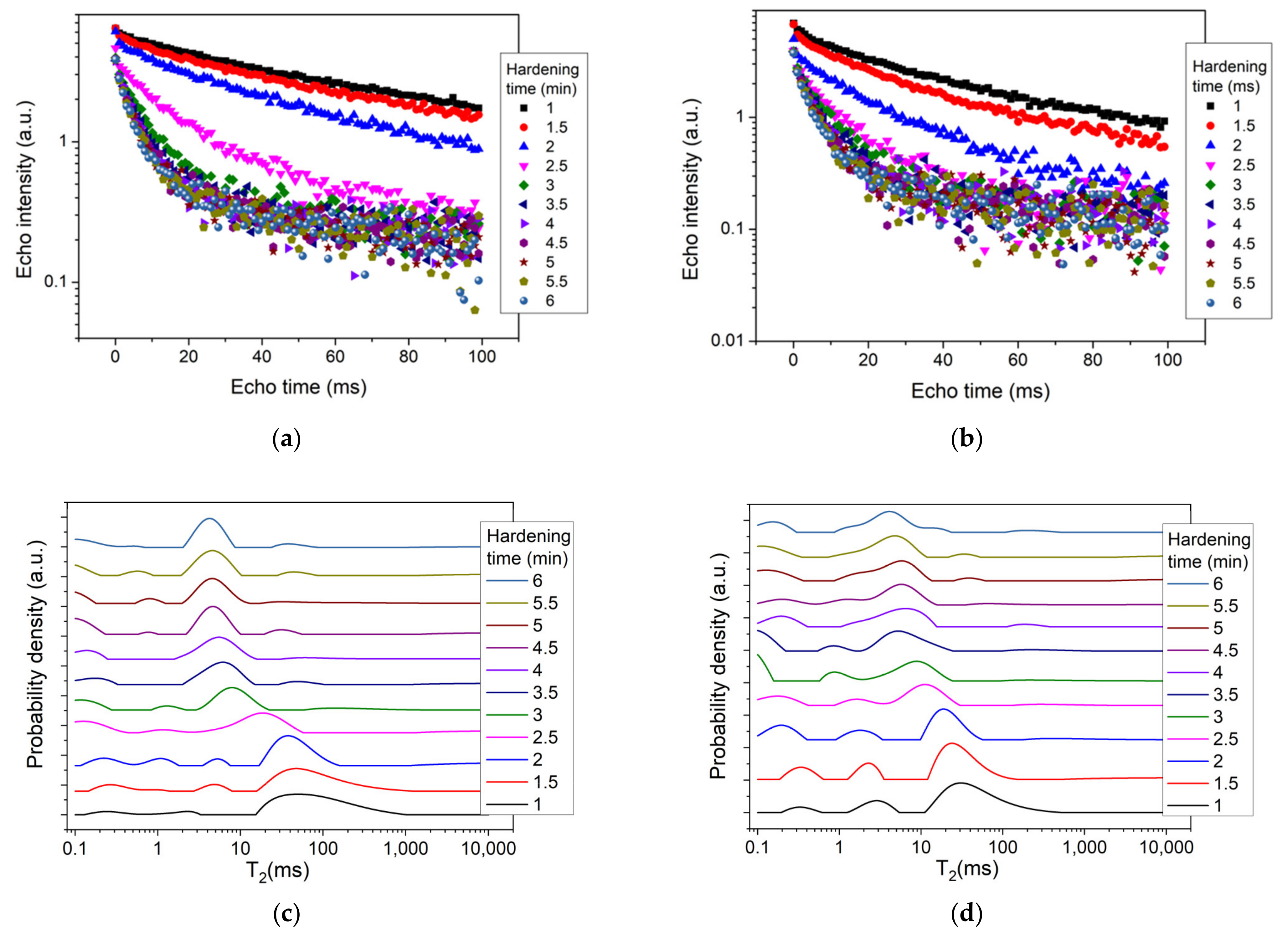
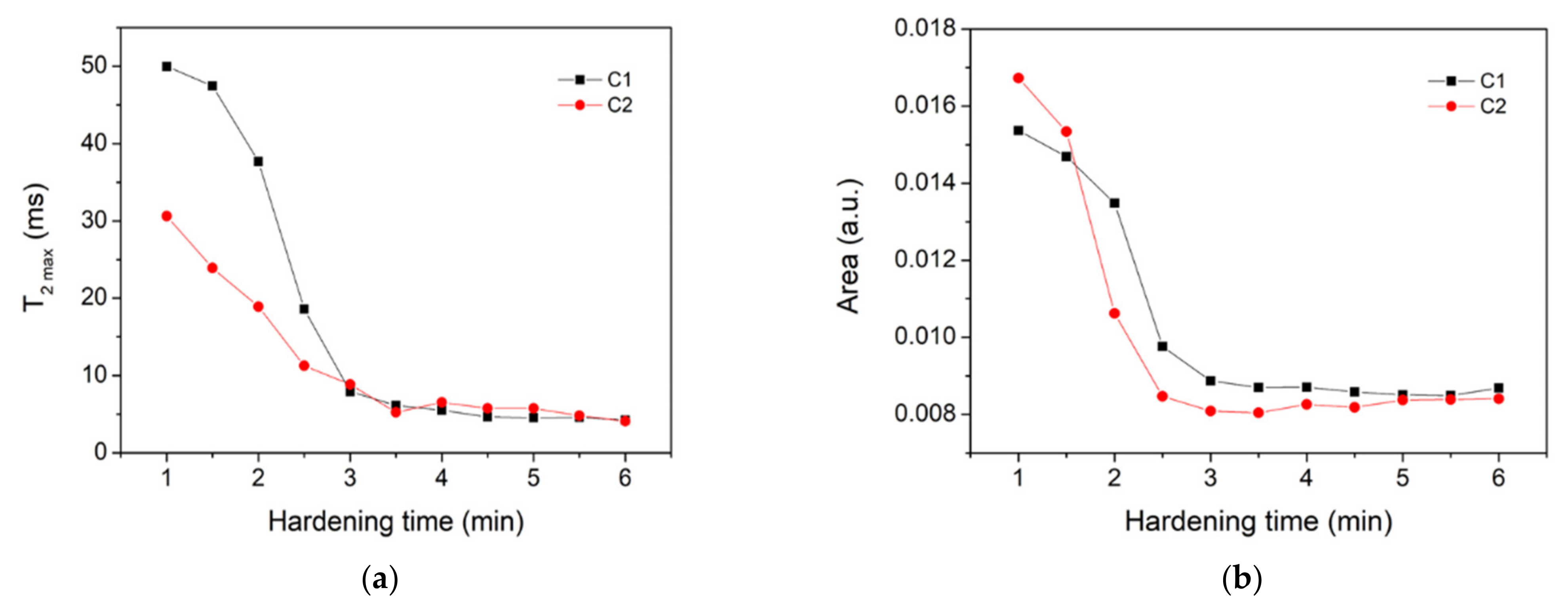
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials Design for Bone-Tissue Engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Ginebra, M.-P.; Montufar, E.B. Cements as Bone Repair Materials, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 9780081024515. [Google Scholar]
- Zapata, M.E.V.; Hernandez, J.H.M.; Tovar, C.D.G. Acrylic Bone Cement Incorporated with Low Chitosan Loadings. Polymers 2020, 12, 1617. [Google Scholar] [CrossRef] [PubMed]
- Zapata, M.E.V.; Hernandez, J.H.M.; Tovar, C.D.G.; Llano, C.H.V.; Escobar, J.A.D.; Vázquez-Lasa, B.; Román, J.S.; Rojo, L. Novel Bioactive and Antibacterial Acrylic Bone Cement Nanocomposites Modified with Graphene Oxide and Chitosan. Int. J. Mol. Sci. 2019, 20, 2938. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, A.M. A Review of Calcium Phosphate Cements and Acrylic Bone Cements as Injectable Materials for Bone Repair and Implant Fixation. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019872594. [Google Scholar] [CrossRef]
- Yin, X.; Stott, M.J.; Rubio, A. α- and β-Tricalcium Phosphate: A Density Functional Study. Phys. Rev. B-Condens. Matter Mater. Phys. 2003, 68, 1–8. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-Tricalcium Phosphate for Bone Substitution: Synthesis and Properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Hameed, A.Z.; Raj, S.A.; Kandasamy, J.; Baghdadi, M.A.; Shahzad, M.A. Chitosan: A Sustainable Material for Multifarious Applications. Polymers 2022, 14, 2335. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan Based Bioactive Materials in Tissue Engineering Applications-A Review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Kołodziejska, M.; Jankowska, K.; Klak, M.; Wszoła, M. Chitosan as an Underrated Polymer in Modern Tissue Engineering. Nanomaterials 2021, 11, 3019. [Google Scholar] [CrossRef]
