Synthesis Characterization and Highly Protective Efficiency of Tetraglycidyloxy Pentanal Epoxy Prepolymer as a Potential Corrosion Inhibitor for Mild Steel in 1 M HCl Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of 2,3,4,5-Tetraglycidyloxy Pentanal (TGP)
2.2. Tested Material
2.3. Characterization Techniques
3. Results
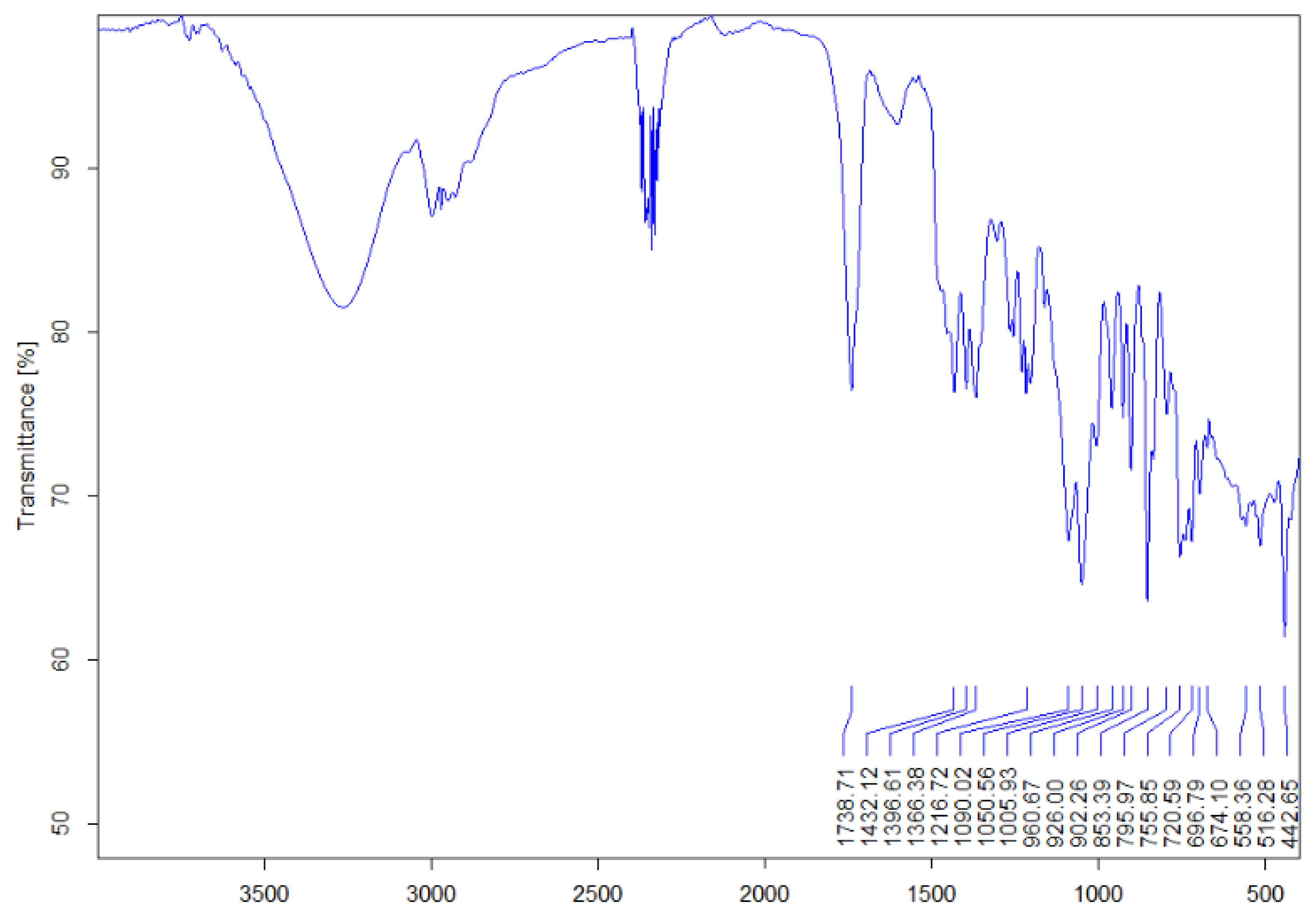
3.1. FTIR Characterization
3.2. NMR Characterization
3.3. PDP Analysis
3.4. EIS Analysis
3.5. SEM/EDS Characterization
3.6. Contact Angle Characterization
3.7. Adsorption Study
3.8. Temperature Effect and Thermodynamic Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rbaa, M.; Benhiba, F.; Hssisou, R.; Lakhrissi, Y.; Lakhrissi, B.; Touhami, M.E.; Warad, I.; Zarrouk, A. Green synthesis of novel carbohydrate polymer chitosan oligosaccharide grafted on d-glucose derivative as bio-based corrosion inhibitor. J. Mol. Liq. 2021, 322, 114549. [Google Scholar] [CrossRef]
- Galai, M.; Rbaa, M.; Ouakki, M.; Abousalem, A.S.; Ech-Chihbi, E.; Dahmani, K.; Dkhireche, N.; Lakhrissi, B.; EbnTouhami, M. Chemically functionalized of 8-hydroxyquinoline derivatives as efficient corrosion inhibition for steel in 1.0 M HCl solution: Experimental and theoretical studies. Surf. Interfaces 2020, 21, 100695. [Google Scholar] [CrossRef]
- Benhiba, F.; Hsissou, R.; Benzekri, Z.; Belghiti, M.E.; Lamhamdi, A.; Bellaouchou, A.; Guenbour, A.; Boukhris, S.; Oudda, H.; Warad, I.; et al. Nitro substituent effect on the electronic behavior and inhibitory performance of two quinoxaline derivatives in relation to the corrosion of mild steel in 1 M HCl. J. Mol. Liq. 2020, 312, 113367. [Google Scholar] [CrossRef]
- Benhiba, F.; Hsissou, R.; Benzekri, Z.; Echihi, S.; El-Blilak, J.; Boukhris, S.; Bellaouchou, A.; Guenbour, A.; Oudda, H.; Warad, I.; et al. DFT/electronic scale, MD simulation and evaluation of 6-methyl-2-(p-tolyl)-1,4-dihydroquinoxaline as a potential corrosion inhibition. J. Mol. Liq. 2021, 335, 116539. [Google Scholar] [CrossRef]
- Lachiri, A.; El Faydy, M.; Benhiba, F.; Zarrok, H.; El Azzouzi, M.; Zertoubi, M.; Azzi, M.; Lakhrissi, B.; Zarrouk, A. Inhibitor effect of new azomethine derivative containing an 8-hydroxyquinoline moiety on corrosion behavior of mild carbon steel in acidic media. Int. J. Corros. Scale Inhib. 2018, 7, 609–632. [Google Scholar]
- Damej, M.; Hsissou, R.; Berisha, A.; Azgaou, K.; Sadiku, M.; Benmessaoud, M.; Labjar, N.; El hajjaji, S. New epoxy resin as a corrosion inhibitor for the protection of carbon steel C38 in 1 M HCl. experimental and theoretical studies (DFT, MC, and MD). J. Mol. Struct. 2022, 1254, 132425. [Google Scholar] [CrossRef]
