Textiles Functionalized with Copper Oxides: A Sustainable Option for Prevention of COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
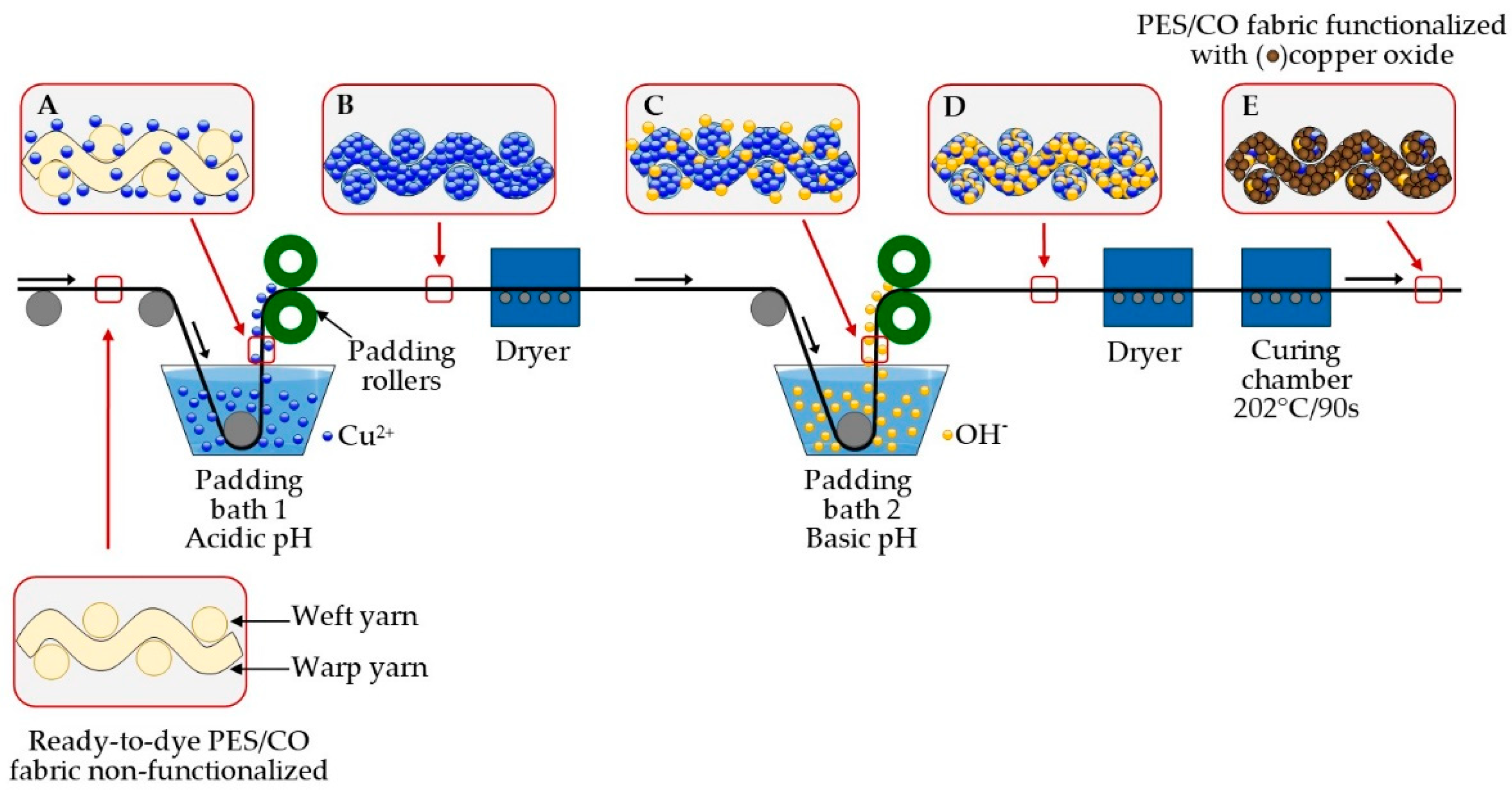
2.2. Functionalization Process at Industrial Scale
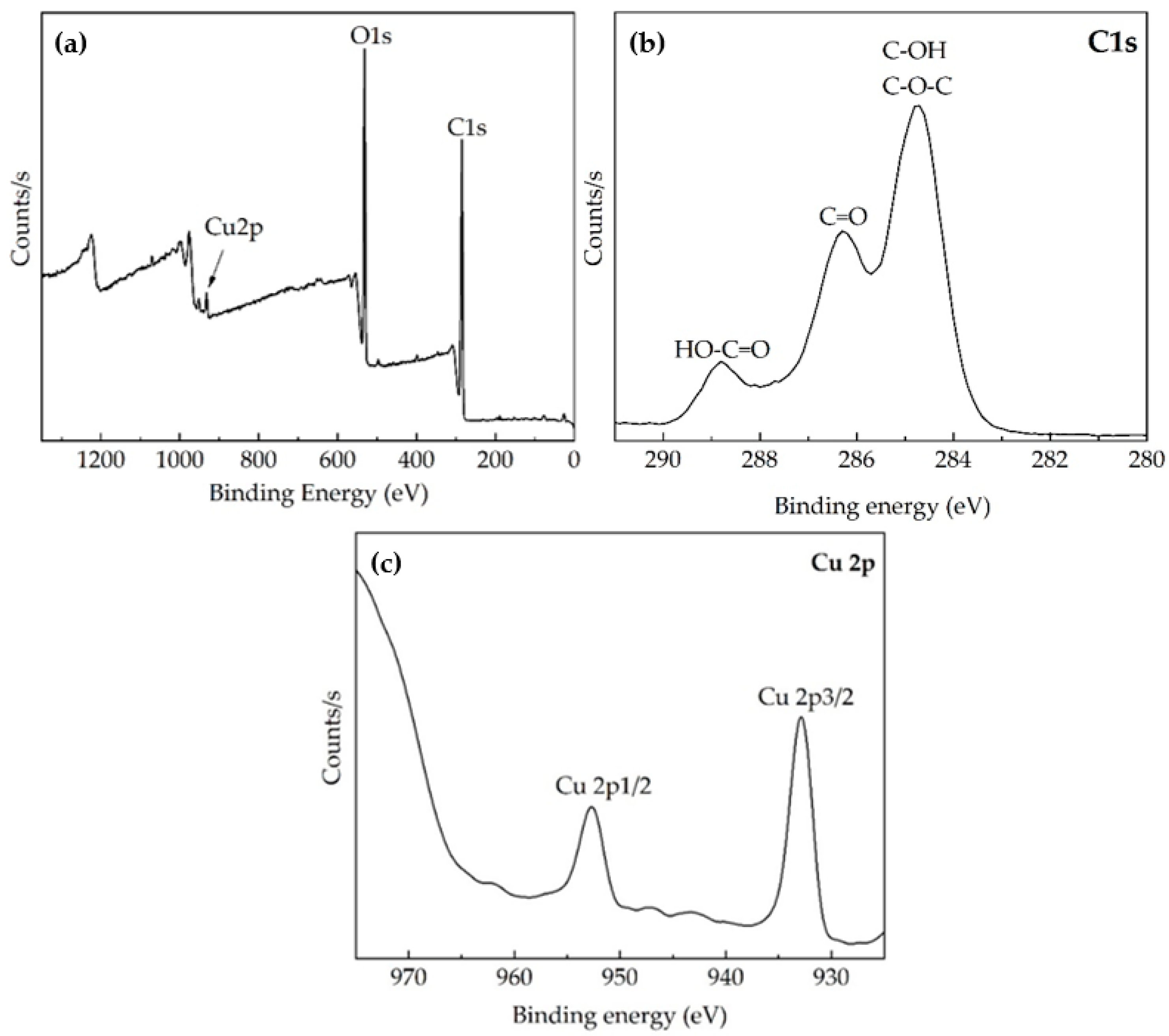
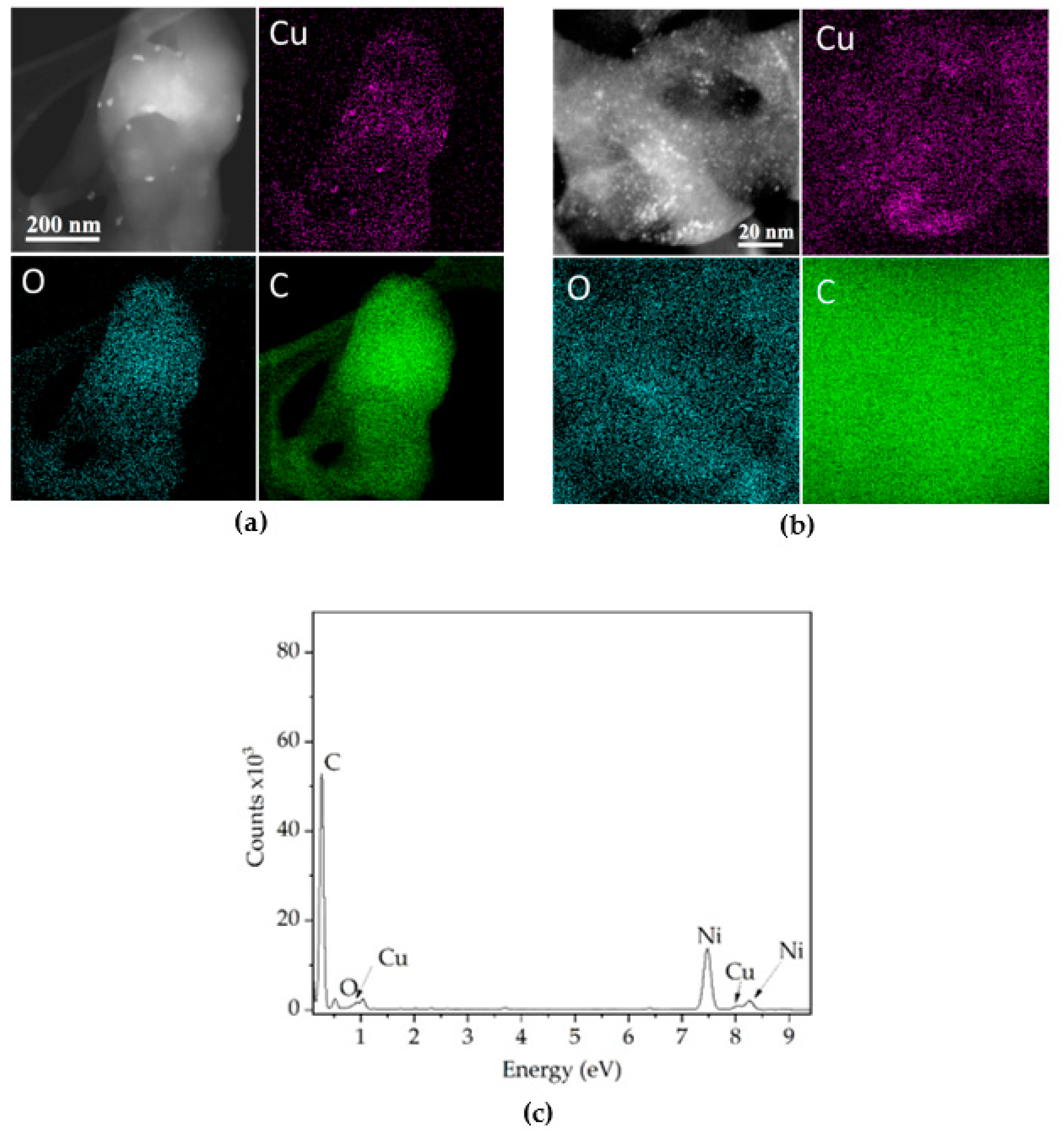
2.3. Characterization Techniques, Quality Control, and Physical Properties
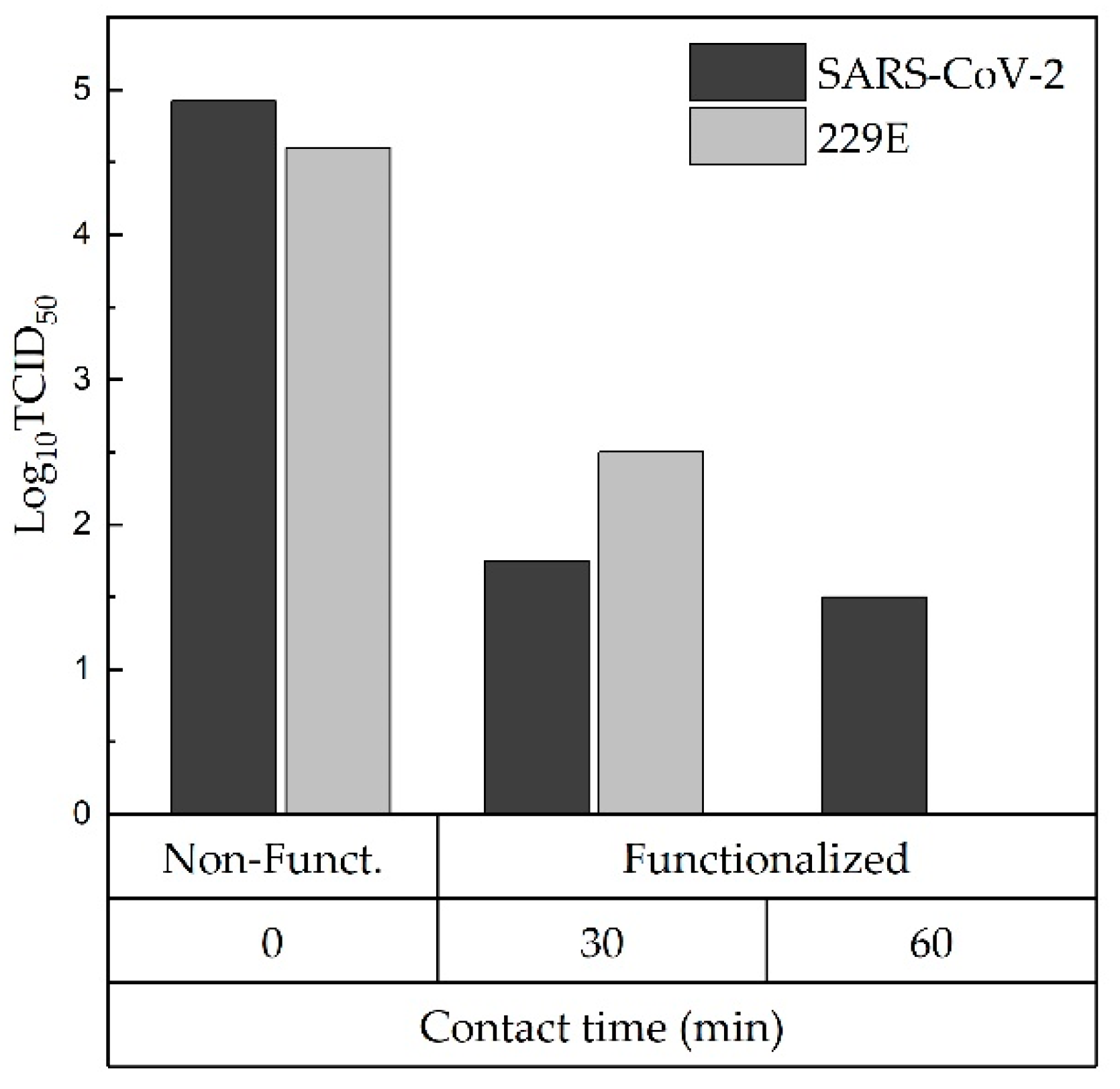
2.4. Antiviral and Antibacterial Evaluations
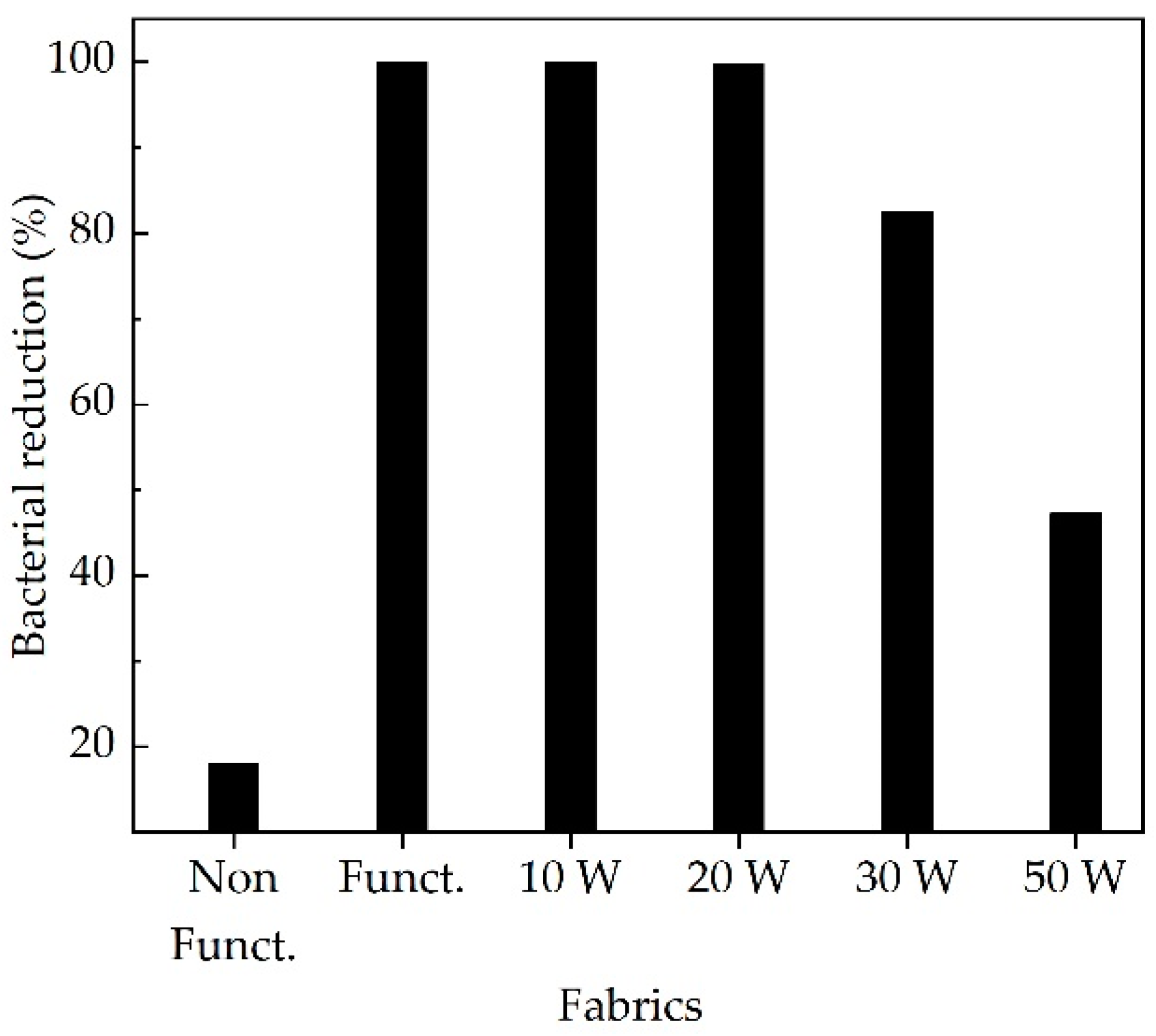
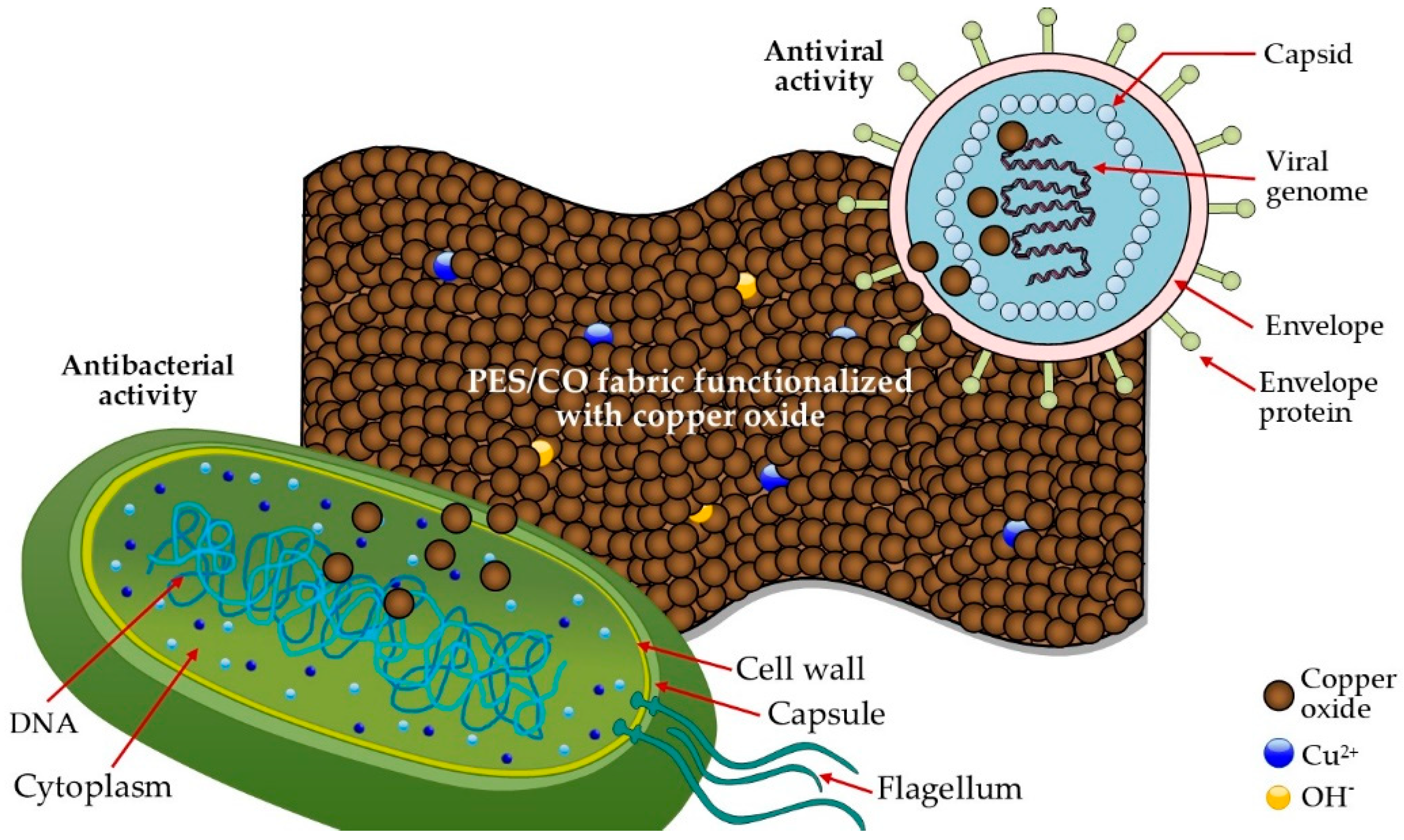
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Travaglio, M.; Yu, Y.; Popovic, R.; Selley, L.; Leal, N.S.; Martins, L.M. Links between air pollution and COVID-19 in England. Environ. Pollut. 2021, 268, 115859. [Google Scholar] [CrossRef]
