Cubosomes: Design, Development, and Tumor-Targeted Drug Delivery Applications
Abstract
:1. Introduction
2. Types of Cubosomes
3. Components of Cubosomes
4. Methods of Preparation for Cubosomes
4.1. Top-Down Approach
4.2. Bottom-Up Approach
4.3. Spray-Drying Method
4.4. Solvent Evaporation Method
5. Physical–Chemical Characterizationsof Cubosomes
6. Cubosomes as Tumor-Targeted Drug Delivery
6.1. Skin Cancer Therapy
6.2. Glioblastoma Multiforme Therapy
6.3. Lung Cancer Treatment
6.4. Colorectal Cancer Therapy
6.5. Liver Cancer Treatment
6.6. Ovarian Cancer Treatment
6.7. Cervical Carcinoma
6.8. Hepatocellular Carcinoma Therapy
6.9. Brain Tumor Therapy
6.10. Breast Cancer Therapy
| Tumor | Cubosomes | Assay | Cell Line | Result | Reference |
|---|---|---|---|---|---|
| Liver Cancer | 5-FU-loaded cubosomes | MTT assay | Hep G2 cell line | Cytotoxicity of 5-FU-loadedcubosomes is much higher than free drug only. | [66] |
| Cisplatin- and paclitaxel-loaded cubosomes | MTT assay | Hep G2 cell line | Cytotoxicity of the uncoated-drug-loaded cubosomeswas more than the coated ones, which may be attributed to the faster release of drugs in the case of the uncoated ones. | [70] | |
| Colorectal Cancer | Cisplatin and metformin nanocubosomes | Sulfo-rhodamine B (SRB) assay | HCT-116 | The harmful effects of the drug-loaded nanoparticles were validated by the fraction of the cell survival values, which were higher than the effects of the individual drug. | [65] |
| Breast Cancer | Folic-acid-modified etoposide cubosomes | MTT assay | MCF-7cell lines | When compared with free medication, ETP-Cubs showed a significant increase in cytotoxicity. | [72] |
| Brain Cancer | Cubosomes loaded with temozolomide (TMZ) and doxorubicin (DOX) | MTS cell proliferation assay | T98G GB-derived cell lines | The viability of A172 and T98G cells was significantly reduced after cells were transfected with miR-7-5p and then treated with TMZ. | [68] |
| DOX-loaded cubosomes | MTS cell proliferation assay | T98G glioblastoma cells | DOX incorporated into cubic nanoparticles at a concentration of 2.3µg/mL exerted higher cytotoxicity than direct DOX delivery. | [71] | |
| AT101-loaded cubosomes | Colorimetric WST-1 assay | A172cell lines | Encapsulated AT101 exhibited stronger cytotoxic effects and more extensive rearrangement of actin fibers in GBM cells than free AT101. | [63] | |
| Ovarian Cancer | Icariin cubosomes | MTT assay | SKOV-3 and Caov 3 | Indicated the cytotoxic potential of ICA-Cubs stops cancer cells from multiplying. | [67] |
| Cervical Cancer | Cubosomes loaded with doxorubicin labeled with 177Lu | MTS assay | HeLa cells | The cytotoxicity enhancement became statistically significant only after shorter incubation times. | [73] |
7. Miscellaneous Drug Delivery by Cubosomes
7.1. Ocular Applications
7.2. Dermatological Applications
7.3. Oral Delivery
8. Drugs Embedded in Cubosomes
9. Challenges and Future Perspectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lübbe, A.S.; Alexiou, C.; Bergemann, C. Clinical applications of magnetic drug targeting. J. Surg. Res. 2001, 95, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Passive and active drug targeting: Drug delivery to tumors as an example. Drug Deliv. 2010, 197, 3–53. [Google Scholar]
- Torchilin, V.P. Drug targeting. Eur. J. Pharm. Sci. 2000, 11, S81–S91. [Google Scholar] [CrossRef]
- Strebhardt, K.; Ullrich, A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer 2008, 8, 473–480. [Google Scholar] [CrossRef]
- Mills, J.K.; Needham, D. Targeted drug delivery. Expert Opin. Ther. Pat. 1999, 9, 1499–1513. [Google Scholar] [CrossRef]
