Anisotropic Microstructure and Performance Characterization of Wild Silkworm Cocoons for Designing Biomimetic Protective Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Specifications Measurements
2.3. Scanning Electron Microscope (SEM) Observation
2.4. Fourier Transform Infrared Spectra (FITR)
2.5. Tensile Properties Test
3. Results and Discussions
3.1. Specifications of Cocoon
3.2. Morphological Characteristics of the Component Layers of Silkworm Cocoon
3.3. FTIR Spectra Analysis
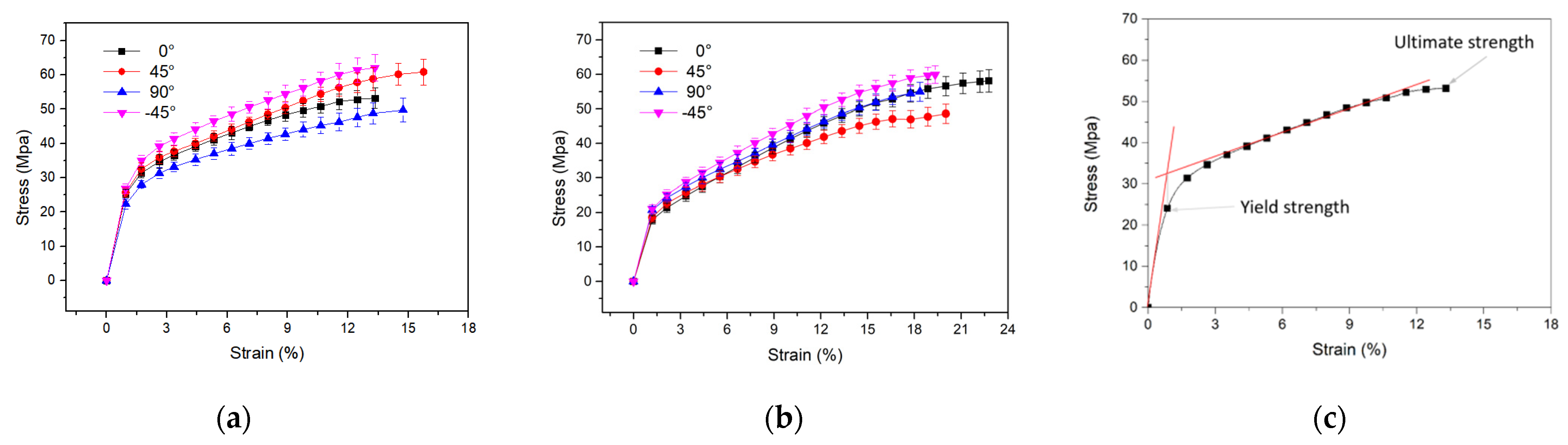
3.4. Tensile Mechanical Properties of Cocoon Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Kaur, J.; Rajkhowa, R.; Li, J.L.; Liu, X.Y.; Wang, X.G. Mechanical properties and structure of silkworm cocoons: A comparative study of Bombyx mori, Antheraea assamensis, Antheraea pernyi and Antheraea mylitta silkworm cocoons. Mater. Sci. Eng. C 2013, 33, 3206–3213. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Steiner, H.; Engstrom, A.; Bennich, H.; Boman, H.G. Insect Immunity : Isolation and Structure of Cecropins B and D from Pupae of the Chinese Oak Silk Moth. Anthevaeapernyi 1982, 224, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, J.; Xing, J.; Zhang, K.; Zhou, Y.; Pan, C.; Wei, Z.; Zhao, Y. Silkworm Cocoon Layer with Gradient Structure as Separator for Lithium-Ion Battery. Energy Technol. 2022, 10, 2100996. [Google Scholar] [CrossRef]