- Hoang, H.T.T.; Duong, T.T.; Nguyen, K.T.; Le, Q.T.P.; Luu, M.T.N.; Trinh, D.A.; Le, A.H.; Ho, C.T.; Dang, K.D.; Nemery, J.; et al. Impact of anthropogenic activities on water quality and plankton communities in the Day River (Red River Delta, Vietnam). Environ. Monit. Assess. 2018, 190, 67. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, M.; Liu, X.; Singh, D.K.; Fu, X. Quantifying the impact of climate change and anthropogenic activities on runoff and sediment load reduction in a typical Loess Plateau watershed. J. Hydrol. Regional Stud. 2022, 39, 100992. [Google Scholar] [CrossRef]
- Perveen, N.; Muzaffar, S.B.; Al-Deeb, M.A. Exploring human-animal host interactions and emergence of COVID-19: Evolutionary and ecological dynamics. Saudi J. Biol. Sci. 2021, 28, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Perrings, C.; Kinzig, A.; Collins, J.P.; Minteer, B.A.; Daszak, P. Economic growth, urbanization, globalization, and the risks of emerging infectious diseases in China: A review. Ambio 2017, 46, 18–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, L.W.; Hamilton, W.L.; Warne, B.; Houldcroft, C.J.; Hosmillo, M.; Jahun, A.S.; Curran, M.D.; Parmar, S.; Caller, L.G.; Caddy, S.L.; et al. Rapid implementation of SARS-CoV-2 sequencing to investigate cases of health-care associated COVID-19: A prospective genomic surveillance study. Lancet Infect. Dis. 2020, 20, 1263–1272. [Google Scholar] [CrossRef]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [Green