- Theek, B.; Gremse, F.; Kunjachan, S.; Fokong, S.; Pola, R.; Pechar, M.; Deckers, R.; Storm, G.; Ehling, J.; Kiessling, F. Characterizing EPR-mediated passive drug targeting using contrast-enhanced functional ultrasound imaging. J. Control. Release 2014, 182, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Béduneau, A.; Saulnier, P.; Hindré, F.; Clavreul, A.; Leroux, J.; Benoit, J. Design of targeted lipid nanocapsules by conjugation of whole antibodies and antibody Fab’fragments. Biomaterials 2007, 28, 4978–4990. [Google Scholar] [CrossRef]
- Hong, M.; Zhu, S.; Jiang, Y.; Tang, G.; Pei, Y. Efficient tumor targeting of hydroxycamptothecin loaded PEGylated niosomes modified with transferrin. J. Control. Release 2009, 133, 96–102. [Google Scholar] [CrossRef]
- Canal, F.; Vicent, M.J.; Pasut, G.; Schiavon, O. Relevance of folic acid/polymer ratio in targeted PEG–epirubicin conjugates. J. Control. Release 2010, 146, 388–399. [Google Scholar] [CrossRef]
- Kirpotin, D.B.; Drummond, D.C.; Shao, Y.; Shalaby, M.R.; Hong, K.; Nielsen, U.B.; Marks, J.D.; Benz, C.C.; Park, J.W. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006, 66, 6732–6740. [Google Scholar] [CrossRef] [Green Version]
- Mikhail, A.S.; Allen, C. Block copolymer micelles for delivery of cancer therapy: Transport at the whole body, tissue and cellular levels. J. Control. Release 2009, 138, 214–223. [Google Scholar] [CrossRef]
- Alavi, M.; Nokhodchi, A. Micro-and nanoformulations of paclitaxel based on micelles, liposomes, cubosomes, and lipid nanoparticles: Recent advances and challenges. Drug Discov. Today 2022, 27, 576–584. [Google Scholar] [CrossRef]
- Faria, A.R.; Silvestre, O.F.; Maibohm, C.; Adão, R.M.; Silva, B.F.; Nieder, J.B. Cubosome nanoparticles for enhanced delivery of mitochondria anticancer drug elesclomol and therapeutic monitoring via sub-cellular NAD (P) H multi-photon fluorescence lifetime imaging. Nano Res. 2019, 12, 991–998. [Google Scholar] [CrossRef]
- Chaudhary, K.; Sharma, D. Cubosomes: A Potential Drug Delivery System. Asian J. Pharm. Res. Dev. 2021, 9, 93–101. [Google Scholar] [CrossRef]
- Lee, K.W.; Nguyen, T.-H.; Hanley, T.; Boyd, B.J. Nanostructure of liquid crystalline matrix determines in vitro sustained release and in vivo oral absorption kinetics for hydrophilic model drugs. Int. J. Pharm. 2009, 365, 190–199. [Google Scholar] [CrossRef]
- Wörle, G.; Siekmann, B.; Koch, M.H.; Bunjes, H. Transformation of vesicular into cubic nanoparticles by autoclaving of aqueous monoolein/poloxamer dispersions. Eur. J. Pharm. Sci. 2006, 27, 44–53. [Google Scholar] [CrossRef]
- Fong, W.-K.; Negrini, R.; Vallooran, J.J.; Mezzenga, R.; Boyd, B.J. Responsive self-assembled nanostructured lipid systems for drug delivery and diagnostics. J. Colloid Interface Sci. 2016, 484, 320–339. [Google Scholar] [CrossRef]
- Barauskas, J.; Johnsson, M.; Joabsson, F.; Tiberg, F. Cubic phase nanoparticles (cubosome): Principles for controlling size, structure, and stability. Langmuir 2005, 21, 2569–2577. [Google Scholar] [CrossRef]
- Caboi, F.; Amico, G.S.; Pitzalis, P.; Monduzzi, M.; Nylander, T.; Larsson, K. Addition of hydrophilic and lipophilic compounds of biological relevance to the monoolein/water system. I. Phase behavior. Chem. Phys. Lipids 2001, 109, 47–62. [Google Scholar] [CrossRef]
- Garg, G.; Saraf, S.; Saraf, S. Cubosomes: An overview. Biol. Pharm. Bull. 2007, 30, 350–353. [Google Scholar] [CrossRef] [Green Version]
- Landau, E.M.; Rosenbusch, J.P. Lipidic cubic phases: A novel concept for the crystallization of membrane proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 14532–14535. [Google Scholar] [CrossRef] [Green Version]