- Dagdag, O.; Safi, Z.; Hsissou, R.; Erramli, H.; El Bouchti, M.; Wazzan, N.; Guo, L.; Verma, C.; Ebenso, E.; El Harfi, A. Epoxy pre-polymers as new and effective materials for corrosion inhibition of carbon steel in acidic medium: Computational and experimental studies. Sci. Rep. 2019, 9, 11715. [Google Scholar] [CrossRef]
- Dagdag, O.; Hsissou, R.; El Harfi, A.; El Gana, L.; Safi, Z.; Guo, L.; Verma, C.; Ebenso, E.E.; El Gouri, M. Development and Anti-corrosion Performance of Polymeric Epoxy Resin and their Zinc Phosphate Composite on 15CDV6 Steel in 3wt% NaCl: Experimental and Computational Studies. J. Bio- Tribo-Corros. 2020, 6, 112. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Akpan, E.D.; Quraishi, M.A.; Dagdag, O.; El Gouri, M.; Sherif, E.-S.M.; Ebenso, E.E. Epoxy resins as anticorrosive polymeric materials: A review. React. Funct. Polym. 2020, 156, 104741. [Google Scholar] [CrossRef]
- Hssissou, R.; Benzidia, B.; Hajjaji, N.; Elharfi, A. Elaboration, Electrochemical Investigation and morphological Study of the Coating Behavior of a new Polymeric Polyepoxide Architecture: Crosslinked and Hybrid Decaglycidyl of Phosphorus Penta methylene Dianiline on E24 Carbon Steel in 3.5% NaCl. Port. Electrochim. Acta 2019, 37, 179–191. [Google Scholar] [CrossRef]
- Hsissou, R.; Benzidia, B.; Rehioui, M.; Berradi, M.; Berisha, A.; Assouag, M.; Hajjaji, N.; Elharfi, A. Anticorrosive property of hexafunctional epoxy polymer HGTMDAE for E24 carbon steel corrosion in 1.0 M HCl: Gravimetric, electrochemical, surface morphology and molecular dynamic simulations. Polym. Bull. 2020, 77, 3577–3601. [Google Scholar] [CrossRef]
- Hsissou, R.; Bekhta, A.; Elharfi, A.; Benzidia, B.; Hajjaji, N. Theoretical and electrochemical studies of the coating behavior of a new epoxy polymer: Hexaglycidyl ethylene of methylene dianiline (HGEMDA) on E24 steel in 3.5% NaCl. Port. Electrochim. Acta 2018, 36, 101–117. [Google Scholar] [CrossRef]
- Yuan, W.; Hu, Q.; Zhang, J.; Huang, F.; Liu, J. Hydrophobic Modification of Graphene Oxide and Its Effect on the Corrosion Resistance of Silicone-Modified Epoxy Resin. Metals 2021, 11, 89. [Google Scholar] [CrossRef]
- Xiong, G.; Kang, P.; Zhang, J.; Li, B.; Yang, J.; Chen, G.; Zhou, Z.; Li, Q. Improved adhesion, heat resistance, anticorrosion properties of epoxy resins/POSS/methyl phenyl silicone coatings. Prog. Org. Coat. 2019, 135, 454–464. [Google Scholar] [CrossRef]
- Hsissou, R.; Abbout, S.; Benhiba, F.; Seghiri, R.; Safi, Z.; Kaya, S.; Briche, S.; Serdaroğlu, G.; Erramli, H.; Elbachiri, A.; et al. Insight into the corrosion inhibition of novel macromolecular epoxy resin as highly efficient inhibitor for carbon steel in acidic mediums: Synthesis, characterization, electrochemical techniques, AFM/UV–Visible and computational investigations. J. Mol. Liq. 2021, 337, 116492. [Google Scholar] [CrossRef]