Version]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Dolci, M.; Signorini, L.; Cason, C.; Campisciano, G.; Kunderfranco, P.; Pariani, E.; Galli, C.; Petix, V.; Ferrante, P.; Delbue, S.; et al. Circulation of SARS-CoV-2 variants among children from november 2020 to january 2022 in Trieste (Italy). Microorganisms 2022, 10, 612. [Google Scholar] [CrossRef]
- Sturdy, A.; Basarab, M.; Cotter, M.; Hager, K.; Shakespeare, D.; Shah, N.; Randall, P.; Spray, D.; Arnold, A. Severe COVID-19 and healthcare-associated infections on the ICU: Time to remember the basics? J. Hosp. Infect. 2020, 105, 593–595. [Google Scholar] [CrossRef]
- Liu, H.; Zhong, W.; Zhang, X.; Lin, D.; Wu, J. Nanomedicine as a promising strategy for the theranostics of infectious diseases. J. Mater. Chem. B 2021, 9, 7878–7908. [Google Scholar] [CrossRef] [PubMed]
- Zingg, W.; Holmes, A.; Dettenkofer, M.; Goetting, T.; Secci, F.; Clack, L.; Allegranzi, B.; Magiorakos, A.-P.; Pittet, D. Hospital organisation, management, and structure for prevention of health-care-associated infection: A systematic review and expert consensus. Lancet Infect. Dis. 2015, 15, 212–224. [Google Scholar] [CrossRef]
- Ammendolia, J.; Saturno, J.; Brooks, A.L.; Jacobs, S.; Jambeck, J.R. An emerging source of plastic pollution: Environmental presence of plastic personal protective equipment (PPE) debris related to COVID-19 in a metropolitan city. Environ. Pollut. 2021, 269, 116160. [Google Scholar] [CrossRef]
- Pan American Health Organization-PAHO. About Coronaviruses. Available online: https://www.paho.org/en/topics/coronavirus-infections (accessed on 16 January 2022).