- Esposito, E.; Cortesi, R.; Drechsler, M.; Paccamiccio, L.; Mariani, P.; Contado, C.; Stellin, E.; Menegatti, E.; Bonina, F.; Puglia, C. Cubosome dispersions as delivery systems for percutaneous administration of indomethacin. Pharm. Res. 2005, 22, 2163–2173. [Google Scholar] [CrossRef]
- Rao, S.V.; Sravya, B.N.; Padmalatha, K. A review on cubosome: The novel drug delivery system. GSC Biol. Pharm. Sci. 2018, 5, 076–081. [Google Scholar]
- Higuchi, W.I. Diffusional models useful in biopharmaceutics: Drug release rate processes. J. Pharm. Sci. 1967, 56, 315–324. [Google Scholar] [CrossRef]
- Allen, T.M.; Mehra, T.; Hansen, C.; Chin, Y.C. Stealth liposomes: An improved sustained release system for 1-β-D-arabinofuranosylcytosine. Cancer Res. 1992, 52, 2431–2439. [Google Scholar]
- Spicer, P.T.; Small, W.B.; Lynch, M.L.; Burns, J.L. Dry powder precursors of cubic liquid crystalline nanoparticles (cubosomes). J. Nanoparticle Res. 2002, 4, 297–311. [Google Scholar] [CrossRef]
- Kaasgaard, T.; Drummond, C.J. Ordered 2-D and 3-D nanostructured amphiphile self-assembly materials stable in excess solvent. Phys. Chem. Chem. Phys. 2006, 8, 4957–4975. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.; Ljusberg-Wahren, H.; Almgren, M.; Larsson, K. Cubic lipid—Water phase dispersed into submicron particles. Langmuir 1996, 12, 4611–4613. [Google Scholar] [CrossRef]
- Dong, Y.-D.; Larson, I.; Hanley, T.; Boyd, B.J. Bulk and dispersed aqueous phase behavior of phytantriol: Effect of vitamin E acetate and F127 polymer on liquid crystal nanostructure. Langmuir 2006, 22, 9512–9518. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Laggner, P.; Almgren, M.; Rappolt, M. Self-assembly in monoelaidin aqueous dispersions: Direct vesicles to cubosomes transition. PLoS ONE 2008, 3, e3747. [Google Scholar] [CrossRef]
- Abraham, T.; Hato, M.; Hirai, M. Polymer-Dispersed Bicontinuous Cubic Glycolipid Nanoparticles. Biotechnol. Prog. 2005, 21, 255–262. [Google Scholar] [CrossRef]
- Chong, J.Y.; Mulet, X.; Waddington, L.J.; Boyd, B.J.; Drummond, C.J. Steric stabilisation of self-assembled cubic lyotropic liquid crystalline nanoparticles: High throughput evaluation of triblock polyethylene oxide-polypropylene oxide-polyethylene oxide copolymers. Soft Matter 2011, 7, 4768–4777. [Google Scholar] [CrossRef]
- Chong, J.Y.; Mulet, X.; Waddington, L.J.; Boyd, B.J.; Drummond, C.J. High-throughput discovery of novel steric stabilizers for cubic lyotropic liquid crystal nanoparticle dispersions. Langmuir 2012, 28, 9223–9232. [Google Scholar] [CrossRef]
- Rosa, M.; Rosa Infante, M.; Miguel, M.d.G.; Lindman, B. Spontaneous formation of vesicles and dispersed cubic and hexagonal particles in amino acid-based catanionic surfactant systems. Langmuir 2006, 22, 5588–5596. [Google Scholar] [CrossRef]
- Muller, F.; Salonen, A.; Glatter, O. Monoglyceride-based cubosomes stabilized by Laponite: Separating the effects of stabilizer, pH and temperature. Colloids Surf. A Physicochem. Eng. Asp. 2010, 358, 50–56. [Google Scholar] [CrossRef]
- Dumanli, I. Mechanistic Studies to Elucidate the Role of Lipid Vehicles on Solubility, Formulation and Bioavailability of Poorly Soluble Compounds; University of Rhode Island: Kingston, RI, USA, 2002. [Google Scholar]
- Milak, S.; Zimmer, A. Glycerol monooleate liquid crystalline phases used in drug delivery systems. Int. J. Pharm. 2015, 478, 569–587. [Google Scholar] [CrossRef]