- Hsissou, R.; Abbout, S.; Safi, Z.; Benhiba, F.; Wazzan, N.; Guo, L.; Nouneh, K.; Briche, S.; Erramli, H.; Touhami, M.E.; et al. Synthesis and anticorrosive properties of epoxy polymer for CS in [1 M] HCl solution: Electrochemical, AFM, DFT and MD simulations. Constr. Build. Mater. 2021, 270, 121454. [Google Scholar] [CrossRef]
- El-Aouni, N.; Hsissou, R.; Safi, Z.; Abbout, S.; Benhiba, F.; El Azzaoui, J.; Haldhar, R.; Wazzan, N.; Guo, L.; Erramli, H.; et al. Performance of two new epoxy resins as potential corrosion inhibitors for carbon steel in 1MHCl medium: Combining experimental and computational approaches. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127066. [Google Scholar] [CrossRef]
- Molhi, A.; Hsissou, R.; Damej, M.; Berisha, A.; Thaçi, V.; Belafhaili, A.; Benmessaoud, M.; Labjar, N.; El Hajjaji, S. Contribution to the corrosion inhibition of C38 steel in 1 M hydrochloric acid medium by a new epoxy resin PGEPPP. Int. J. Corros. Scale Inhib 2021, 10, 399–418. [Google Scholar]
- Molhi, A.; Hsissou, R.; Damej, M.; Berisha, A.; Bamaarouf, M. Performance of two epoxy resins against corrosion of C38 steel in 1 M HCl: Electrochemical, thermodynamic and theoretical assessment. Int. J. Corros. Scale Inhib. 2021, 10, 812–837. [Google Scholar]
- Abbout, S.; Hsissou, R.; Erramli, H.; Chebabe, D.; Salim, R.; Kaya, S.; Hajjaji, N. Gravimetric, electrochemical and theoretical study, and surface analysis of novel epoxy resin as corrosion inhibitor of carbon steel in 0.5 M H2SO4 solution. J. Mol. Struct. 2021, 1245, 131014. [Google Scholar] [CrossRef]
- Abbout, S.; Hsissou, R.; Chebabe, D.; Erramli, H.; Hajjaji, N. Investigation of the anti-corrosion properties of Galactomannan as additive in epoxy coatings for carbon steel: Rheological and electrochemical study. Inorg. Chem. Commun. 2021, 134, 108971. [Google Scholar] [CrossRef]
- Hsissou, R.; Azogagh, M.; Benhiba, F.; Echihi, S.; Galai, M.; Shaim, A.; Bahaj, H.; Briche, S.; Kaya, S.; Serdaroğlu, G.; et al. Insight of development of two cured epoxy polymer composite coatings as highly protective efficiency for carbon steel in sodium chloride solution: DFT, RDF, FFV and MD approaches. J. Mol. Liq. 2022, 360, 119406. [Google Scholar] [CrossRef]
- Guo, L.; Safi, Z.S.; Kaya, S.; Shi, W.; Tüzün, B.; Altunay, N.; Kaya, C. Anticorrosive Effects of Some Thiophene Derivatives Against the Corrosion of Iron: A Computational Study. Front. Chem. 2018, 6, 155. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, Ş.; Safi, Z.S.; Kaya, S.; Işın, D.Ö.; Guo, L.; Kaya, C. A computational study on corrosion inhibition performances of novel quinoline derivatives against the corrosion of iron. J. Mol. Struct. 2017, 1134, 751–761. [Google Scholar] [CrossRef]