- Chen, F.; Porter, D.; Vollrath, F. Structure and physical properties of silkworm cocoons. J. R. Soc. Interface 2012, 9, 2299–2308. [Google Scholar] [CrossRef] [Green Version]
- Kwak, H.W.; Eom, J.; Cho, S.Y.; Lee, M.E.; Jin, H.J. High-toughness natural polymer nonwoven preforms inspired by silkworm cocoon structure. Int. J. Biol. Macromol. 2019, 127, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, Y.; Liang, C.; Wang, P.; Hu, D. The preparation and characteristics of high puncture resistant composites inspired by natural silk cocoon. Compos. Part A Appl. Sci. Manuf. 2021, 149, 106537. [Google Scholar] [CrossRef]
- Kaur, J.; Rajkhowa, R.; Tsuzuki, T.; Wang, X. Crystals in Antheraea assamensis silkworm cocoon: Their removal, recovery and roles. Mater. Des. 2015, 88, 236–244. [Google Scholar] [CrossRef]
- Zhao, H.P.; Feng, X.Q.; Yu, S.W.; Cui, W.Z.; Zou, F.Z. Mechanical properties of silkworm cocoons. Polymer 2005, 46, 9192–9201. [Google Scholar] [CrossRef]
- Zhao, H.P.; Feng, X.Q.; Cui, W.Z.; Zou, F.Z. Mechanical properties of silkworm cocoon pelades. Eng. Fract. Mech. 2007, 74, 1953–1962. [Google Scholar] [CrossRef]
- Chen, F.; Porter, D.; Vollrath, F. Silk cocoon (Bombyx mori): Multi-layer structure and mechanical properties. Acta Biomater. 2012, 8, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tan, Y.; Chen, L.; Jing, L.; Wu, D.; Wang, T. High fibre-volume silkworm cocoon composites with strong structure bonded by polyurethane elastomer for high toughness. Compos. Part A Appl. Sci. Manuf. 