- Rizwan, S.; Hanley, T.; Boyd, B.J.; Rades, T.; Hook, S. Liquid crystalline systems of phytantriol and glyceryl monooleate containing a hydrophilic protein: Characterisation, swelling and release kinetics. J. Pharm. Sci. 2009, 98, 4191–4204. [Google Scholar] [CrossRef]
- Spicer, P.T.; Hayden, K.L.; Lynch, M.L.; Ofori-Boateng, A.; Burns, J.L. Novel process for producing cubic liquid crystalline nanoparticles (cubosomes). Langmuir 2001, 17, 5748–5756. [Google Scholar] [CrossRef]
- Dong, Y.-D.; Larson, I.; Barnes, T.J.; Prestidge, C.A.; Allen, S.; Chen, X.; Roberts, C.J.; Boyd, B.J. Understanding the interfacial properties of nanostructured liquid crystalline materials for surface-specific delivery applications. Langmuir 2012, 28, 13485–13495. [Google Scholar] [CrossRef]
- Dan, Y.; Poo, M.-m. Spike timing-dependent plasticity of neural circuits. Neuron 2004, 44, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, S.B.; Boyd, B.J. Cubosomes: Structure, preparation and use as an antigen delivery system. In Subunit Vaccine Delivery; Springer: Berlin, Germany, 2015; pp. 125–140. [Google Scholar]
- Esposito, E.; Eblovi, N.; Rasi, S.; Drechsler, M.; Di Gregorio, G.M.; Menegatti, E.; Cortesi, R. Lipid-based supramolecular systems for topical application: A preformulatory study. Aaps Pharmsci. 2003, 5, 62–76. [Google Scholar] [CrossRef]
- Um, J.Y.; Chung, H.; Kim, K.S.; Kwon, I.C.; Jeong, S.Y. In vitro cellular interaction and absorption of dispersed cubic particles. Int. J. Pharm. 2003, 253, 71–80. [Google Scholar] [CrossRef]
- Mezzenga, R.; Meyer, C.; Servais, C.; Romoscanu, A.I.; Sagalowicz, L.; Hayward, R.C. Shear rheology of lyotropic liquid crystals: A case study. Langmuir 2005, 21, 3322–3333. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Dawoud, M. Sorbitol based powder precursor of cubosomes as an oral delivery system for improved bioavailability of poorly water soluble drugs. J. Drug Deliv. Sci. Technol. 2016, 35, 106–113. [Google Scholar] [CrossRef]
- Murgia, S.; Falchi, A.M.; Meli, V.; Schillén, K.; Lippolis, V.; Monduzzi, M.; Rosa, A.; Schmidt, J.; Talmon, Y.; Bizzarri, R. Cubosome formulations stabilized by a dansyl-conjugated block copolymer for possible nanomedicine applications. Colloids Surf. B Biointerfaces 2015, 129, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Boge, L.; Hallstensson, K.; Ringstad, L.; Johansson, J.; Andersson, T.; Davoudi, M.; Larsson, P.T.; Mahlapuu, M.; Håkansson, J.; Andersson, M. Cubosomes for topical delivery of the antimicrobial peptide LL-37. Eur. J. Pharm. Biopharm. 2019, 134, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Bessone, C.D.V.; Akhlaghi, S.P.; Tártara, L.I.; Quinteros, D.A.; Loh, W.; Allemandi, D.A. Latanoprost-loaded phytantriol cubosomes for the treatment of glaucoma. Eur. J. Pharm. Sci. 2021, 160, 105748. [Google Scholar] [CrossRef]
- Patil, S.M.; Sawant, S.S.; Kunda, N.K. Inhalable bedaquiline-loaded cubosomes for the treatment of non-small cell lung cancer (NSCLC). Int. J. Pharm. 2021, 607, 121046. [Google Scholar] [CrossRef]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; El-Zahaby, S.A. Norfloxacin loaded nano-cubosomes for enhanced management of otitis externa: In vitro and in vivo evaluation. Int. J. Pharm. 2021, 600, 120490. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Banerjee, S.; Khan, S.; Jha, P.N.; Gupta, G.; Dua, K.; Hasnain, M.S.; Nayak, A.K.; Dubey, S.K.; Singhvi, G. QbD-driven formulation development and evaluation of topical hydrogel containing ketoconazole loaded cubosomes. Mater. Sci. Eng. C 2021, 119, 111548. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Gu, P.; Wusiman, A.; Ni, H.; Xu, S.; Zhang, Y.; Zhu, T.; He, J.; Liu, Z.; Hu, Y. Immunoenhancement effects of chitosan-modified ginseng stem-leaf saponins-encapsulated cubosomes as an ajuvant. Colloids Surf. B Biointerfaces 2021, 204, 111799. [Google Scholar] [CrossRef] [PubMed]