- Dagdag, O.; Hsissou, R.; El Harfi, A.; Safi, Z.; Berisha, A.; Verma, C.; Ebenso, E.E.; Quraishi, M.A.; Wazzan, N.; Jodeh, S.; et al. Epoxy resins and their zinc composites as novel anti-corrosive materials for copper in 3% sodium chloride solution: Experimental and computational studies. J. Mol. Liq. 2020, 315, 113757. [Google Scholar] [CrossRef]
- Hsissou, R.; Dagdag, O.; Berradi, M.; El Bouchti, M.; Assouag, M.; El Bachiri, A.; Elharfi, A. Investigation of structure and rheological behavior of a new epoxy polymer pentaglycidyl ether pentabisphenol A of phosphorus and of its composite with natural phosphate. SN Appl. Sci. 2019, 1, 869. [Google Scholar] [CrossRef] [Green Version]
- Hsissou, R.; Berradi, M.; El Bouchti, M.; El Bachiri, A.; El Harfi, A. Synthesis characterization rheological and morphological study of a new epoxy resin pentaglycidyl ether pentaphenoxy of phosphorus and their composite (PGEPPP/MDA/PN). Polym. Bull. 2019, 76, 4859–4878. [Google Scholar] [CrossRef]
- Benhiba, F.; Benzekri, Z.; Guenbour, A.; Tabyaoui, M.; Bellaouchou, A.; Boukhris, S.; Oudda, H.; Warad, I.; Zarrouk, A. Combined electronic/atomic level computational, surface (SEM/EDS), chemical and electrochemical studies of the mild steel surface by quinoxalines derivatives anti-corrosion properties in 1 mol⋅L-1 HCl solution. Chin. J. Chem. Eng. 2020, 28, 1436–1458. [Google Scholar] [CrossRef]
- Hsissou, R.; Benhiba, F.; Abbout, S.; Dagdag, O.; Benkhaya, S.; Berisha, A.; Erramli, H.; Elharfi, A. Trifunctional epoxy polymer as corrosion inhibition material for carbon steel in 1.0 M HCl: MD simulations, DFT and complexation computations. Inorg. Chem. Commun. 2020, 115, 107858. [Google Scholar] [CrossRef]
- Hsissou, R.; Benhiba, F.; Dagdag, O.; El Bouchti, M.; Nouneh, K.; Assouag, M.; Briche, S.; Zarrouk, A.; Elharfi, A. Development and potential performance of prepolymer in corrosion inhibition for carbon steel in 1.0 M HCl: Outlooks from experimental and computational investigations. J. Colloid Interface Sci. 2020, 574, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Echihi, S.; Hsissou, R.; Benzbiria, N.; Afrokh, M.; Boudalia, M.; Bellaouchou, A.; Guenbour, A.; Azzi, M.; Tabyaoui, M. Performance of Methanolic Extract of Artemisia herba alba as a Potential Green Inhibitor on Corrosion Behavior of Mild Steel in Hydrochloric Acid Solution. Biointerface Res. Appl. Chem. 2021, 11, 14751–14763. [Google Scholar]
- Dagdag, O.; Hsissou, R.; Berisha, A.; Erramli, H.; Hamed, O.; Jodeh, S.; El Harfi, A. Polymeric-based epoxy cured with a polyaminoamide as an anticorrosive coating for aluminum 2024-T3 surface: Experimental studies supported by computational modeling. J. Bio- Tribo-Corros. 2019, 5, 58. [Google Scholar] [CrossRef]