- Onishi, H.; Machida, Y. Biodegradation and Distribution of Water-Soluble Chitosan in Mice. Biomaterials 1999, 20, 175–182. [Google Scholar] [CrossRef]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A Versatile Biopolymer for Orthopaedic Tissue-Engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef]
- Fang, C.H.; Lin, Y.W.; Sun, J.S.; Lin, F.H. The Chitosan/Tri-Calcium Phosphate Bio-Composite Bone Cement Promotes Better Osteo-Integration: An in Vitro and in Vivo Study. J. Orthop. Surg. Res. 2019, 14, 1–9. [Google Scholar] [CrossRef]
- Ni, Q.; Chen, S. Assessment of Structural Changes of Human Teeth by Low-Field Nuclear Magnetic Resonance (NMR). Meas. Sci. Technol. 2010, 21, 015803. [Google Scholar] [CrossRef] [Green Version]
- Pop, A.; Ardelean, I. Monitoring the Size Evolution of Capillary Pores in Cement Paste during the Early Hydration via Diffusion in Internal Gradients. Cem. Concr. Res. 2015, 77, 76–81. [Google Scholar] [CrossRef]
- Pop, A.; Badea, C.; Ardelean, I. The Effects of Different Superplasticizers and Water-to-Cement Ratios on the Hydration of Gray Cement Using T2-NMR. Appl. Magn. Reson. 2013, 44, 1223–1234. [Google Scholar] [CrossRef]
- Ni, Q.; Nicolella, D.P. The Characterization of Human Cortical Bone Microdamage by Nuclear Magnetic Resonance. Meas. Sci. Technol. 2005, 16, 659–668. [Google Scholar] [CrossRef]
- Jang, H.J.; Shin, C.Y.; Kim, K.B. Safety Evaluation of Polyethylene Glycol (PEG) Compounds for Cosmetic Use. Toxicol. Res. 2015, 31, 105–136. [Google Scholar] [CrossRef] [Green Version]
- Morúa, O.C.; Cardoso, M.J.B.; da Silva, H.N.; Carrodeguas, R.G.; Rodríguez, M.A.; Fook, M.V.L. Synthesis of Brushite/Polyethylene Glycol Cement for Filler in Bone Tissue Injuries. Ceramica 2021, 67, 289–294. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef] [Green Version]