- Sanjana, A.; Ahmed, M.G.; BH, J.G. Development and evaluation of dexamethasone loaded cubosomes for the treatment of vitiligo. Mater. Today: Proc. 2022, 50, 197–205. [Google Scholar] [CrossRef]
- Elsenosy, F.M.; Abdelbary, G.A.; Elshafeey, A.H.; Elsayed, I.; Fares, A.R. Brain Targeting of Duloxetine HCL via Intranasal Delivery of Loaded Cubosomal Gel: In vitro Characterization, ex vivo Permeation, and in vivo Biodistribution Studies. Int. J. Nanomed. 2020, 15, 9517–9537. [Google Scholar] [CrossRef]
- Leaf, C. Why we’re losing the war on cancer (and how to win it). Fortune (Eur. Ed.) 2004, 149, 42–55. [Google Scholar]
- Fan, C.; Gao, W.; Chen, Z.; Fan, H.; Li, M.; Deng, F.; Chen, Z. Tumor selectivity of stealth multi-functionalized superparamagnetic iron oxide nanoparticles. Int. J. Pharm. 2011, 404, 180–190. [Google Scholar] [CrossRef]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.J.; Foy, K.C.; Kaumaya, P.T. Cancer immunotherapy: Present status, future perspective, and a new paradigm of peptide immunotherapeutics. Discov. Med. 2013, 15, 166–176. [Google Scholar]
- Zhai, J.; Tan, F.H.; Luwor, R.B.; Srinivasa Reddy, T.; Ahmed, N.; Drummond, C.J.; Tran, N. In Vitro and In Vivo Toxicity and Biodistribution of Paclitaxel-Loaded Cubosomes as a Drug Delivery Nanocarrier: A Case Study Using an A431 Skin Cancer Xenograft Model. ACS Appl. Bio Mater. 2020, 3, 4198–4207. [Google Scholar] [CrossRef]
- Aleandri, S.; Bandera, D.; Mezzenga, R.; Landau, E.M. Biotinylated cubosomes: A versatile tool for active targeting and codelivery of paclitaxel and a fluorescein-based lipid dye. Langmuir 2015, 31, 12770–12776. [Google Scholar] [CrossRef]
- Flak, D.K.; Adamski, V.; Nowaczyk, G.; Szutkowski, K.; Synowitz, M.; Jurga, S.; Held-Feindt, J. AT101-loaded cubosomes as an alternative for improved glioblastoma therapy. Int. J. Nanomed. 2020, 15, 7415–7431. [Google Scholar] [CrossRef]
- Yang, C.; Merlin, D. Lipid-based drug delivery nanoplatforms for colorectal cancer therapy. Nanomaterials 2020, 10, 1424. [Google Scholar] [CrossRef]
- Saber, M.M.; Al-Mahallawi, A.M.; Nassar, N.N.; Stork, B.; Shouman, S.A. Targeting colorectal cancer cell metabolism through development of cisplatin and metformin nano-cubosomes. BMC Cancer 2018, 18, 822. [Google Scholar] [CrossRef] [Green Version]
- Nasr, M.; Ghorab, M.K.; Abdelazem, A. In vitro and in vivo evaluation of cubosomes containing 5-fluorouracil for liver targeting. Acta Pharm. Sin. B 2015, 5, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Fahmy, U.A.; Fahmy, O.; Alhakamy, N.A. Optimized Icariin Cubosomes Exhibit Augmented Cytotoxicity against SKOV-3 Ovarian Cancer Cells. Pharmaceutics 2021, 13, 20. [Google Scholar] [CrossRef]
- Gajda, E.; Godlewska, M.; Mariak, Z.; Nazaruk, E.; Gawel, D. Combinatory treatment with miR-7-5p and drug-loaded cubosomes effectively impairs cancer cells. Int. J. Mol. Sci. 2020, 21, 5039. [Google Scholar] [CrossRef]
- Saber, S.; Nasr, M.; Saad, A.S.; Mourad, A.A.; Gobba, N.A.; Shata, A.; Hafez, A.-M.; Elsergany, R.N.; Elagamy, H.I.; El-Ahwany, E. Albendazole-loaded cubosomes interrupt the ERK1/2-HIF-1α-p300/CREB axis in mice intoxicated with diethylnitrosamine: A new paradigm in drug repurposing for the inhibition of hepatocellular carcinoma progression. Biomed. Pharmacother. 2021, 142, 112029. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Tian, D.; Sun, L.; Wang, X.; Tian, M. Theranostic combinatorial drug-loaded coated cubosomes for enhanced targeting and efficacy against cancer cells. Cell Death Dis. 2020, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Nazaruk, E.; Majkowska-Pilip, A.; Bilewicz, R. Lipidic cubic-phase nanoparticles—cubosomes for efficient drug delivery to cancer cells. ChemPlusChem 2017, 82, 570–575. [Google Scholar] [CrossRef]