2019, 125, 105553. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, H. Structure and functions of cocoons constructed by Eri Silkworm. Polymers 2020, 12, 2701. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, H.; Zhou, H.; Wang, K.; Wang, S.; Zhou, W. Comparative Analysis of Structure and Properties of Stereoscopic Cocoon and Flat Cocoon. Autex Res. J. 2021, 21, 1–7. [Google Scholar] [CrossRef]
- Chen, F.; Porter, D.; Vollrath, F. Morphology and structure of silkworm cocoons. Mater. Sci. Eng. C 2012, 32, 772–778. [Google Scholar] [CrossRef]
- Guan, J.; Zhu, W.; Liu, B.; Yang, K.; Vollrath, F.; Xu, J. Comparing the microstructure and mechanical properties of Bombyx mori and Antheraea pernyi cocoon composites. Acta Biomater. 2017, 47, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, C.; Wang, Z. Investigation of the microstructural characteristics and the tensile strength of silkworm cocoons using X-ray micro computed tomography. Mater. Des. 2021, 199, 109436. [Google Scholar] [CrossRef]
- Zhang, J.; Rajkhowa, R.; Li, J.L.; Liu, X.Y.; Wang, X.G. Silkworm cocoon as natural material and structure for thermal insulation. Mater. Des. 2013, 49, 842–849. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, J.; Gao, W.; Li, J.; Wang, X. Cocoon of the silkworm Antheraea pernyi as an example of a thermally insulating biological interface. Biointerphases 2014, 9, 031013. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, J.; Jin, X.; Du, S.; Kaur, J.; Wang, X. Natural and highly protective composite structures—Wild silkworm cocoons. Compos. Commun. 2017, 4, 1–4. [Google Scholar] [CrossRef]