- Abou Elmaaty, T.; Sayed-Ahmed, K.; Elsisi, H.; Ramadan, S.M.; Sorour, H.; Magdi, M.; Abdeldayem, S.A. Novel antiviral and antibacterial durable polyester fabrics printed with selenium nanoparticles (SeNPs). Polymers 2022, 14, 955. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Nath, K.; Parekh, Y.; Enayathullah, M.G.; Bokara, K.K.; Sinhamahapatra, A. Antimicrobial silver nanoparticle-photodeposited fabrics for SARS-CoV-2 destruction. Colloid Interface Sci. Commun. 2021, 45, 100542. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.S.; Shamim, S.U.D.; Rahman, M.K.; Ahmed, F.; Gafur, M.A. The development of ZnO nanoparticle coated cotton fabrics for antifungal and antibacterial applications. Mater. Sci. Appl. 2020, 11, 601–610. [Google Scholar] [CrossRef]
- Pakdel, E.; Fang, J.; Sun, L.; Wang, X. Nanocoatings for smart textiles. In Smart Textiles: Wearable Nanotechnology; Yilmaz, N.D., Ed.; Scrivener Publishing: Salem, MA, USA, 2019; pp. 247–300. [Google Scholar]
- Román, L.E.; Gomez, E.D.; Solís, J.L.; Gómez, M.M. Antibacterial cotton fabric functionalized with copper oxide nanoparticles. Molecules 2020, 25, 5802. [Google Scholar] [CrossRef]
- Perelshtein, I.; Applerot, G.; Perkas, N.; Wehrschuetz-Sigl, E.; Hasmann, A.; Guebitz, G.; Gedanken, A. CuO–cotton nanocomposite: Formation, morphology, and antibacterial activity. Surf. Coat. Technol. 2009, 204, 54–57. [Google Scholar] [CrossRef]
- Zarbaf, D.; Montazer, M.; Sadeghian Maryan, A. In-situ synthesis of nano-copper on denim garment along with nano-clay for antibacterial and decoloration purposes. Cellulose 2017, 24, 4083–4095. [Google Scholar] [CrossRef]
- Román, L.E.; Amézquita, M.J.; Uribe, C.L.; Maurtua, D.J.; Costa, S.A.; Costa, S.M.; Keiski, R.; Solís, J.L.; Gómez, M.M. In situ growth of CuO nanoparticles onto cotton textiles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 025009. [Google Scholar] [CrossRef]
- Gordon, S.; Hsieh, Y.L. Cotton: Science and Technology; Woodhead Publishing: Cambridge, UK, 2007. [Google Scholar]
- Clark, M. Handbook of Textile and Industrial Dyeing. Volumen 1: Principles, Processes and Types of Dyes; Woodhead Publishing: Cambridge, UK, 2011. [Google Scholar]
- Bourbigot, S. Flame retardancy of textiles: New approaches. In Advances in Fire Retardant Materials; Horrocks, A.R., Price, D., Eds.; Woodhead Publishing: Cambridge, UK, 2008; pp. 9–40. [Google Scholar]
- Vigneswaran, C.; Ananthasubramanian, M.; Kandhavadivu, P. Bioprocessing of synthetic fibres. In Bioprocessing of Textiles: Fundamentals for Applications and Research Perspective; Vigneswaran, C., Ananthasubramanian, M., Kandhavadivu, P., Eds.; Woodhead Publishing: New Dheli, India, 2014; pp. 189–250. [Google Scholar]
- Gandhi, K.L. Woven Textiles: Principles, Technologies and Applications; Woodhead Publishing: Duxford, UK, 2020. [Google Scholar]
- Hu, J. Fabric Testing; Woodhead Publishing: Cambridge, UK, 2008. [Google Scholar]
- AATCC. Evaluation Procedure 1-2007-Gray Scale for Color Change; American Association of Textile Chemists and Colorists: Research Triangle Park, NC, USA, 2009. [Google Scholar]
- Gómez, M.M.; Uribe, C.L.; Maurtua, D.; Román, L.E.; Castro, F.V.; Amézquita, M. Materiales Textiles Antimicrobianos Con Protección a La Radiación UV; y Métodos de Funcionalización Para la Obtención de Los Mismos; INDECOPI, Ed.; Universidad Nacional de Ingeniería: Lima, Peru, 2015. [Google Scholar]
- American Association of Textile Chemists and Colorists. Test Method 61-Colorfastness to Laundering: Accelerated; American Association of Textile Chemists & Colorists (AATCC): Research Triangle Park, NC, USA, 2020. [Google Scholar]
- American Association of Textile Chemists and Colorists. Test Method 15-Colorfastness to Perspiration; American Association of Textile Chemists & Colorists (AATCC): Research Triangle Park, NC, USA, 2012. [Google Scholar]
- American Association of Textile Chemists and Colorists. Test Method 8-Colorfastness to Crocking: Crockmeter Method; American Association of Textile Chemists & Colorists (AATCC): Research Triangle Park, NC, USA, 2016. [Google Scholar]
- American Association of Textile Chemists and Colorists. Test Method 16-Colorfastness to Light: Xenon-arc; American Association of Textile Chemists & Colorists (AATCC): Research Triangle Park, NC, USA, 2020. [Google Scholar]
- ASTM D3775; Standard Test Method for End (Warp) and Pick (Filling) Count of Woven Fabrics. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D1424; Standard Test Method for Tearing Strength of Fabrics by Falling-Pendulum (Elmendorf-Type) Apparatus. ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM D5034; Standard Test Method for Breaking Strength and Elongation of Textile Fabrics (Grab Test). ASTM International: West Conshohocken, PA, USA, 2017.