- Tian, Y.; Li, J.-c.; Zhu, J.-x.; Zhu, N.; Zhang, H.-m.; Liang, L.; Sun, L. Folic acid-targeted etoposide cubosomes for theranostic application of cancer cell imaging and therapy. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 2426–2435. [Google Scholar] [CrossRef] [Green Version]
- Cytryniak, A.; Nazaruk, E.; Bilewicz, R.; Górzyńska, E.; Żelechowska-Matysiak, K.; Walczak, R.; Mames, A.; Bilewicz, A.; Majkowska-Pilip, A. Lipidic cubic-phase nanoparticles (cubosomes) loaded with doxorubicin and labeled with 177Lu as a potential tool for combined chemo and internal radiotherapy for cancers. Nanomaterials 2020, 10, 2272. [Google Scholar] [CrossRef] [PubMed]
- Anbarasan, B.; Grace, X.F.; Shanmuganathan, S. An overview of cubosomes—Smart drug delivery system. Sri. Ramachandra J. Med. 2015, 8, 1–4. [Google Scholar]
- Gan, L.; Han, S.; Shen, J.; Zhu, J.; Zhu, C.; Zhang, X.; Gan, Y. Self-assembled liquid crystalline nanoparticles as a novel ophthalmic delivery system for dexamethasone: Improving preocular retention and ocular bioavailability. Int. J. Pharm. 2010, 396, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Rattanapak, T.; Birchall, J.; Young, K.; Ishii, M.; Meglinski, I.; Rades, T.; Hook, S. Transcutaneous immunization using microneedles and cubosomes: Mechanistic investigations using Optical Coherence Tomography and Two-Photon Microscopy. J. Control. Release 2013, 172, 894–903. [Google Scholar] [CrossRef]
- Thadanki, M.; Kumari, P.S.; Prabha, K.S. Overview of cubosomes: A nano particle. Int. J. Res. Pharm. Chem. 2011, 1, 535–541. [Google Scholar]
- Chung, H.; Kim, J.-s.; Um, J.; Kwon, I.C.; Jeong, S. Self-assembled “nanocubicle” as a carrier for peroral insulin delivery. Diabetologia 2002, 45, 448–451. [Google Scholar] [CrossRef] [Green Version]
- Rarokar, N.R.; Saoji, S.D.; Raut, N.A.; Taksande, J.B.; Khedekar, P.B.; Dave, V.S. Nanostructured cubosomes in a thermoresponsive depot system: An alternative approach for the controlled delivery of docetaxel. AAPS Pharmscitech 2016, 17, 436–445. [Google Scholar] [CrossRef] [Green Version]
- Ali, Z.; Sharma, P.K.; Warsi, M.H. Fabrication and evaluation of ketorolac loaded cubosome for ocular drug delivery. J. Appl. Pharm. Sci. 2016, 6, 204–208. [Google Scholar] [CrossRef] [Green Version]
- Morsi, N.M.; Abdelbary, G.A.; Ahmed, M.A. Silver sulfadiazine based cubosome hydrogels for topical treatment of burns: Development and in vitro/in vivo characterization. Eur. J. Pharm. Biopharm. 2014, 86, 178–189. [Google Scholar] [CrossRef]
- Boyd, B.J.; Khoo, S.-M.; Whittaker, D.V.; Davey, G.; Porter, C.J. A lipid-based liquid crystalline matrix that provides sustained release and enhanced oral bioavailability for a model poorly water soluble drug in rats. Int. J. Pharm. 2007, 340, 52–60. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Novel piperine-loaded Tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in Alzheimer’s disease: Pharmaceutical, biological, and toxicological studies. Int. J. Nanomed. 2015, 10, 5459–5473. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.-R.; Li, Q.; Wan, T.; He, B.; Han, J.; Chen, H.-X.; Yang, F.-X.; Wang, W.; Xu, H.-Z.; Ye, T. Galactosylated chitosan/5-fluorouracil nanoparticles inhibit mouse hepatic cancer growth and its side effects. World J. Gastroenterol. WJG 2012, 18, 6076–6087. [Google Scholar] [CrossRef]