- Zhou, X.; Song, W.; Lu, Y. Investigation on the microstructural characteristics and quasi-static puncture resistance of both domesticated and wild silkworm cocoons. Text. Res. J. 2020, 90, 2714–2726. [Google Scholar] [CrossRef]
- Kaur, J.; Rajkhowa, R.; Tsuzuki, T.; Millington, K.; Zhang, J.; Wang, X. Photoprotection by silk cocoons. Biomacromolecules 2013, 14, 3660–3667. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, H.; Zhao, J.; Chen, J.; Zhou, H. Study on Structure and Anti-Uv Properties of Sericin Cocoons. Autex Res. J. 2022, 22, 1–7. [Google Scholar] [CrossRef]
- Du, S.; Li, J.; Zhang, J.; Wang, X. Microstructure and mechanical properties of silk from different components of the Antheraea pernyi cocoon. Mater. Des. 2015, 65, 766–771. [Google Scholar] [CrossRef]
- Dai, Z.J.; Sun, W.; Zhang, Z. Comparative analysis of iTRAQ-based proteomes for cocoons between the domestic silkworm (Bombyx mori) and wild silkworm (Bombyx mandarina). J. Proteom. 2019, 192, 366–373. [Google Scholar] [CrossRef]
- Khodarahmi Borujeni, E.; Shaikhzadeh Najar, S.; Kamali Dolatabadi, M. The study on structural properties and tensile strength of reared silkworm cocoon. J. Text. Inst. 2018, 109, 195–201. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, D.; Meng, Z.; Dong, Z.; Lin, Y.; Chen, S.; Xia, Q.; Zhao, P. Wild silkworm cocoon contains more metabolitesthan domestic silkworm cocoon to improve its protection. J. Insect Sci. 2017, 17, 105. [Google Scholar] [CrossRef]
- Lee, Y. Silk Reeling and Testing Manual; p23/316; Food and Agriculture Organization of the United Nations: Rome, Italy, 1999. [Google Scholar]
- Li, J.Y.; Ye, L.P.; Che, J.Q.; Song, J.; You, Z.Y.; Yun, K.C.; Wang, S.H.; Zhong, B.X. Comparative proteomic analysis of the silkworm middle silk gland reveals the importance of ribosome biogenesis in silk protein production. J. Proteom. 2015, 126, 109–120. [Google Scholar] [CrossRef]
- Takasu, Y.; Yamada, H.; Tsubouchi, K. Isolation of Three Main Sericin Components from the Cocoon of the Silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 2002, 66, 2715–2718. [Google Scholar] [CrossRef]
- Roy, M.; Kumar, S.; Tejas, M.; Kusurkar, S. Carbondioxide Gating in Silk Cocoon. Biointerphases 2012, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhang, Y.; Chen, H.; Zhang, R.; Yang, X.; Wang, P.; Ma, P. The Analysis and Comparison of the Needle-puncture Property of Four Types of Silkworm Cocoons. Fibers Polym. 2020, 21, 1868–1877. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Li, Y.; Xu, Y.; Liao, Y.; Yang, X.; Wang, P. A new perspective on the needle-puncture property of silkworm cocoons with a hierarchical multi-layer structure. Compos. Commun. 2020, 22, 100539. [Google Scholar] [CrossRef]