- Hsissou, R.; Dagdag, O.; Berradi, M.; El Bouchti, M.; Assouag, M.; Elharfi, A. Development rheological and anti-corrosion property of epoxy polymer and its composite. Heliyon 2019, 5, e02789. [Google Scholar] [CrossRef] [PubMed]
- Hsissou, R.; Dagdag, O.; Abbout, S.; Benhiba, F.; Berradi, M.; El Bouchti, M.; Berisha, A.; Hajjaji, N.; Elharfi, A. Novel derivative epoxy resin TGETET as a corrosion inhibition of E24 carbon steel in 1.0 M HCl solution. Experimental and computational (DFT and MD simulations) methods. J. Mol. Liq. 2019, 284, 182–192. [Google Scholar] [CrossRef]
- Ouass, A.; Galai, M.; Ouakki, M.; Ech-Chihbi, E.; Kadiri, L.; Hsissou, R.; Essaadaoui, Y.; Berisha, A.; Cherkaoui, M.; Lebkiri, A.; et al. Poly(sodium acrylate) and Poly(acrylic acid sodium) as an eco-friendly corrosion inhibitor of mild steel in normal hydrochloric acid: Experimental, spectroscopic and theoretical approach. J. Appl. Electrochem. 2021, 51, 1009–1032. [Google Scholar] [CrossRef]
- Hsissou, R.; Benhiba, F.; Echihi, S.; Benzidia, B.; Cherrouf, S.; Haldhar, R.; Alvi, P.A.; Kaya, S.; Serdaroğlu, G.; Zarrouk, A. Performance of curing epoxy resin as potential anticorrosive coating for carbon steel in 3.5% NaCl medium: Combining experimental and computational approaches. Chem. Phys. Lett. 2021, 783, 139081. [Google Scholar] [CrossRef]
- Hsissou, R.; Benhiba, F.; Echihi, S.; Benkhaya, S.; Hilali, M.; Berisha, A.; Briche, S.; Zarrouk, A.; Nouneh, K.; Elharfi, A. New epoxy composite polymers as a potential anticorrosive coatings for carbon steel in 3.5% NaCl solution: Experimental and computational approaches. Chem. Data Collect. 2021, 31, 100619. [Google Scholar] [CrossRef]
- Abd-El-Nabey, B.; Abdel-Gaber, A.; Elewady, G.; Sadeek, M.; Abd-El-Rhman, H. Inhibitive action of benzaldehyde thiosemicarbazones on the corrosion of mild steel in 3 MH3PO4. Int. J. Electrochem. Sci 2012, 7, 11718–11733. [Google Scholar]
- Abdallah, M.; Zaafarany, I.; Fouda, A.; El-Kader, A. Inhibition of zinc corrosion by some benzaldehyde derivatives in HCl solution. J. Mater. Eng. Perform. 2012, 21, 995–1002. [Google Scholar] [CrossRef]
- Hsissou, R.; Abbout, S.; Seghiri, R.; Rehioui, M.; Berisha, A.; Erramli, H.; Assouag, M.; Elharfi, A. Evaluation of corrosion inhibition performance of phosphorus polymer for carbon steel in [1 M] HCl: Computational studies (DFT, MC and MD simulations). J. Mater. Res. Technol. 2020, 9, 2691–2703. [Google Scholar] [CrossRef]










| Con. (M) | −Ecorr (mVSCE) | icorr (µA cm−2) | −βc (mV dec−1) | Βa (mV dec−1) | ƞPDP (%) | |
|---|---|---|---|---|---|---|
| HCl | 1 | 498 | 983.0 | 92.0 | 104.0 | - |
| TGP | 10−6 | 477 | 227.24 | 185.5 | 113.5 | 76.88 |
| 10−5 | 473 | 192.30 | 117.3 | 91.2 | 80.43 | |
| 10−4 | 464 | 154.1 | 141.1 | 89.8 | 84.24 | |