- Karami, Z.; Hamidi, M. Cubosomes: Remarkable drug delivery potential. Drug Discov. Today 2016, 21, 789–801. [Google Scholar] [CrossRef]
- Alavi, M.; Webster, T.J. Nano liposomal and cubosomal formulations with platinum-based anticancer agents: Therapeutic advances and challenges. Nanomedicine 2020, 15, 2399–2410. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B.; Drechsler, M.; Garamus, V.M.; Lesieur, S. Protein entrapment in PEGylated lipid nanoparticles. Int. J. Pharm. 2013, 454, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Wibroe, P.P.; Azmi, I.D.M.; Nilsson, C.; Yaghmur, A.; Moghimi, S.M. Citrem modulates internal nanostructure of glyceryl monooleate dispersions and bypasses complement activation: Towards development of safe tunable intravenous lipid nanocarriers. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1909–1914. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [Green Version]
- Murgia, S.; Biffi, S.; Mezzenga, R. Recent advances of non-lamellar lyotropic liquid crystalline nanoparticles in nanomedicine. Curr. Opin. Colloid Interface Sci. 2020, 48, 28–39. [Google Scholar] [CrossRef]
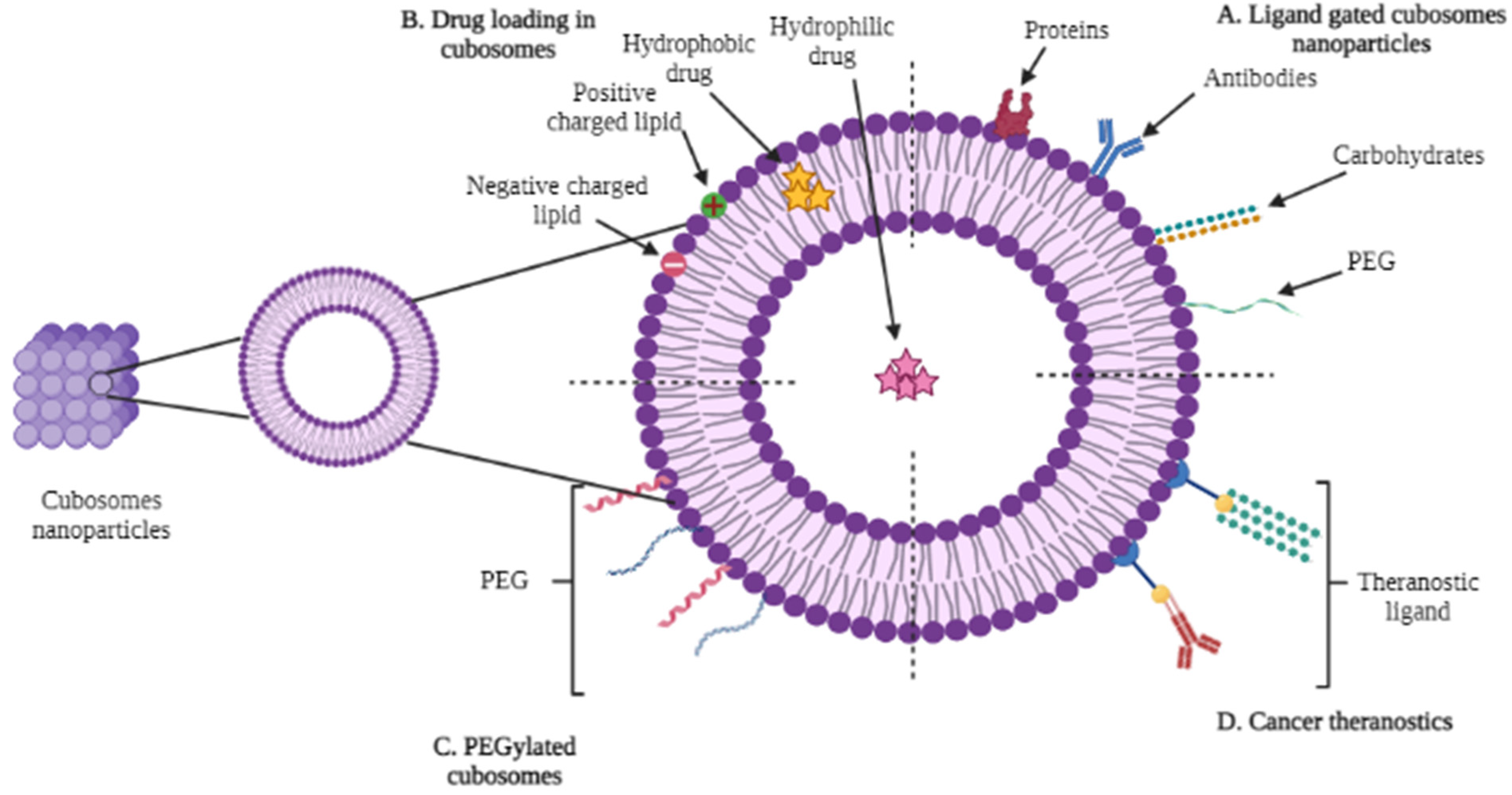
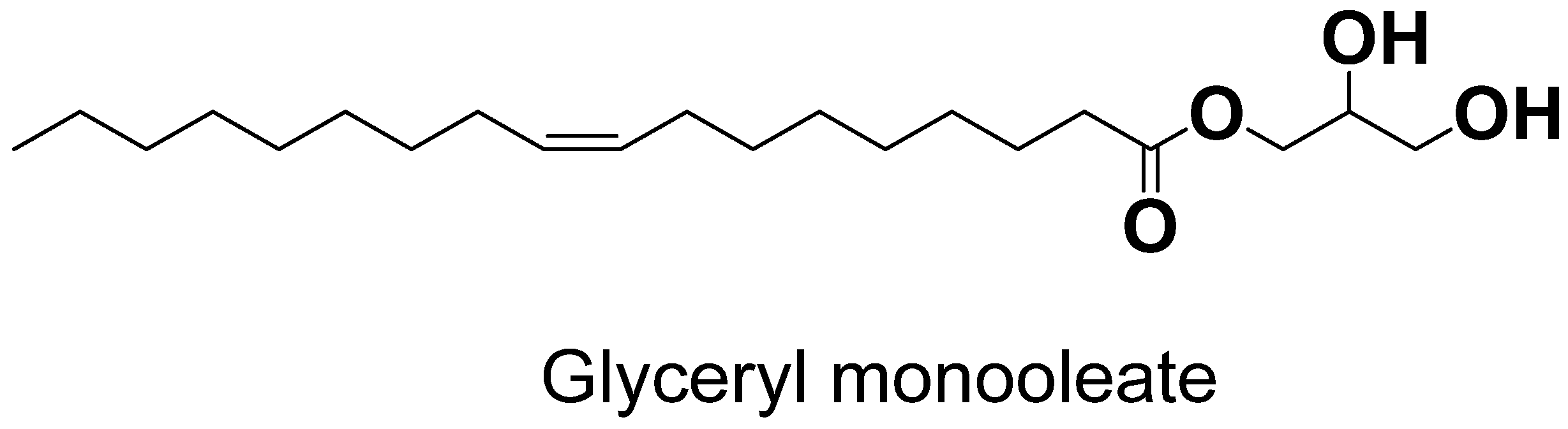
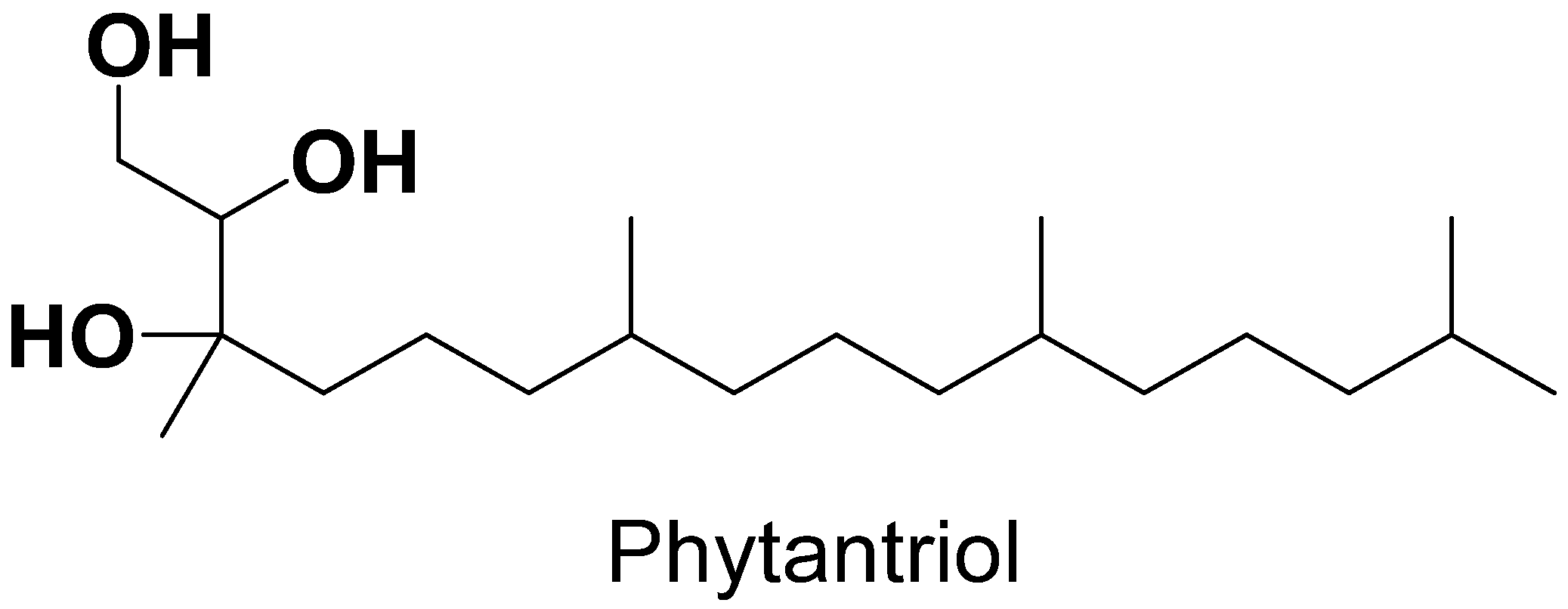
| Lipids | Stabilizer | Reference |
|---|---|---|
| Monoolein | Pluronic F127 | [29] |
| Phytantriol | Pluronic F127 | [30] |
| Monoelaidin | Pluronic F127 | [31] |
| β-XP (1-O-phytanyl-β-d-xyloside) | Pluronic F127 | [32] |
| Monoolein or phytantriol | Pluronic F108 | [33] |
| Phytantriol | Myrj 59 | [34] |
| Monoolein | Modified starch | [27] |
| Sodium octyl sulfate (SCS) | Arginine-based cationicsurfactant | [35] |
| Monoolein | Laponite XLG | [36] |
| Techniques | Benefit | Drawback |
|---|---|---|
| Top-down approach | Formulation has stability against aggregation for up to one year. | It requires high energy input to disperse the aggregates into cubosomes. |
| Bottom-up approach | It requires low energy input; thus, it can be safely used with temperature-sensitive agents. | Preferable for only thermo-sensitive reactants, and preparations are stable for less time. |
| Spray-drying method | The technique is a highly versatile, cheap, and scalable method. It is well-suited for drying labile products, such as vaccines and proteins. | The mixture was difficult to spray-dry as a cubic phase is immediately formed upon hydration of monoolein. |
| Solvent evaporation method | Cubosomes formed using solvent evaporation approach are smaller, with higher physical stability. | High polydispersity of particle sizes is reported due to large-scale mixing of ethanol and water. |
| Cubosomes | Composition of Cubosomes | Particle Size (nm) | Zeta Potential (mV) | Encapsulation Efficiency (EE%) | Polydispersity Index (PDI) | Reference |
|---|---|---|---|---|---|---|
| Cubosomes loaded with antimicrobial peptides | GMO, Poloxamer 407, antimicrobial peptide LL-37 | 191.7 ± 12.0 | −24.8 ± 3.4 | 60.0 | 0.05 ± 0.02 | [49] |
| Latanoprost-loaded phytantriolcubosomes | Phytantriol, F127, latanoprost | 209.3 ± 5.1 | −24.5 ± 0.6 | 94.00 ± 3.16 | 0.11 ± 0.01 | [50] |
| Inhalable-bedaquiline-loaded cubosomes | - | 150.2 ± 5.1 | 35.4 ± 2.3 | 51.85 ± 4.83 | 0.24 ± 0.02 | [51] |
| Norfloxacin-loaded nanocubosomes | GMO, F108, Cremophor | 216.8 ± 2.5 | −41.2 ± 2.3 | 94.3 ± 1.4 | 0.3 ± 0.0 | [52] |
| Ketoconazole-loaded cubosomes | GMO, Poloxamer 407, PVA | 381 ± 2.082 | - | 2.22 ± 1.08 | 0.918 ± 0.0 | [53] |