- Garside, P.; Lahlil, S.; Wyeth, P. Characterization of historic silk by polarized attenuated total reflectance fourier transform infrared spectroscopy for informed conservation. Appl. Spectrosc. 2005, 59, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- de Palaminy, L.; Daher, C.; Moulherat, C. Development of a non-destructive methodology using ATR-FTIR and chemometrics to discriminate wild silk species in heritage collections. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2022, 270, 120788. [Google Scholar] [CrossRef]
- Ling, S.; Qi, Z.; Knight, D.P.; Huang, Y.; Huang, L.; Zhou, H.; Shao, Z.; Chen, X. Insight into the structure of single Antheraea pernyi silkworm fibers using synchrotron FTIR microspectroscopy. Biomacromolecules 2013, 14, 1885–1892. [Google Scholar] [CrossRef]
- You, Q.; Li, Q.; Zheng, H.; Hu, Z.; Zhou, Y.; Wang, B. Discerning Silk Produced by Bombyx mori from Those Produced by Wild Species using ELISA Combined with Conventional Methods. J. Agric. Food Chem. 2017, 65, 7805–7812. [Google Scholar] [CrossRef]
- Magoshi, J.; Mizuide, M.; Magoshi, Y. Physical Properties and Structure of Silk. VI. Conformational Changes in Silk Fibroin Induced by Immersion in Water at 2 to 130 °C. J. Polym. Sci. Polym. Phys. Ed. 1979, 17, 515–520. [Google Scholar] [CrossRef]