| 10−3 | 453 | 147.42 | 194.3 | 151.2 | 85.0 |
| Concentrations (M) | Rs (Ω cm2) | Rct (Ω cm2) | Q (µF sn−1) | ndl | Cdl (µF/cm2) | ƞEIS (%) | |
|---|---|---|---|---|---|---|---|
| HCl | 1 | 1.107 | 34.81 | 420.0 | 0.772 | 121.0 | - |
| TGP | 10−6 | 1.903 | 165.9 | 183.4 | 0.810 | 74.31 | 79.01 |
| 10−5 | 1.622 | 166.5 | 180.9 | 0.808 | 73.00 | 79.09 | |
| 10−4 | 1.930 | 194.5 | 149.7 | 0.804 | 63.00 | 82.14 | |
| 10−3 | 2.271 | 210.2 | 133.1 | 0.798 | 54.33 | 87.17 |
| TGP | Techniques | R2 | Slopes | Intercept (10−6) | Kads (M−1) | ΔGads (KJ mol−1) |
|---|---|---|---|---|---|---|
| PDP | 1 | 1.1759 | 0.622719 | 3.87 | −42.13 | |
| EIS | 0.9999 | 1.14491 | 2.71049 | 1.95 | −43.74 |
| T K | −Ecorr mVAg/AgCl | icorr µA cm−2 | −βc mV dec−1 | % | Ea kJ mol−1 | ΔHa kJ mol−1 | −ΔSa J mol−1K−1 |
|---|---|---|---|---|---|---|---|
| MS electrode/1 M HCl solution | |||||||
| 298 | 498 | 983 | 92 | - | 21.0 | 18.5 | 126.0 |
| 308 | 491 | 1200 | 184 | - | |||
| 318 | 475 | 1450 | 171 | - | |||
| 328 | 465 | 2200 | 161 | - | |||
| Inhibitory resin/MS electrode/1 M HCl solution | |||||||
| 298 | 453 | 147.42 | 194.3 | 85.0 | 26.53 | 23.14 | 123.37 |
| 308 | 486 | 798.0 | 157.2 | 83.5 | |||
| 318 | 494 | 255.0 | 167.1 | 82.2 | |||
| 328 | 499 | 404.19 | 171.4 | 81.6 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsissou, R.; Lachhab, R.; El Magri, A.; Echihi, S.; Vanaei, H.R.; Galai, M.; Ebn Touhami, M.; Rafik, M. Synthesis Characterization and Highly Protective Efficiency of Tetraglycidyloxy Pentanal Epoxy Prepolymer as a Potential Corrosion Inhibitor for Mild Steel in 1 M HCl Medium. Polymers 2022, 14, 3100. https://doi.org/10.3390/polym14153100
Hsissou R, Lachhab R, El Magri A, Echihi S, Vanaei HR, Galai M, Ebn Touhami M, Rafik M. Synthesis Characterization and Highly Protective Efficiency of Tetraglycidyloxy Pentanal Epoxy Prepolymer as a Potential Corrosion Inhibitor for Mild Steel in 1 M HCl Medium. Polymers. 2022; 14(15):3100. https://doi.org/10.3390/polym14153100
Chicago/Turabian StyleHsissou, Rachid, Redouane Lachhab, Anouar El Magri, Siham Echihi, Hamid Reza Vanaei, Mouhsine Galai, Mohamed Ebn Touhami, and Mohamed Rafik. 2022. "Synthesis Characterization and Highly Protective Efficiency of Tetraglycidyloxy Pentanal Epoxy Prepolymer as a Potential Corrosion Inhibitor for Mild Steel in 1 M HCl Medium" Polymers 14, no. 15: 3100. https://doi.org/10.3390/polym14153100
APA StyleHsissou, R., Lachhab, R., El Magri, A., Echihi, S., Vanaei, H. R., Galai, M., Ebn Touhami, M., & Rafik, M. (2022). Synthesis Characterization and Highly Protective Efficiency of Tetraglycidyloxy Pentanal Epoxy Prepolymer as a Potential Corrosion Inhibitor for Mild Steel in 1 M HCl Medium. Polymers, 14(15), 3100. https://doi.org/10.3390/polym14153100