| Chitosan-modified ginseng stem–leaf-saponins-encapsulated cubosomes | GMO, Poloxamer 407, chitosan, ginseng stem–leaf saponins | 204.93 ± 5.80 | 29.90 ± 0.551 | 60.47 ± 4.72 | 0.160 ± 0.015 | [54] |
| Dexamethasone-loaded cubosomes | GMO, Poloxamer 407, oleic acid | 250.40 nm | −36.10 ± 2.56 | 93.8 | - | [55] |
| Duloxetine-HCL-loaded cubosomalgel | GMO, glycerol tripalmitate, Pluronic F68 and F127 | 145.8 ± 4.8 | 1.6 ± 0.21 | 98.57 ± 0.51 | 1 ± 0.1 | [56] |
| Drugs | Objective of Study | Outcome of Study | Reference |
|---|---|---|---|
| Docetaxel | Synthesis and evaluation of controlled release of cubosomes incorporated with docetaxel as thermo-sensitive depot. | The depot offered gradual drug release, preparation wasfree-flowing at room temperature, and changed to the depot at bodytemperature. | [79] |
| Antimicrobial peptide LL-37 | The antimicrobial potential of cubosomal LL-37 was evaluated using in vitro andexvivo skin irritation models. | The formulation provides superior protection to LL-37 against enzymatic degradation and significant bactericidal effects, and ensures a controlled release. Cubic nanoparticles reduce skin irritation due to LL-37. | [49] |
| Ketorolac | Monoolein and poloxamer cubicnanoparticles for ocular delivery of ketorolac. | Optimized cubosomes loaded with Ketorolac provided transcorneal permeation and retention. | [80] |
| Indomethacin | Evaluation of Indomethacin-fabricated cubosomes for anti-inflammatoryactivity. | Homogenized-monoolein- andpoloxamer-containing-cubosomes prolonged the delivery of lipophilic drugthrough the skin. | [23] |
| Flurbiprofen (FB) | NSAID used for treatment of ocular inflammation. | The formulation expressed less ocular irritation and enhanced trans-corneal permeation of FB. | [80] |
| Erythromycin | Treatment and prevention of several types of acne as a result of its bacteriostaticactivity againstPropionibacterium acnes. | The formulation prevents the acne due to the topical application of erythromycin impregnated with cubosomes. | [81] |
| Insulin | Tested against the C-Type-1-diabetic-induced rat (insulin-dependent diabetes). | Cubosomes provide shield to insulin against proteolysis. It is found to be stable at normal temperature and controlled the hyperglycemia in a reproducible manner. | [82] |
| 20(S)protopanaxadiol (PPD) | To improve the bioavailability of antitumor drug. | Cubosomes enhanced the oral bioavailability of PPD as a result of enhanced absorption of sparinglywater-soluble drug. | [83] |
| Dacarbazine | To reduce the side effectsagainst melanoma. | Dacarbazinedelivered through cubosomes decreases the side effects of intravenous delivery. It also enhanced drug efficacy, safety, and shelf life. | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umar, H.; Wahab, H.A.; Gazzali, A.M.; Tahir, H.; Ahmad, W. Cubosomes: Design, Development, and Tumor-Targeted Drug Delivery Applications. Polymers 2022, 14, 3118. https://doi.org/10.3390/polym14153118
Umar H, Wahab HA, Gazzali AM, Tahir H, Ahmad W. Cubosomes: Design, Development, and Tumor-Targeted Drug Delivery Applications. Polymers. 2022; 14(15):3118. https://doi.org/10.3390/polym14153118
Chicago/Turabian StyleUmar, Hassaan, Habibah A. Wahab, Amirah Mohd Gazzali, Hafsa Tahir, and Waqas Ahmad. 2022. "Cubosomes: Design, Development, and Tumor-Targeted Drug Delivery Applications" Polymers 14, no. 15: 3118. https://doi.org/10.3390/polym14153118
APA StyleUmar, H., Wahab, H. A., Gazzali, A. M., Tahir, H., & Ahmad, W. (2022). Cubosomes: Design, Development, and Tumor-Targeted Drug Delivery Applications. Polymers, 14(15), 3118. https://doi.org/10.3390/polym14153118