- ISO 18184; Textiles-Determination of Antiviral Activity of Textile Products. ISO: Geneva, Switzerland, 2019.
- ASTM E2149; Standard Test Method for Determining the Antimicrobial Activity of Antimicrobial Agents under Dynamic Contact Conditions. ASTM International: West Conshohocken, PA, USA, 2013.
- Schindler, W.D.; Hauser, P.J. Chemical Finishing of Textiles; Woodhead Publishing: Cambridge, UK, 2004; ISBN 1-85573-905-4. [Google Scholar]
- Yu, M.; Wang, Q.; Yang, W.; Xu, Y.; Zhang, M.; Deng, Q.; Liu, G. Facile fabrication of magnetic, durable and superhydrophobic cotton for efficient oil/water separation. Polymers 2019, 11, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Chen, S.; Wang, W.; Tian, M.; Ning, N.; Zhang, L. Polyester (PET) fabrics coated with environmentally friendly adhesive and its interface structure and adhesive properties with rubber. Compos. Sci. Technol. 2020, 195, 108171. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Fouda, A.; Salem, S.S. Integration of cotton fabrics with biosynthesized CuO nanoparticles for bactericidal activity in the terms of their cytotoxicity assessment. Ind. Eng. Chem. Res. 2021, 60, 1553–1563. [Google Scholar] [CrossRef]
- Ashraf, M.; Champagne, P.; Campagne, C.; Perwuelz, A.; Dumont, F.; Leriche, A. Study the multi self-cleaning characteristics of ZnO nanorods functionalized polyester fabric. J. Ind. Text. 2014, 45, 1440–1456. [Google Scholar] [CrossRef]
- Harifi, T.; Montazer, M. Application of sonochemical technique for sustainable surface modification of polyester fibers resulting in durable nano-sonofinishing. Ultrason. Sonochem. 2017, 37, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Shaikh, A.; Dusane, R.O.; Parida, S. Copper oxide nanosheets and nanowires grown by one-step linear sweep voltammetry for supercapacitor application. J. Energy Storage 2020, 31, 101631. [Google Scholar] [CrossRef]
- Vogel, A. Química Analítica Cualitativa; Kapelusz: Buenos Aires, Argentina, 1991; ISBN 978-9-5013-3550-7. [Google Scholar]
- Huang, M.-L.; Cai, Z.; Wu, Y.-Z.; Lu, S.-G.; Luo, B.-S.; Li, Y.-H. Metallic coloration on polyester fabric with sputtered copper and copper oxides films. Vacuum 2020, 178, 109489. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Ghosh, S.; Bhaumik, S. Tearing and tensile strength behaviour of military khaki fabrics from grey to finished process. IJCST 2006, 18, 247–264. [Google Scholar] [CrossRef]
- Camero, M.; Lanave, G.; Catella, C.; Lucente, M.S.; Decaro, N.; Martella, V.; Buonavoglia, C. Evaluation of virucidal activity of fabrics using feline coronavirus. J. Virol. Methods 2021, 295, 114214. [Google Scholar] [CrossRef]
- Hewawaduge, C.; Senevirathne, A.; Jawalagatti, V.; Kim, J.W.; Lee, J.H. Copper-impregnated three-layer mask efficiently inactivates SARS-CoV2. Environ. Res. 2021, 196, 110947. [Google Scholar] [CrossRef]
- Behzadinasab, S.; Chin, A.; Hosseini, M.; Poon, L.; Ducker, W.A. A surface coating that rapidly inactivates SARS-CoV-2. ACS Appl. Mater. Interfaces 2020, 12, 34723–34727. [Google Scholar] [CrossRef]
- Hosseini, M.; Chin, A.W.H.; Behzadinasab, S.; Poon, L.L.M.; Ducker, W.A. Cupric oxide coating that rapidly reduces infection by SARS-CoV-2 via solids. ACS Appl. Mater. Interfaces 2021, 13, 5919–5928. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, F.; Amiri, A.; Bajuri, M.Y.; Yuhana, N.Y.; Ferrara, M. Role of different types of nanomaterials against diagnosis, prevention and therapy of COVID-19. Sustain. Cities Soc. 2021, 72, 103046. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.T.J.; Boisvert, L.N.; Zuo, Y.Y. Face masks against COVID-19: Standards, efficacy, testing and decontamination methods. Adv. Colloid Interface Sci. 2021, 292, 102435. [Google Scholar] [CrossRef]