- Poza, P.; Pérez-Rigueiro, J.; Elices, M.; LLorca, J. Fractographic analysis of silkworm and spider silk. Eng. Fract. Mech. 2002, 69, 1035–1048. [Google Scholar] [CrossRef]
- Zhang, J.; Du, S.; Kafi, A.; Fox, B.; Li, J.L.; Liu, X.Y.; Rajkhowa, R.; Wang, X.G. Surface energy of silk fibroin and mechanical properties of silk cocoon composites. RSC Adv. 2015, 5, 1640–1647. [Google Scholar] [CrossRef]
- Miura, M.; Kato, H.; Iwasa, M.; Morikawa, H. Analysis of the construction process of cocoon shape by Bombyx mori. J. Sericultural Sci. Jpn. 1997, 66, 23–30. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, W.; Gao, X.; Meng, W.; Guan, J. Strain rate and anisotropic microstructure dependent mechanical behaviors of silkworm cocoon shells. PLoS ONE 2016, 11, e0149931. [Google Scholar] [CrossRef] [Green Version]

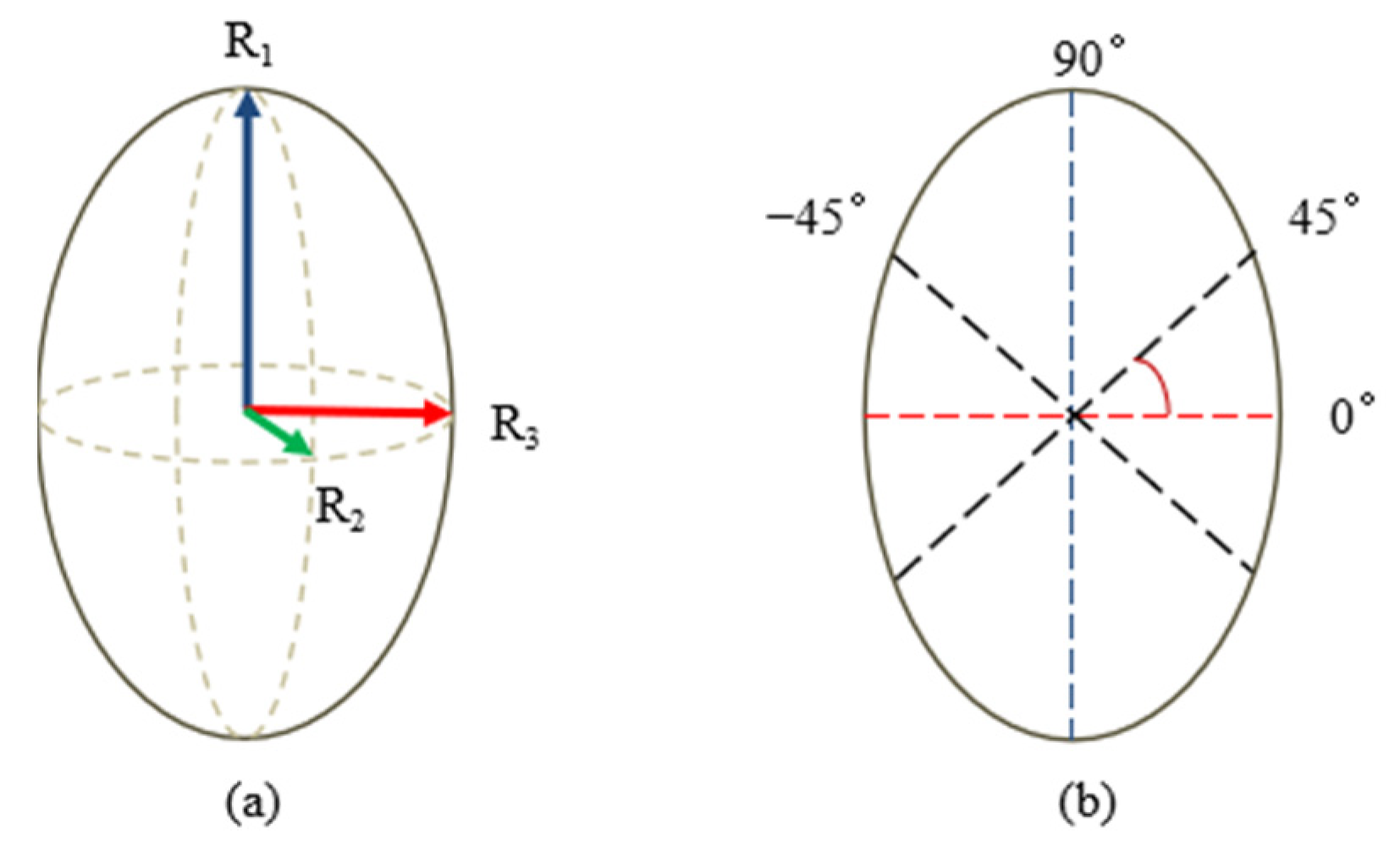
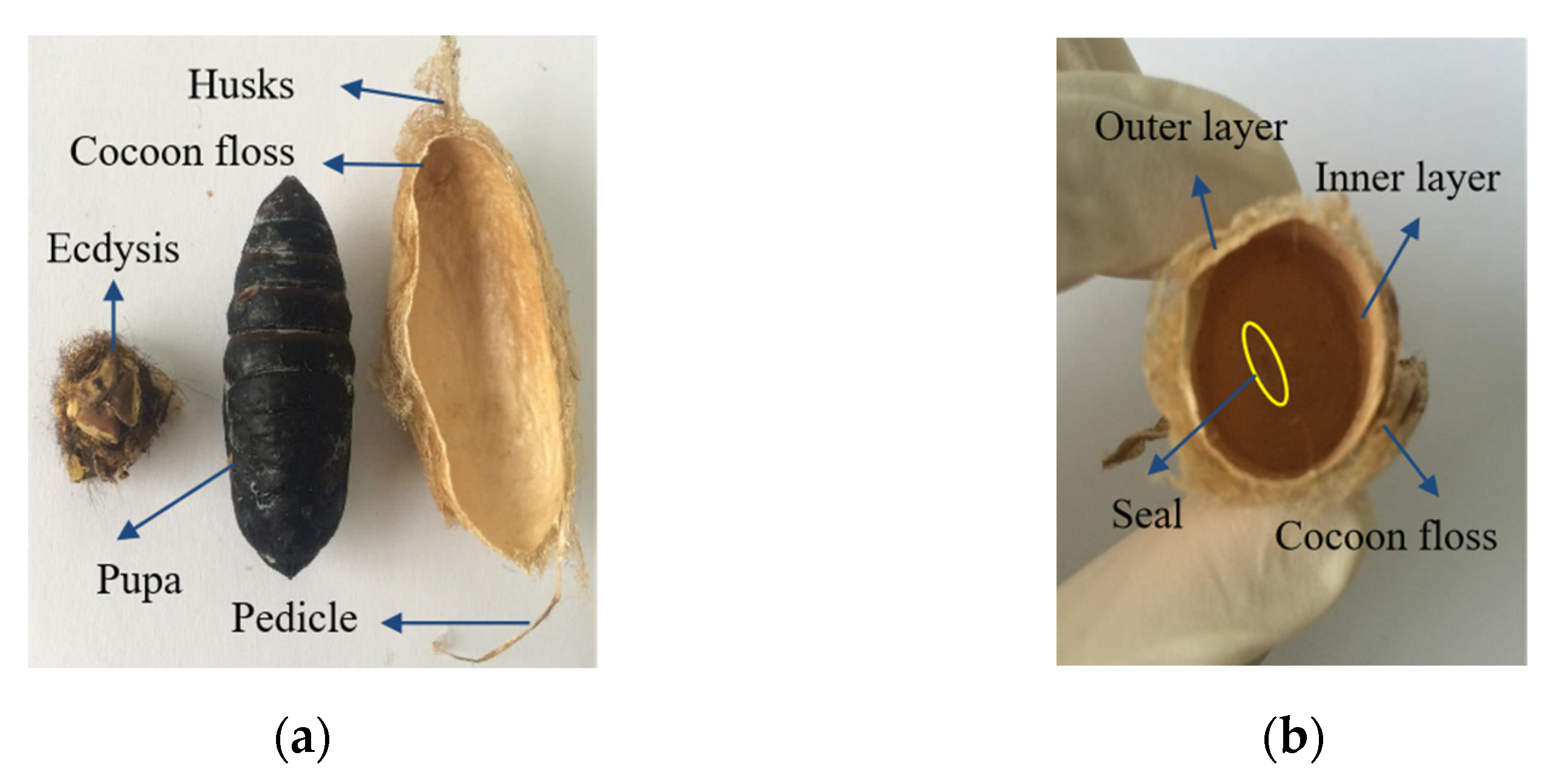

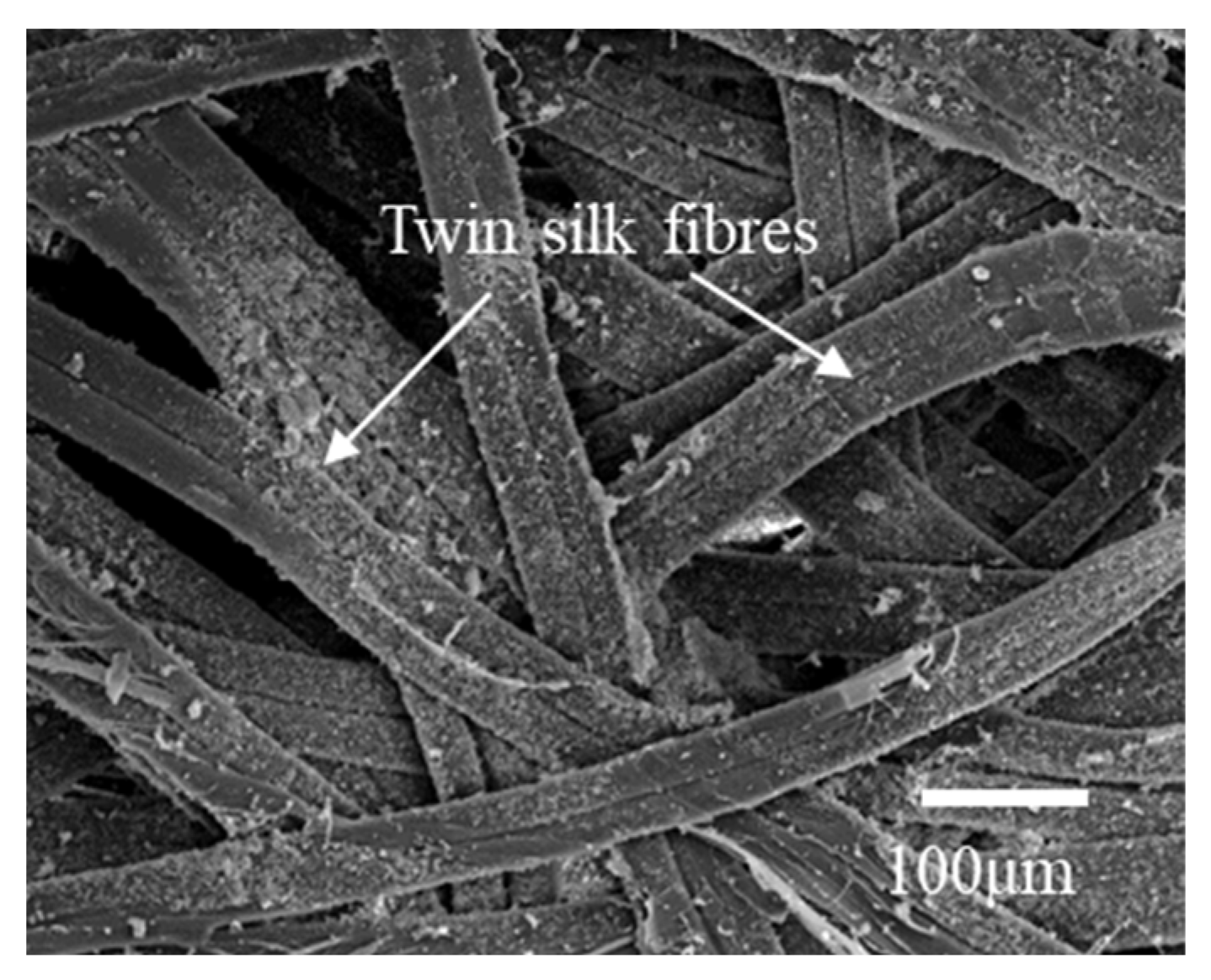

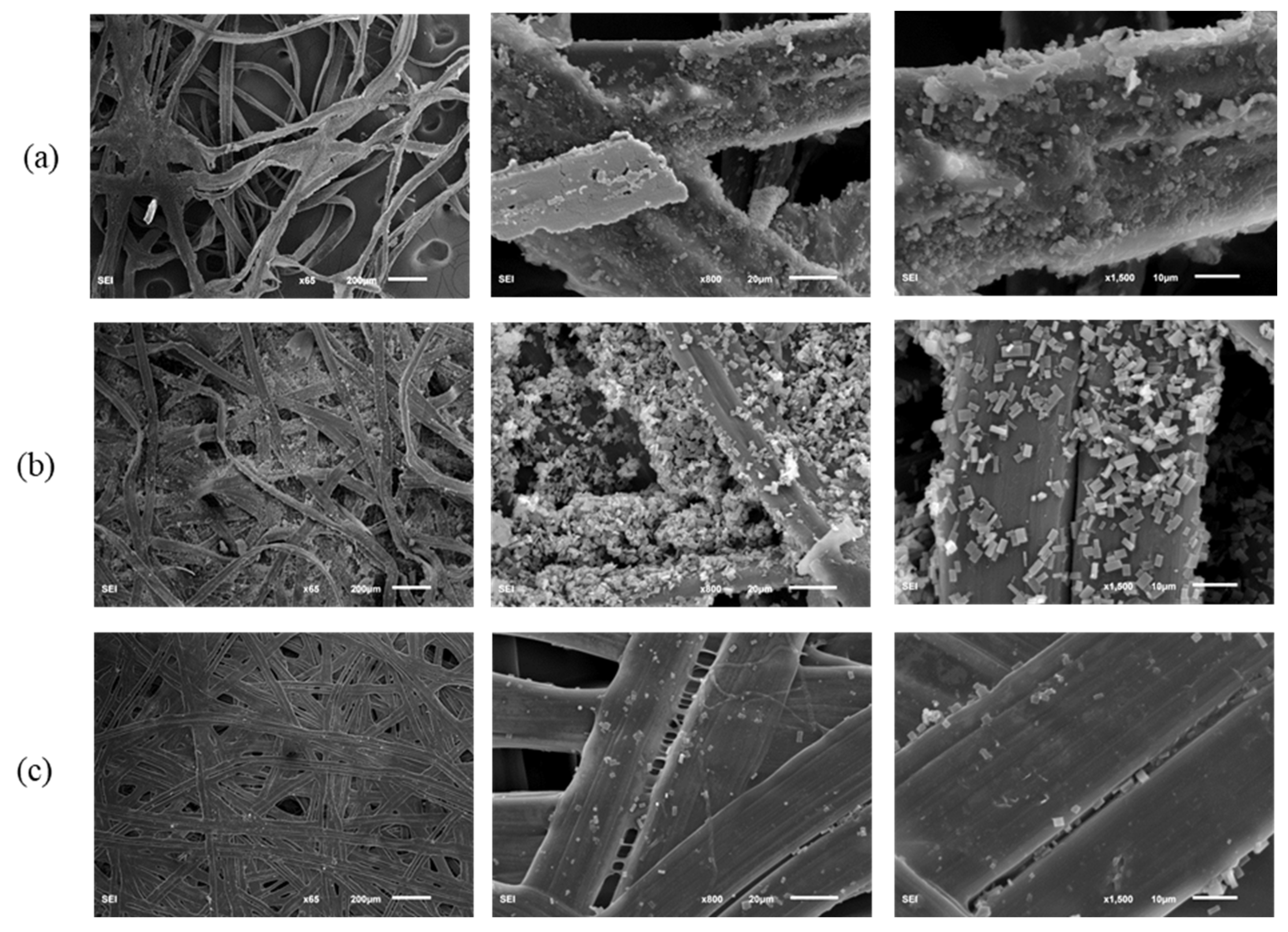
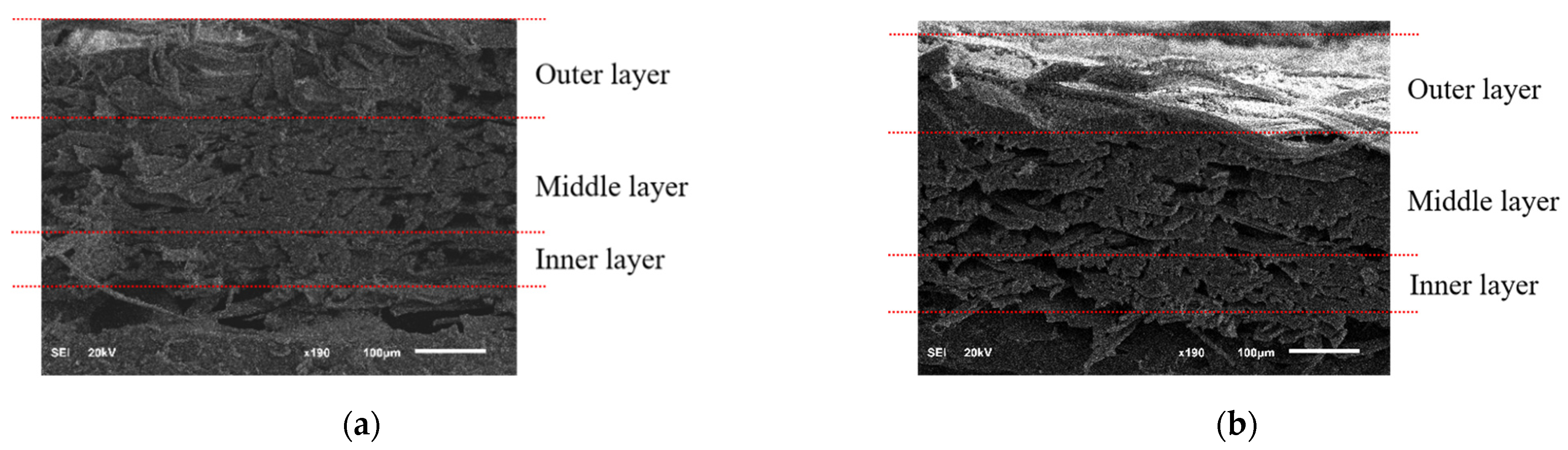

| Weight (g) | Thickness (mm) | Length (mm) | Width (mm) | Ratio | |||
|---|---|---|---|---|---|---|---|
| 2R1 | 2R2 | 2R3 | R1/R2 | R1/R3 | |||
| A. pernyi (H) | 0.54 ± 0.04 | 0.39 ± 0.08 | 41.45 ± 3.60 | 21.32 ± 1.43 | 22.51 ± 1.38 | 1.94 | 1.84 |
| A. pernyi (L) | 0.84 ± 0.05 | 0.45 ± 0.06 | 46.52 ± 3.10 | 23.89 ± 1.52 | 24.67 ± 1.55 | 1.95 | 1.89 |
| p-value | 1.14 × 10−7 | 7.30 × 10−4 | 2.11 × 10−5 | 4.98 × 10−7 | 2.11 × 10−6 | - | - |
| Fiber Bonding Length (μm) | Porosity (%) | ||
|---|---|---|---|