- Provencher, S.W. CONTIN: A General Purpose Constrained Regularization Program for Inverting Noisy Linear Algebraic and Integral Equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- Venkataramanan, L.; Song, Y.; Hürlimann, M.D. Solving Fredholm Integrals of the First Kind with Tensor Product Structure in 2 and 2.5 Dimensions. IEEE Trans. Signal Process. 2002, 50, 1017–1026. [Google Scholar] [CrossRef]
- Khashaba, R.M.; Moussa, M.M.; Mettenburg, D.J.; Rueggeberg, F.A.; Chutkan, N.B.; Borke, J.L. Polymeric-Calcium Phosphate Cement Composites-Material Properties: In Vitro and In Vivo Investigations. Int. J. Biomater. 2010, 2010, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Christel, T.; Kuhlmann, M.; Vorndran, E.; Groll, J.; Gbureck, U. Dual Setting α-Tricalcium Phosphate Cements. J. Mater. Sci. Mater. Med. 2013, 24, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Rödel, M.; Teßmar, J.; Groll, J.; Gbureck, U. Tough and Elastic α-Tricalcium Phosphate Cement Composites with Degradable PEG-Based Cross-Linker. Materials 2018, 12, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Cement | Organic Phase (%) | Inorganic Phase (%) | Initiation System (%) | ||||
|---|---|---|---|---|---|---|---|
| UDMA | HEMA | PEG 400 | TCP | Chitosan | BPO | DHEPT | |
| C1 | 3 | 25 | 22 | 50 | 0 | 2 | 0.75 |
| C2 | 3 | 25 | 22 | 25 | 25 | 2 | 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacan, I.; Moldovan, M.; Sarosi, C.; Ardelean, I. Chitosan Effect on Hardening Dynamics of Calcium Phosphate Cement: Low-Field NMR Relaxometry Investigations. Polymers 2022, 14, 3042. https://doi.org/10.3390/polym14153042
Lacan I, Moldovan M, Sarosi C, Ardelean I. Chitosan Effect on Hardening Dynamics of Calcium Phosphate Cement: Low-Field NMR Relaxometry Investigations. Polymers. 2022; 14(15):3042. https://doi.org/10.3390/polym14153042
Chicago/Turabian StyleLacan, Ioana, Mărioara Moldovan, Codruța Sarosi, and Ioan Ardelean. 2022. "Chitosan Effect on Hardening Dynamics of Calcium Phosphate Cement: Low-Field NMR Relaxometry Investigations" Polymers 14, no. 15: 3042. https://doi.org/10.3390/polym14153042