- Shionoiri, N.; Sato, T.; Fujimori, Y.; Nakayama, T.; Nemoto, M.; Matsunaga, T.; Tanaka, T. Investigation of the antiviral properties of copper iodide nanoparticles against feline calicivirus. J. Biosci. Bioeng. 2012, 113, 580–586. [Google Scholar] [CrossRef]
- Metwally, R.A.; El Sikaily, A.; El-Sersy, N.A.; Ghozlan, H.A.; Sabry, S.A. Antimicrobial activity of textile fabrics dyed with prodigiosin pigment extracted from marine Serratia rubidaea RAM_Alex bacteria. Egypt. J. Aquat. Res. 2021, 47, 301–305. [Google Scholar] [CrossRef]
- Tamayo, L.; Azocar, M.; Kogan, M.; Riveros, A.; Paez, M. Copper-polymer nanocomposites: An excellent and cost-effective biocide for use on antibacterial surfaces. Mater. Sci. Eng. C 2016, 69, 1391–1409. [Google Scholar] [CrossRef]
- Stanić, V.; Tanasković, S.B. Antibacterial activity of metal oxide nanoparticles. In Nanotoxicity, 1st ed.; Rajendran, S., Mukherjee, A., Nguyen, T.A., Godugu, C., Shukla, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 241–274. ISBN 978-0-12-819943-5. [Google Scholar]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [Green Version]
- Rani, I.; Goyal, A.; Bhatnagar, M.; Manhas, S.; Goel, P.; Pal, A.; Prasad, R. Potential molecular mechanisms of zinc- and copper-mediated antiviral activity on COVID-19. Nutr. Res. 2021, 92, 109–128. [Google Scholar] [CrossRef]
- Erxleben, A. Interactions of copper complexes with nucleic acids. Coord. Chem. Rev. 2018, 360, 92–121. [Google Scholar] [CrossRef]

| Evaluation | Colorfastness | |||||
|---|---|---|---|---|---|---|
| Laundering | Perspiration | Crocking | Light | |||
| Staining | Multifiber test fabrics | Acetate | 5 | 4.5 | --- | --- |
| Cotton | 4.5 | 4.5 | --- | --- | ||
| Nylon | 5 | 4.5 | --- | --- | ||
| Polyester | 5 | 4.5 | --- | --- | ||
| Acrylic | 4.5 | 4.5 | --- | --- | ||
| Wool | 2.5 | 4.5 | --- | --- | ||
| White test | Dry | --- | --- | 5 | --- | |
| Wet | --- | --- | 4 | --- | ||
| Color change | 3 | 2.3 | --- | 2 | ||
| Fabric | Yarn Density | Breaking Strength (N) | Tearing Strength (N) | |||
|---|---|---|---|---|---|---|
| Ends/In | Picks/In | Warp | Weft | Warp | Weft | |
| Non-functionalized | 147 | 82 | 769 | 368 | 22 | 12.5 |
| Functionalized | 155 | 81 | 792 | 393 | 17 | 8.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Román, L.E.; Villalva, C.; Uribe, C.; Paraguay-Delgado, F.; Sousa, J.; Vigo, J.; Vera, C.M.; Gómez, M.M.; Solís, J.L. Textiles Functionalized with Copper Oxides: A Sustainable Option for Prevention of COVID-19. Polymers 2022, 14, 3066. https://doi.org/10.3390/polym14153066
Román LE, Villalva C, Uribe C, Paraguay-Delgado F, Sousa J, Vigo J, Vera CM, Gómez MM, Solís JL. Textiles Functionalized with Copper Oxides: A Sustainable Option for Prevention of COVID-19. Polymers. 2022; 14(15):3066. https://doi.org/10.3390/polym14153066
Chicago/Turabian StyleRomán, Luz Esmeralda, Cleny Villalva, Carmen Uribe, Francisco Paraguay-Delgado, José Sousa, Johnny Vigo, Concepción Mercedes Vera, Mónica Marcela Gómez, and José Luis Solís. 2022. "Textiles Functionalized with Copper Oxides: A Sustainable Option for Prevention of COVID-19" Polymers 14, no. 15: 3066. https://doi.org/10.3390/polym14153066
APA StyleRomán, L. E., Villalva, C., Uribe, C., Paraguay-Delgado, F., Sousa, J., Vigo, J., Vera, C. M., Gómez, M. M., & Solís, J. L. (2022). Textiles Functionalized with Copper Oxides: A Sustainable Option for Prevention of COVID-19. Polymers, 14(15), 3066. https://doi.org/10.3390/polym14153066