| A. pernyi (H) | Outer layer | 51 ± 9 | 25.65 ± 1.84 |
| Middle layer | 61 ± 4 | 10.25 ± 1.02 | |
| Inner layer | 63 ± 5 | 5.21 ± 1.01 | |
| A. pernyi (L) | Outer layer | 55 ± 4 | 11.62 ± 2.67 |
| Middle layer | 65 ± 4 | 5.61 ± 0.66 | |
| Inner layer | 66 ± 5 | 3.70 ± 0.90 |
| RA1265 | RA1235 | Crystallization Index | |
|---|---|---|---|
| A. pernyi (H) | 0.98 | 0.97 | 1.01 |
| A. pernyi (L) | 0.85 | 0.81 | 1.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Luo, J.; Xiong, Y.; Wu, J. Anisotropic Microstructure and Performance Characterization of Wild Silkworm Cocoons for Designing Biomimetic Protective Materials. Polymers 2022, 14, 3072. https://doi.org/10.3390/polym14153072
Li M, Luo J, Xiong Y, Wu J. Anisotropic Microstructure and Performance Characterization of Wild Silkworm Cocoons for Designing Biomimetic Protective Materials. Polymers. 2022; 14(15):3072. https://doi.org/10.3390/polym14153072
Chicago/Turabian StyleLi, Mengru, Jie Luo, Yi Xiong, and Jisong Wu. 2022. "Anisotropic Microstructure and Performance Characterization of Wild Silkworm Cocoons for Designing Biomimetic Protective Materials" Polymers 14, no. 15: 3072. https://doi.org/10.3390/polym14153072
APA StyleLi, M., Luo, J., Xiong, Y., & Wu, J. (2022). Anisotropic Microstructure and Performance Characterization of Wild Silkworm Cocoons for Designing Biomimetic Protective Materials. Polymers, 14(15), 3072. https://doi.org/10.3390/polym14153072





